Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging
Abstract
1. Introduction
2. Flavonoids
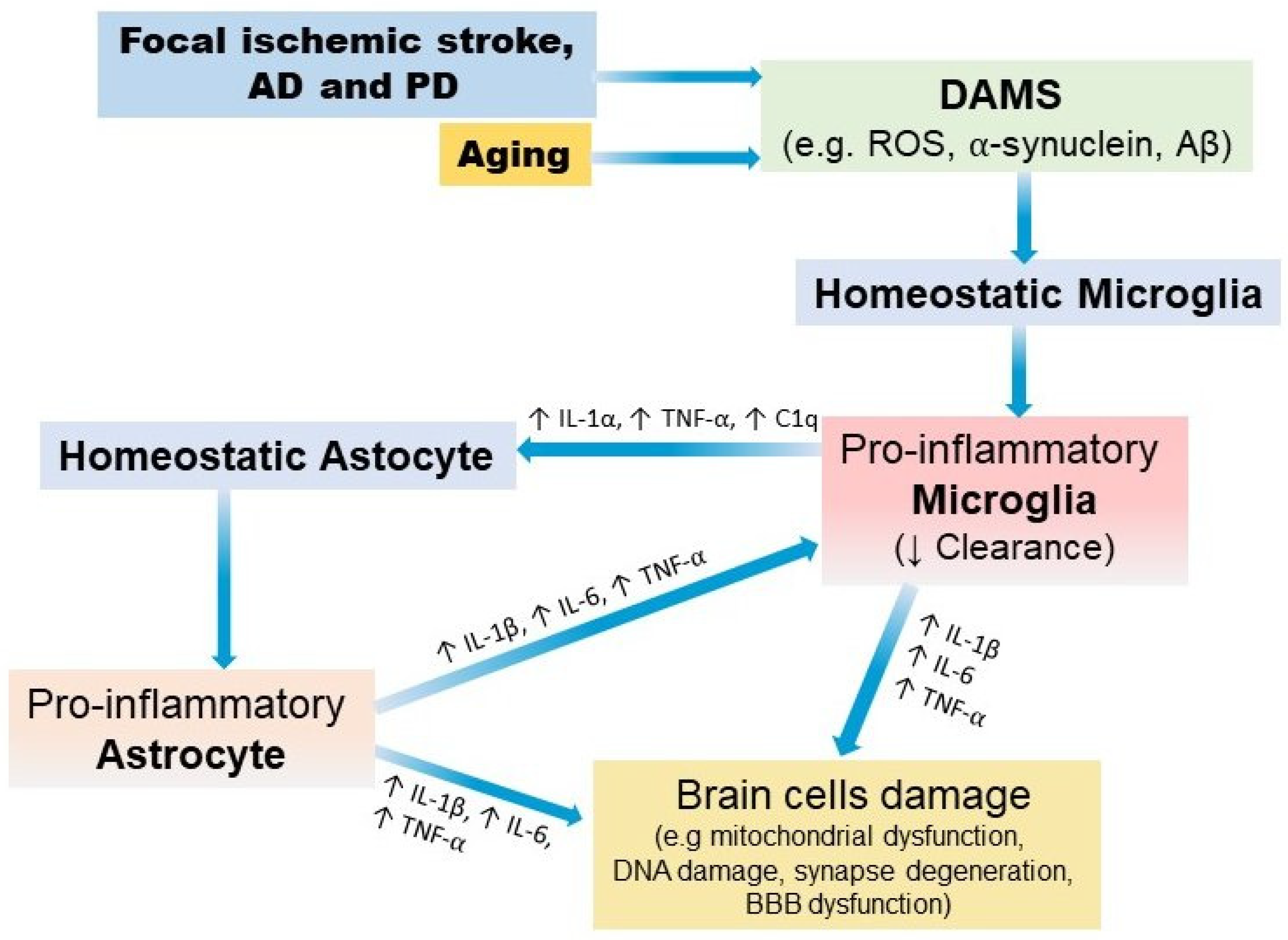
3. Neuroinflammation
4. Aging
5. Basic Physiopathology of Focal Ischemic Stroke, AD, and PD
5.1. Focal Ischemic Stroke
5.2. Alzheimer’s Disease (AD)
5.3. Parkinson’s Disease (PD)
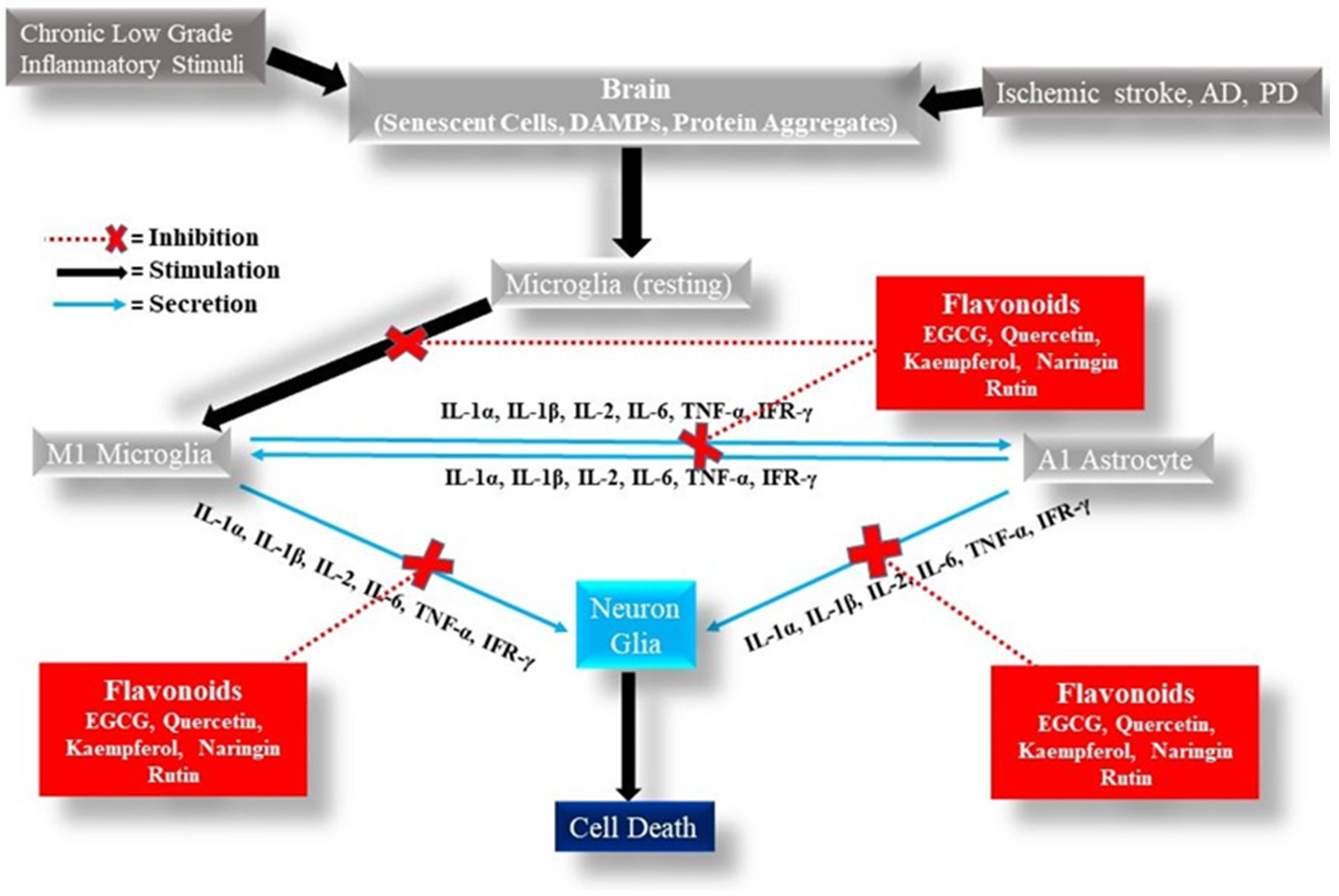
6. Anti-Inflammatory Effects of Flavonoids in Focal Ischemic Stroke, AD, and PD
6.1. Anti-Inflammatory Effects of Flavonoids in Focal Ischemic Stroke
6.1.1. In Vitro Studies
6.1.2. Animal Model
Synergistic Effect of Flavonoids with rtPA
Preventive Treatment with Flavonoids
6.1.3. Clinical
6.2. Anti-Inflammatory Effects of Flavonoids in AD
6.2.1. In Vitro Studies
6.2.2. Animal Model Studies
6.2.3. Clinical
6.3. Anti-Inflammatory Effects of Flavonoids in PD
6.3.1. In Vitro Studies
6.3.2. Animal Model Studies
6.3.3. Clinical
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirkwood, T.B.L. Understanding the Odd Science of Aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- López-Valdés, H.E.; Martínez-Coria, H. The Role of Neuroinflammation in Age-Related Dementias. Rev. Investig. Clín. Organo Hosp. Enferm. Nutr. 2016, 68, 40–48. [Google Scholar]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- Ramzan, I.; Li, G.Q. Phytotherapies—Past, Present, and Future. In Phytotherapies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–17. ISBN 978-1-119-00603-9. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Foshati, S. Bioavailability and Metabolism of Flavonoids: A Review. Int. J. Nutr. Sci. 2017, 2, 180–184. [Google Scholar]
- Menendez, C.; Dueñas, M.; Galindo, P.; González-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodríguez-Gómez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular Deconjugation of Quercetin Glucuronide: The Flavonoid Paradox Revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Kaila Srai, S.; Rice-Evans, C.; Spencer, J.P.E. Absorption, Tissue Distribution and Excretion of Pelargonidin and Its Metabolites Following Oral Administration to Rats. Br. J. Nutr. 2006, 95, 51–58. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.-L.; Rémésy, C. Anthocyanin Metabolism in Rats and Their Distribution to Digestive Area, Kidney, and Brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- El Mohsen, M.M.A.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and Metabolism of Epicatechin and Its Access to the Brain after Oral Ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in Neurological Disorders: Pharmacotherapeutic Targets from Bench to Bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Wheeler, M.A.; Quintana, F.J. Function and Therapeutic Value of Astrocytes in Neurological Diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Calvo-Rodriguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like Receptors in Neuroinflammation, Neurodegeneration, and Alcohol-Induced Brain Damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hauenstein, A.V. The NLRP3 Inflammasome: Mechanism of Action, Role in Disease and Therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Brooks, A.J.; Dehkhoda, F.; Kragelund, B.B. Cytokine Receptors. In Principles of Endocrinology and Hormone Action; Endocrinology; Belfiore, A., LeRoith, D., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 157–185. ISBN 978-3-319-44675-2. [Google Scholar]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Offner, H.; Matejuk, S.; Matejuk, A. Microglia and Astrocyte Involvement in Neurodegeneration and Brain Cancer. J. Neuroinflamm. 2021, 18, 298. [Google Scholar] [CrossRef]
- MacNee, W.; Rabinovich, R.A.; Choudhury, G. Ageing and the Border between Health and Disease. Eur. Respir. J. 2014, 44, 1332–1352. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Blinkouskaya, Y.; Caçoilo, A.; Gollamudi, T.; Jalalian, S.; Weickenmeier, J. Brain Aging Mechanisms with Mechanical Manifestations. Mech. Ageing Dev. 2021, 200, 111575. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re Only as Old as Your Arteries: Translational Strategies for Preserving Vascular Endothelial Function with Aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Torres-Querol, C.; Torres, P.; Vidal, N.; Portero-Otín, M.; Arque, G.; Purroy, F. Acute Ischemic Stroke Triggers a Cellular Senescence-Associated Secretory Phenotype. Sci. Rep. 2021, 11, 15752. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of Aging and Immunosenescence: Connecting the Dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Chen, S.; Zhu, J.; Ma, J.; Zhai, Y. The Emerging Role of Central and Peripheral Immune Systems in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 872134. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Cruz, M.-E.; López-Valdés, H.E. Physiopathology of Ischemic Stroke and Its Modulation Using Memantine: Evidence from Preclinical Stroke. Neural Regen. Res. 2021, 16, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and Ischemic Stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Bartley, A.C.; Alsawas, M.; Sheely, H.L.; Shultz, J.; Takahashi, P.Y.; Young, N.P.; Murad, M.H. Impact of Multiple Chronic Conditions in Patients Hospitalized with Stroke and Transient Ischemic Attack. J. Stroke 2017, 26, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Lyden, S.; Wold, J. Acute Treatment of Ischemic Stroke. Neurol. Clin. 2022, 40, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling Pathways Involved in Ischemic Stroke: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Gelderblom, M.; Sobey, C.G.; Kleinschnitz, C.; Magnus, T. Danger Signals in Stroke. Ageing Res. Rev. 2015, 24, 77–82. [Google Scholar] [CrossRef]
- Grønberg, N.V.; Johansen, F.F.; Kristiansen, U.; Hasseldam, H. Leukocyte Infiltration in Experimental Stroke. J. Neuroinflamm. 2013, 10, 892. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion after Focal Cerebral Ischemia. Stroke J. Cereb. Circ. 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Neumann, J.; Gunzer, M.; Gutzeit, H.O.; Ullrich, O.; Reymann, K.G.; Dinkel, K. Microglia Provide Neuroprotection after Ischemia. FASEB J. 2006, 20, 714–716. [Google Scholar] [CrossRef]
- Denes, A.; Vidyasagar, R.; Feng, J.; Narvainen, J.; McColl, B.W.; Kauppinen, R.A.; Allan, S.M. Proliferating Resident Microglia after Focal Cerebral Ischaemia in Mice. J. Cereb. Blood Flow Metab. 2007, 27, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional Differences between Microglia and Monocytes after Ischemic Stroke. J. Neuroinflamm. 2015, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Gregersen, R.; Meldgaard, M.; Clausen, B.H.; Heibøl, E.K.; Ladeby, R.; Knudsen, J.; Frandsen, A.; Owens, T.; Finsen, B. A Role for Interferon-Gamma in Focal Cerebral Ischemia in Mice. J. Neuropathol. Exp. Neurol. 2004, 63, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Tyrrell, P.J.; Rothwell, N.J. Interleukin-1 and Neuronal Injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef]
- Sims, N.R.; Yew, W.P. Reactive Astrogliosis in Stroke: Contributions of Astrocytes to Recovery of Neurological Function. Neurochem. Int. 2017, 107, 88–103. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The Diversity and Disparity of the Glial Scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Ding, S. Dynamic Reactive Astrocytes after Focal Ischemia. Neural Regen. Res. 2014, 9, 2048–2052. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. A New Perspective on Alzheimer’s Disease as a Brain Expression of a Complex Metabolic Disorder. In Alzheimer’s Disease; Wisniewski, T., Ed.; Codon Publications: Brisbane, Australia, 2019; ISBN 978-0-646-80968-7. [Google Scholar]
- Pons, V.; Rivest, S. Targeting Systemic Innate Immune Cells as a Therapeutic Avenue for Alzheimer Disease. Pharmacol. Rev. 2022, 74, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Golde, T.E. Alzheimer’s Disease—The Journey of a Healthy Brain into Organ Failure. Mol. Neurodegener. 2022, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Hyman, B.T.; Spires-Jones, T.L. Beyond the Neuron-Cellular Interactions Early in Alzheimer Disease Pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia Show Altered Morphology and Reduced Arborization in Human Brain during Aging and Alzheimer’s Disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-Vivo Measurement of Activated Microglia in Dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef]
- Edison, P.; Archer, H.A.; Gerhard, A.; Hinz, R.; Pavese, N.; Turkheimer, F.E.; Hammers, A.; Tai, Y.F.; Fox, N.; Kennedy, A.; et al. Microglia, Amyloid, and Cognition in Alzheimer’s Disease: An [11C](R)PK11195-PET and [11C]PIB-PET Study. Neurobiol. Dis. 2008, 32, 412–419. [Google Scholar] [CrossRef]
- Schuitemaker, A.; Kropholler, M.A.; Boellaard, R.; van der Flier, W.M.; Kloet, R.W.; van der Doef, T.F.; Knol, D.L.; Windhorst, A.D.; Luurtsema, G.; Barkhof, F.; et al. Microglial Activation in Alzheimer’s Disease: An (R)-[11C]PK11195 Positron Emission Tomography Study. Neurobiol. Aging 2013, 34, 128–136. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial Activation Correlates in Vivo with Both Tau and Amyloid in Alzheimer’s Disease. Brain J. Neurol. 2018, 141, 2740–2754. [Google Scholar] [CrossRef]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An Early and Late Peak in Microglial Activation in Alzheimer’s Disease Trajectory. Brain J. Neurol. 2017, 140, 792–803. [Google Scholar] [CrossRef]
- Malpetti, M.; Kievit, R.A.; Passamonti, L.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; Mak, E.; Nicastro, N.; Bevan-Jones, W.R.; Su, L.; et al. Microglial Activation and Tau Burden Predict Cognitive Decline in Alzheimer’s Disease. Brain 2020, 143, 1588–1602. [Google Scholar] [CrossRef]
- Nicastro, N.; Malpetti, M.; Mak, E.; Williams, G.B.; Bevan-Jones, W.R.; Carter, S.F.; Passamonti, L.; Fryer, T.D.; Hong, Y.T.; Aigbirhio, F.I.; et al. Gray Matter Changes Related to Microglial Activation in Alzheimer’s Disease. Neurobiol. Aging 2020, 94, 236–242. [Google Scholar] [CrossRef]
- Bologna, M.; Truong, D.; Jankovic, J. The Etiopathogenetic and Pathophysiological Spectrum of Parkinsonism. J. Neurol. Sci. 2022, 433, 120012. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Parkinson Disease. Park. Relat. Disord. 2018, 46 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- Gerhard, A.; Watts, J.; Trender-Gerhard, I.; Turkheimer, F.; Banati, R.B.; Bhatia, K.; Brooks, D.J. In Vivo Imaging of Microglial Activation with [11C](R)-PK11195 PET in Corticobasal Degeneration. Mov. Disord. 2004, 19, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical Analysis of Tumor Necrosis Factor and Its Receptors in Parkinson’s Disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Daniel, S.E.; Blunt, S.B. Glial Pathology but Absence of Apoptotic Nigral Neurons in Long-Standing Parkinson’s Disease. Mov. Disord. 1998, 13, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Vawter, M.P.; Dillon-Carter, O.; Tourtellotte, W.W.; Carvey, P.; Freed, W.J. TGFβ1 and TGFβ2 Concentrations Are Elevated in Parkinson’s Disease in Ventricular Cerebrospinal Fluid. Exp. Neurol. 1996, 142, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Tieu, K.; Choi, D.-K.; Wu, D.-C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 Is Instrumental in Parkinson’s Disease Neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ Lymphocytes into the Brain Contributes to Neurodegeneration in a Mouse Model of Parkinson Disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Griffin, W.S.T.; Liu, L.; Li, Y.; Mrak, R.E.; Barger, S.W. Interleukin-1 Mediates Alzheimer and Lewy Body Pathologies. J. Neuroinflamm. 2006, 3, 5. [Google Scholar] [CrossRef]
- Klegeris, A.; Pelech, S.; Giasson, B.I.; Maguire, J.; Zhang, H.; McGeer, E.G.; McGeer, P.L. Alpha-Synuclein Activates Stress Signaling Protein Kinases in THP-1 Cells and Microglia. Neurobiol. Aging 2008, 29, 739–752. [Google Scholar] [CrossRef]
- Holloway, P.M.; Gavins, F.N.E. Modeling Ischemic Stroke In Vitro: Status Quo and Future Perspectives. Stroke 2016, 47, 561–569. [Google Scholar] [CrossRef]
- Tasca, C.I.; Dal-Cim, T.; Cimarosti, H. In Vitro Oxygen-Glucose Deprivation to Study Ischemic Cell Death. In Neuronal Cell Death; Methods in Molecular Biology; Lossi, L., Merighi, A., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1254, pp. 197–210. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Guo, S.; Shi, L.; He, Q.; Zhang, P.; Yu, S.; Zhao, R. Myricetin Ameliorated Ischemia/Reperfusion-Induced Brain Endothelial Permeability by Improvement of ENOS Uncoupling and Activation ENOS/NO. J. Pharmacol. Sci. 2019, 140, 62–72. [Google Scholar] [CrossRef]
- Le, K.; Song, Z.; Deng, J.; Peng, X.; Zhang, J.; Wang, L.; Zhou, L.; Bi, H.; Liao, Z.; Feng, Z. Quercetin Alleviates Neonatal Hypoxic-Ischemic Brain Injury by Inhibiting Microglia-Derived Oxidative Stress and TLR4-Mediated Inflammation. Inflamm. Res. 2020, 69, 1201–1213. [Google Scholar] [CrossRef]
- Wang, C.-P.; Li, J.-L.; Zhang, L.-Z.; Zhang, X.-C.; Yu, S.; Liang, X.-M.; Ding, F.; Wang, Z.-W. Isoquercetin Protects Cortical Neurons from Oxygen-Glucose Deprivation-Reperfusion Induced Injury via Suppression of TLR4-NF-κB Signal Pathway. Neurochem. Int. 2013, 63, 741–749. [Google Scholar] [CrossRef]
- Wang, C.-P.; Shi, Y.-W.; Tang, M.; Zhang, X.-C.; Gu, Y.; Liang, X.-M.; Wang, Z.-W.; Ding, F. Isoquercetin Ameliorates Cerebral Impairment in Focal Ischemia Through Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Effects in Primary Culture of Rat Hippocampal Neurons and Hippocampal CA1 Region of Rats. Mol. Neurobiol. 2017, 54, 2126–2142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, J.; Ma, L.; Yuan, Z.; Wang, Y.; Wang, X.; Xing, D.; Lei, F.; Du, L. Comprehensive Study of Baicalin Down-Regulating NOD2 Receptor Expression of Neurons with Oxygen-Glucose Deprivation in Vitro and Cerebral Ischemia-Reperfusion In Vivo. Eur. J. Pharmacol. 2010, 649, 92–99. [Google Scholar] [CrossRef]
- Zheng, W.-X.; He, W.-Q.; Zhang, Q.-R.; Jia, J.-X.; Zhao, S.; Wu, F.-J.; Cao, X.-L. Baicalin Inhibits NLRP3 Inflammasome Activity via the AMPK Signaling Pathway to Alleviate Cerebral Ischemia-Reperfusion Injury. Inflammation 2021, 44, 2091–2105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hou, J.; Fu, J.; Li, D.; Zhang, C.; Liu, J. Baicalin Protects Rat Brain Microvascular Endothelial Cells Injured by Oxygen-Glucose Deprivation via Anti-Inflammation. Brain Res. Bull. 2013, 97, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Wang, B.; Li, M.; Liu, J.; Yang, H.; Shi, Y. Baicalin and Geniposide Inhibit Polarization and Inflammatory Injury of OGD/R-Treated Microglia by Suppressing the 5-LOX/LTB4 Pathway. Neurochem. Res. 2021, 46, 1844–1858. [Google Scholar] [CrossRef]
- Ran, Y.; Qie, S.; Gao, F.; Ding, Z.; Yang, S.; Tian, G.; Liu, Z.; Xi, J. Baicalein Ameliorates Ischemic Brain Damage through Suppressing Proinflammatory Microglia Polarization via Inhibiting the TLR4/NF-ΚB and STAT1 Pathway. Brain Res. 2021, 1770, 147626. [Google Scholar] [CrossRef]
- Ye-Hao, Z.; Lan, M.; Peng, Z.; Guang-Yu, L.; Jian-Xun, L. Effect of baicalin on inflammatory response and TLR4/NF-κB signaling pathway of human brain microvascular endothelial cell after hypoxia-reoxygenation injury. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2020, 45, 4686–4691. [Google Scholar] [CrossRef]
- Hou, J.; Wang, J.; Zhang, P.; Li, D.; Zhang, C.; Zhao, H.; Fu, J.; Wang, B.; Liu, J. Baicalin Attenuates Proinflammatory Cytokine Production in Oxygen-Glucose Deprived Challenged Rat Microglial Cells by Inhibiting TLR4 Signaling Pathway. Int. Immunopharmacol. 2012, 14, 749–757. [Google Scholar] [CrossRef]
- Mo, Z.-T.; Zheng, J.; Liao, Y.-L. Icariin Inhibits the Expression of IL-1β, IL-6 and TNF-α Induced by OGD/R through the IRE1/XBP1s Pathway in Microglia. Pharm. Biol. 2021, 59, 1473–1479. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, J.; Li, W.; Zhang, L.; Wang, X.; Liu, Q. Casticin Protected against Neuronal Injury and Inhibited the TLR4/NF-ΚB Pathway after Middle Cerebral Artery Occlusion in Rats. Pharmacol. Res. Perspect. 2021, 9, e00752. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, Y.; Deng, W.; Teng, J. Pratensein Mitigates Oxidative Stress and NLRP3 Inflammasome Activation in OGD/R-Injured HT22 Cells by Activating Nrf2-Anti-Oxidant Signaling. Neurotox. Res. 2022, 40, 384–394. [Google Scholar] [CrossRef]
- Yao, L.; Yang, M.; Zhang, J.; Wang, F.; Liu, Q.; Xie, X.; Liu, Z.; Guo, Q.; Su, H.; Zhai, J.; et al. Tectorigenin Attenuates the OGD/R-Induced HT-22 Cell Damage through Regulation of the PI3K/AKT and the PPARγ/NF-ΚB Pathways. Hum. Exp. Toxicol. 2021, 40, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Xue, L.; Yang, Y.; Zhi, F. Astilbin Protects against Cerebral Ischaemia/Reperfusion Injury by Inhibiting Cellular Apoptosis and ROS-NLRP3 Inflammasome Axis Activation. Int. Immunopharmacol. 2020, 84, 106571. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Pan, Z.; Zhu, Y.; Tuo, J.; Meng, Q.; Dai, G.; Yang, G.; Pan, Y. Anthocyanin Ameliorates Hypoxia and Ischemia Induced Inflammation and Apoptosis by Increasing Autophagic Flux in SH-SY5Y Cells. Eur. J. Pharmacol. 2020, 883, 173360. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, X.; Yan, M.; Li, D.; Zou, R. Tricin Attenuates Cerebral Ischemia/Reperfusion Injury through Inhibiting Nerve Cell Autophagy, Apoptosis and Inflammation by Regulating the PI3K/Akt Pathway. Hum. Exp. Toxicol. 2022, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, J.; Bi, F.; Bai, Z. Diosmetin Alleviates Cerebral Ischemia-Reperfusion Injury through Keap1-Mediated Nrf2/ARE Signaling Pathway Activation and NLRP3 Inflammasome Inhibition. Environ. Toxicol. 2022, 37, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wu, J.; Chen, J.; Zhou, Y.; Chen, X.; Wu, Q.; Xu, Y.; Tu, W.; Lou, X.; Yang, G.; et al. Schaftoside Ameliorates Oxygen Glucose Deprivation-Induced Inflammation Associated with the TLR4/Myd88/Drp1-Related Mitochondrial Fission in BV2 Microglia Cells. J. Pharmacol. Sci. 2019, 139, 15–22. [Google Scholar] [CrossRef]
- Kumar, A.; Aakriti; Gupta, V. A Review on Animal Models of Stroke: An Update. Brain Res. Bull. 2016, 122, 35–44. [Google Scholar] [CrossRef]
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective Effect of Kaempferol Glycosides against Brain Injury and Neuroinflammation by Inhibiting the Activation of NF-ΚB and STAT3 in Transient Focal Stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Cheng, X.; Yang, Y.-L.; Liu, M.; Zhang, S.-S.; Wang, Y.-H.; Du, G.-H. Kaempferol Attenuates Neuroinflammation and Blood Brain Barrier Dysfunction to Improve Neurological Deficits in Cerebral Ischemia/Reperfusion Rats. Brain Res. 2019, 1722, 146361. [Google Scholar] [CrossRef]
- Gelderblom, M.; Leypoldt, F.; Lewerenz, J.; Birkenmayer, G.; Orozco, D.; Ludewig, P.; Thundyil, J.; Arumugam, T.V.; Gerloff, C.; Tolosa, E.; et al. The Flavonoid Fisetin Attenuates Postischemic Immune Cell Infiltration, Activation and Infarct Size after Transient Cerebral Middle Artery Occlusion in Mice. J. Cereb. Blood Flow Metab. 2012, 32, 835–843. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Xu, H.; Li, G.; Ma, Y.; Pang, Y.J. Morin Mitigates Oxidative Stress, Apoptosis and Inflammation in Cerebral Ischemic Rats. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 348–355. [Google Scholar] [CrossRef]
- Khamchai, S.; Chumboatong, W.; Hata, J.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Morin Protects the Blood-Brain Barrier Integrity against Cerebral Ischemia Reperfusion through Anti-Inflammatory Actions in Rats. Sci. Rep. 2020, 10, 13379. [Google Scholar] [CrossRef]
- Zhang, F.; Li, N.; Jiang, L.; Chen, L.; Huang, M. Neuroprotective Effects of (−)-Epigallocatechin-3-Gallate Against Focal Cerebral Ischemia/Reperfusion Injury in Rats through Attenuation of Inflammation. Neurochem. Res. 2015, 40, 1691–1698. [Google Scholar] [CrossRef]
- Li, L.; Pan, G.; Fan, R.; Li, D.; Guo, L.; Ma, L.; Liang, H.; Qiu, J. Luteolin Alleviates Inflammation and Autophagy of Hippocampus Induced by Cerebral Ischemia/Reperfusion by Activating PPAR Gamma in Rats. BMC Complement. Med. Ther. 2022, 22, 176. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X.; Pang, X. Luteoloside Attenuates Neuroinflammation in Focal Cerebral Ischemia in Rats via Regulation of the PPARγ/Nrf2/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, N.; Ishii, T.; Oyama, D.; Fukuta, T.; Agato, Y.; Sato, A.; Shimizu, K.; Asai, T.; Asakawa, T.; Kan, T.; et al. Neuroprotective Effect of Nobiletin on Cerebral Ischemia–Reperfusion Injury in Transient Middle Cerebral Artery-Occluded Rats. Brain Res. 2014, 1559, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.L.; Borba, H.H.L.; de F. Bonetti, A.; Leonart, L.P.; Pontarolo, R. Cytokines and Interferons: Types and Functions. In Autoantibodies and Cytokines; Khan, W.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sapkota, A.; Gaire, B.P.; Cho, K.S.; Jeon, S.J.; Kwon, O.W.; Jang, D.S.; Kim, S.Y.; Ryu, J.H.; Choi, J.W. Eupatilin Exerts Neuroprotective Effects in Mice with Transient Focal Cerebral Ischemia by Reducing Microglial Activation. PLoS ONE 2017, 12, e0171479. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-P. Epigallocatechin Gallate Extends the Therapeutic Window of Recombinant Tissue Plasminogen Activator Treatment in Ischemic Rats. J. Stroke Cerebrovasc. Dis. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.P.; Safhi, M.M.; Islam, F. Neuroprotective Effect of Naringenin Is Mediated through Suppression of NF-ΚB Signaling Pathway in Experimental Stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Chen, L.; Zhang, J.; Zhang, L.; Zhao, X.; Zhao, T.; Zhao, Y. Protective Effect of Naringenin in Experimental Ischemic Stroke: Down-Regulated NOD2, RIP2, NF-ΚB, MMP-9 and up-Regulated Claudin-5 Expression. Neurochem. Res. 2014, 39, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Khuwaja, G.; Tabassum, R.; Javed, H.; Siddiqui, M.S.; Safhi, M.M.; Islam, F. Hesperidin Ameliorates Functional and Histological Outcome and Reduces Neuroinflammation in Experimental Stroke. Brain Res. 2011, 1420, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.A.; Salam, R.M.A.; Kenawy, S.A.; Attia, A.S. Pinocembrin Attenuates Hippocampal Inflammation, Oxidative Perturbations and Apoptosis in a Rat Model of Global Cerebral Ischemia Reperfusion. Pharmacol. Rep. 2015, 67, 115–122. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, T.; Huang, M.; Cao, L.; Li, Y.; Cheng, H.; Jin, H.; Yu, D. Neuroprotection by the Soy Isoflavone, Genistein, via Inhibition of Mitochondria-Dependent Apoptosis Pathways and Reactive Oxygen Induced-NF-ΚB Activation in a Cerebral Ischemia Mouse Model. Neurochem. Int. 2012, 60, 759–767. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J. Sanggenon C Ameliorates Cerebral Ischemia-Reperfusion Injury by Inhibiting Inflammation and Oxidative Stress through Regulating RhoA-ROCK Signaling. Inflammation 2020, 43, 1476–1487. [Google Scholar] [CrossRef]
- Li, T.-F.; Ma, J.; Han, X.-W.; Jia, Y.-X.; Yuan, H.-F.; Shui, S.-F.; Guo, D.; Yan, L. Chrysin Ameliorates Cerebral Ischemia/Reperfusion (I/R) Injury in Rats by Regulating the PI3K/Akt/MTOR Pathway. Neurochem. Int. 2019, 129, 104496. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Z.; Peng, X.; Wu, L.; Wu, H.; Ou, Y.; Lai, J. Eupafolin Alleviates Cerebral Ischemia/Reperfusion Injury in Rats via Blocking the TLR4/NF-ΚB Signaling Pathway. Mol. Med. Rep. 2020, 22, 5135–5144. [Google Scholar] [CrossRef]
- Wang, W.; Tang, L.; Li, Y.; Wang, Y. Biochanin A Protects against Focal Cerebral Ischemia/Reperfusion in Rats via Inhibition of P38-Mediated Inflammatory Responses. J. Neurol. Sci. 2015, 348, 121–125. [Google Scholar] [CrossRef]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Wang, X.-H.; You, Y.-P. Epigallocatechin Gallate Extends Therapeutic Window of Recombinant Tissue Plasminogen Activator Treatment for Brain Ischemic Stroke: A Randomized Double-Blind and Placebo-Controlled Trial. Clin. Neuropharmacol. 2017, 40, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG Redirects Amyloidogenic Polypeptides into Unstructured, off-Pathway Oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG Remodels Mature α-Synuclein and Amyloid-β Fibrils and Reduces Cellular Toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.T.; Jung, C.H.; Lee, S.R.; Bae, J.H.; Baek, W.K.; Suh, M.H.; Park, J.; Park, C.W.; Suh, S.I. The Green Tea Polyphenol (−)-Epigallocatechin Gallate Attenuates Beta-Amyloid-Induced Neurotoxicity in Cultured Hippocampal Neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons against Aβ(1–42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Shytle, R.D.; Bai, Y.; Tian, J.; Hou, H.; Mori, T.; Zeng, J.; Obregon, D.; Town, T.; Tan, J. Flavonoid-Mediated Presenilin-1 Phosphorylation Reduces Alzheimer’s Disease β-Amyloid Production. J. Cell. Mol. Med. 2009, 13, 574–588. [Google Scholar] [CrossRef]
- Hirohata, M.; Hasegawa, K.; Tsutsumi-Yasuhara, S.; Ohhashi, Y.; Ookoshi, T.; Ono, K.; Yamada, M.; Naiki, H. The Anti-Amyloidogenic Effect Is Exerted against Alzheimer’s Beta-Amyloid Fibrils in Vitro by Preferential and Reversible Binding of Flavonoids to the Amyloid Fibril Structure. Biochemistry 2007, 46, 1888–1899. [Google Scholar] [CrossRef]
- Song, N.; Zhang, L.; Chen, W.; Zhu, H.; Deng, W.; Han, Y.; Guo, J.; Qin, C. Cyanidin 3-O-β-Glucopyranoside Activates Peroxisome Proliferator-Activated Receptor-γ and Alleviates Cognitive Impairment in the APP(Swe)/PS1(ΔE9) Mouse Model. Biochim. Biophys. Acta 2016, 1862, 1786–1800. [Google Scholar] [CrossRef]
- Huang, D.-S.; Yu, Y.-C.; Wu, C.-H.; Lin, J.-Y. Protective Effects of Wogonin against Alzheimer’s Disease by Inhibition of Amyloidogenic Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 3545169. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein Inhibits Heparin-Induced Tau Aggregation by Initializing Non-Toxic Tau Oligomer Formation. Cell Commun. Signal. 2021, 19, 16. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Balmik, A.A.; Boral, D.; Ramasamy, S.; Chinnathambi, S. Baicalein Suppresses Repeat Tau Fibrillization by Sequestering Oligomers. Arch. Biochem. Biophys. 2019, 675, 108119. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and Rutin Exhibit Antiamyloidogenic and Fibril-Disaggregating Effects in Vitro and Potent Antioxidant Activity in APPswe Cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Preclinical Models of Alzheimer’s Disease: Relevance and Translational Validity. Curr. Protoc. Pharmacol. 2019, 84, e57. [Google Scholar] [CrossRef]
- Vitek, M.P.; Araujo, J.A.; Fossel, M.; Greenberg, B.D.; Howell, G.R.; Rizzo, S.J.S.; Seyfried, N.T.; Tenner, A.J.; Territo, P.R.; Windisch, M.; et al. Translational Animal Models for Alzheimer’s Disease: An Alzheimer’s Association Business Consortium Think Tank. Alzheimer Dement. Transl. Res. Clin. Interv. 2020, 6, e12114. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin Enhanced Cortico-Hippocampal Learning and Memory Capability in Mice with Scopolamine-Induced Amnesia: Role of Antioxidant Defense, Cholinergic and BDNF Signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ramalingayya, G.V.; Nampoothiri, M.; Nayak, P.G.; Kishore, A.; Shenoy, R.R.; Rao, C.M.; Nandakumar, K. Naringin and Rutin Alleviates Episodic Memory Deficits in Two Differentially Challenged Object Recognition Tasks. Pharmacogn. Mag. 2016, 12, S63–S70. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, N.; Ramezani, M.; Khodagholi, F.; Noori, M. Kaempferol Promotes Memory Retention and Density of Hippocampal CA1 Neurons in Intra-Cerebroventricular STZ-Induced Experimental AD Model in Wistar Rats. Biologija 2016, 62, 157–168. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating Effect of Luteolin on Memory Impairment in an Alzheimer’s Disease Model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of Hesperidin on Neurobehavioral, Neuroinflammation, Oxidative Stress and Lipid Alteration in Intracerebroventricular Streptozotocin Induced Cognitive Impairment in Mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.-W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Memory Impairment and Aβ Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Sawmiller, D.; Habib, A.; Li, S.; Darlington, D.; Hou, H.; Tian, J.; Shytle, R.D.; Smith, A.; Giunta, B.; Mori, T.; et al. Diosmin Reduces Cerebral Aβ Levels, Tau Hyperphosphorylation, Neuroinflammation, and Cognitive Impairment in the 3xTg-AD Mice. J. Neuroimmunol. 2016, 299, 98–106. [Google Scholar] [CrossRef]
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin Ameliorates Behavioral Impairments and Neuropathology of Transgenic APP/PS1 Mice. Behav. Brain Res. 2015, 281, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green Tea Epigallocatechin-3-Gallate (EGCG) Modulates Amyloid Precursor Protein Cleavage and Reduces Cerebral Amyloidosis in Alzheimer Transgenic Mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green Tea (−)-Epigallocatechin-3-Gallate Inhibits Beta-Amyloid-Induced Cognitive Dysfunction through Modification of Secretase Activity via Inhibition of ERK and NF-KappaB Pathways in Mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green Tea Epigallocatechin-3-Gallate (EGCG) Reduces Beta-Amyloid Mediated Cognitive Impairment and Modulates Tau Pathology in Alzheimer Transgenic Mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.-J.; Nam, Y.; Kim, H.-C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Cognitive Impairment and Reduces Soluble Aβ Levels in a Triple Transgenic Mouse Model of Alzheimer’s Disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.-T.L.; Shin, E.-J.; Kim, H.-C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid, Ameliorates Cognitive Impairment, Oxidative Burden, and Hyperphosphorylation of Tau in Senescence-Accelerated Mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef]
- Paula, P.-C.; Maria, S.-G.A.; Luis, C.-H.; Patricia, C.-G.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, J.; Qin, M. Protective Effect of Cyanidin 3-O-Glucoside on Beta-Amyloid Peptide-Induced Cognitive Impairment in Rats. Neurosci. Lett. 2013, 534, 285–288. [Google Scholar] [CrossRef]
- Currais, A.; Prior, M.; Dargusch, R.; Armando, A.; Ehren, J.; Schubert, D.; Quehenberger, O.; Maher, P. Modulation of P25 and Inflammatory Pathways by Fisetin Maintains Cognitive Function in Alzheimer’s Disease Transgenic Mice. Aging Cell 2014, 13, 379–390. [Google Scholar] [CrossRef]
- Liang, J.; López-Valdés, H.E.; Martínez-Coria, H.; Lindemeyer, A.K.; Shen, Y.; Shao, X.M.; Olsen, R.W. Erratum to: Dihydromyricetin Ameliorates Behavioral Deficits and Reverses Neuropathology of Transgenic Mouse Models of Alzheimer’s Disease. Neurochem. Res. 2014, 39, 1403. [Google Scholar] [CrossRef]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment: The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing Dentate Gyrus Function with Dietary Flavanols Improves Cognition in Older Adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Neuner, J.; Högen, T.; Schmidt, F.; Kamp, F.; Scerri, C.; Giese, A.; Vassallo, N. Polyphenolic Compounds Are Novel Protective Agents against Lipid Membrane Damage by α-Synuclein Aggregates In Vitro. Biochim. Biophys. Acta 2012, 1818, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ono, K.; Takamura, Y.; Mizuguchi, M.; Ikeda, T.; Nishijo, H.; Yamada, M. Phenolic Compounds Prevent the Oligomerization of α-Synuclein and Reduce Synaptic Toxicity. J. Neurochem. 2015, 134, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Quan, Z.; Wong, W.; Guo, J.; Zhang, R.; Yang, Q.; Dai, R.; McGeer, P.L.; Qing, H. Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease. Neurochem. Res. 2016, 41, 2788–2796. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Jung, S.-C.; Park, D.-B.; Maeng, Y.-H.; Hong, J.Y.; Kim, S.-J.; Lee, S.-R.; Kim, S.-J.; Kim, S.J.; et al. Anti-Neuroinflammatory Activity of Nobiletin on Suppression of Microglial Activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and Luteolin Modulate Microglial Activation via Inhibition of STAT1-Induced CD40 Expression. J. Neuroinflamm. 2008, 5, 41. [Google Scholar] [CrossRef]
- Wu, L.-H.; Lin, C.; Lin, H.-Y.; Liu, Y.-S.; Wu, C.Y.-J.; Tsai, C.-F.; Chang, P.-C.; Yeh, W.-L.; Lu, D.-Y. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016, 53, 1080–1091. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.-Y.; Choi, Y.H. Naringenin Attenuates the Release of Pro-Inflammatory Mediators from Lipopolysaccharide-Stimulated BV2 Microglia by Inactivating Nuclear Factor-ΚB and Inhibiting Mitogen-Activated Protein Kinases. Int. J. Mol. Med. 2012, 30, 204–210. [Google Scholar] [CrossRef]
- Chinta, S.J.; Ganesan, A.; Reis-Rodrigues, P.; Lithgow, G.J.; Andersen, J.K. Anti-Inflammatory Role of the Isoflavone Diadzein in Lipopolysaccharide-Stimulated Microglia: Implications for Parkinson’s Disease. Neurotox. Res. 2013, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Xiong, N.; Wang, H.; Huang, J.; Sun, S.; Wang, T. Morin Exerts Neuroprotective Actions in Parkinson Disease Models In Vitro and In Vivo. Acta Pharmacol. Sin. 2010, 31, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, Y.; Chun, H.J.; Kim, A.H.; Kim, J.Y.; Lee, J.Y.; Ishigami, A.; Lee, J. Neuroprotective and Anti-Inflammatory Effects of Morin in a Murine Model of Parkinson’s Disease. J. Neurosci. Res. 2016, 94, 865–878. [Google Scholar] [CrossRef]
- Cho, N.; Choi, J.H.; Yang, H.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and Anti-Inflammatory Effects of Flavonoids Isolated from Rhus Verniciflua in Neuronal HT22 and Microglial BV2 Cell Lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, P.; Cai, Y. Genkwanin Suppresses MPP+-Induced Cytotoxicity by Inhibiting TLR4/MyD88/NLRP3 Inflammasome Pathway in a Cellular Model of Parkinson’s Disease. Neurotoxicology 2021, 87, 62–69. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.-T. Animal Models of Parkinson’s Disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Pingale, T.; Gupta, G.L. Classic and Evolving Animal Models in Parkinson’s Disease. Pharmacol. Biochem. Behav. 2020, 199, 173060. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Bobrovskaya, L. An Update on the Rotenone Models of Parkinson’s Disease: Their Ability to Reproduce the Features of Clinical Disease and Model Gene-Environment Interactions. Neurotoxicology 2015, 46, 101–116. [Google Scholar] [CrossRef]
- Hu, Q.; Uversky, V.N.; Huang, M.; Kang, H.; Xu, F.; Liu, X.; Lian, L.; Liang, Q.; Jiang, H.; Liu, A.; et al. Baicalein Inhibits α-Synuclein Oligomer Formation and Prevents Progression of α-Synuclein Accumulation in a Rotenone Mouse Model of Parkinson’s Disease. Biochim. Biophys. Acta 2016, 1862, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Huang, H.-J.; Wang, Y.-T.; Lin, A.M.-Y. Baicalein Attenuates α-Synuclein Aggregation, Inflammasome Activation and Autophagy in the MPP+-Treated Nigrostriatal Dopaminergic System in Vivo. J. Ethnopharmacol. 2016, 194, 522–529. [Google Scholar] [CrossRef]
- Lee, E.; Park, H.R.; Ji, S.T.; Lee, Y.; Lee, J. Baicalein Attenuates Astroglial Activation in the 1-Methyl-4-Phenyl-1,2,3,4-Tetrahydropyridine-Induced Parkinson’s Disease Model by Downregulating the Activations of Nuclear Factor-ΚB, ERK, and JNK. J. Neurosci. Res. 2014, 92, 130–139. [Google Scholar] [CrossRef]
- Mu, X.; He, G.-R.; Yuan, X.; Li, X.-X.; Du, G.-H. Baicalein Protects the Brain against Neuron Impairments Induced by MPTP in C57BL/6 Mice. Pharmacol. Biochem. Behav. 2011, 98, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, G.; Mu, X.; Zhang, T.; Li, X.; Hu, J.; Xu, B.; Du, G. Neuroprotective Effect of Baicalein against MPTP Neurotoxicity: Behavioral, Biochemical and Immunohistochemical Profile. Neurosci. Lett. 2008, 441, 16–20. [Google Scholar] [CrossRef]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective Role of Apigenin on Rotenone Induced Rat Model of Parkinson’s Disease: Suppression of Neuroinflammation and Oxidative Stress Mediated Apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Jeon, M.-T.; Kim, H.D.; Jung, U.J.; Jang, M.C.; Chu, J.W.; Yang, S.J.; Choi, I.Y.; Choi, M.-S.; Kim, S.R. Nobiletin Protects Dopaminergic Neurons in the 1-Methyl-4-Phenylpyridinium-Treated Rat Model of Parkinson’s Disease. J. Med. Food 2015, 18, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin Treatment Improves Motor and Cognitive Deficits Seen in MPTP-Induced Parkinson Model Mice. Neuroscience 2014, 259, 126–141. [Google Scholar] [CrossRef]
- Leem, E.; Nam, J.H.; Jeon, M.-T.; Shin, W.-H.; Won, S.-Y.; Park, S.-J.; Choi, M.-S.; Jin, B.K.; Jung, U.J.; Kim, S.R. Naringin Protects the Nigrostriatal Dopaminergic Projection through Induction of GDNF in a Neurotoxin Model of Parkinson’s Disease. J. Nutr. Biochem. 2014, 25, 801–806. [Google Scholar] [CrossRef]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Naringin Treatment Induces Neuroprotective Effects in a Mouse Model of Parkinson’s Disease in Vivo, but Not Enough to Restore the Lesioned Dopaminergic System. J. Nutr. Biochem. 2016, 28, 140–146. [Google Scholar] [CrossRef]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.; Mandel, S. Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate Prevents N-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Dopaminergic Neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Wang, M.-H.; Chang, K.-C.; Soung, H.-S.; Fang, C.-H.; Lin, Y.-W.; Li, K.-Y.; Yang, C.-C.; Tsai, C.-C. Protective Effect of (−)Epigallocatechin-3-Gallate on Rotenone-Induced Parkinsonism-like Symptoms in Rats. Neurotox. Res. 2020, 37, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective Effect of Bioflavonoid Quercetin in 6-Hydroxydopamine-Induced Oxidative Stress Biomarkers in the Rat Striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular Mechanisms Underlying Protective Effects of Quercetin against Mitochondrial Dysfunction and Progressive Dopaminergic Neurodegeneration in Cell Culture and MitoPark Transgenic Mouse Models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Li, S.; Pu, X.-P. Neuroprotective Effect of Kaempferol against a 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of Quercetin in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2012, 2012, 928643. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, K.; Nataraj, J.; Janakiraman, U.; Manivasagam, T.; Essa, M.M. Antioxidant and Anti-Inflammatory Potential of Hesperidin against 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine-Induced Experimental Parkinson’s Disease in Mice. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 294. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. The Flavonoid Hesperetin Alleviates Behavioral Abnormality in 6-Hydroxydopamine Rat Model of Hemi-Parkinsonism. Basic Clin. Neurosci. 2010, 2, 20–23. [Google Scholar]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue Distribution and Neuroprotective Effects of Citrus Flavonoid Tangeretin in a Rat Model of Parkinson’s Disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Khan, M.M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin Protects Dopaminergic Neurons from Oxidative Stress in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Jamali-Raeufy, N.; Zabihnejad, S.; Rabiee, N.; Roghani, M. Troxerutin Exerts Neuroprotection in 6-Hydroxydopamine Lesion Rat Model of Parkinson’s Disease: Possible Involvement of PI3K/ERβ Signaling. Eur. J. Pharmacol. 2017, 801, 72–78. [Google Scholar] [CrossRef]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Myricitrin Ameliorates 6-Hydroxydopamine-Induced Dopaminergic Neuronal Loss in the Substantia Nigra of Mouse Brain. J. Med. Food 2016, 19, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Gao, Z.-Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.-P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants 2021, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Altharawi, A.; Alharthy, K.M.; Althurwi, H.N.; Albaqami, F.F.; Alzarea, S.I.; Al-Abbasi, F.A.; Nadeem, M.S.; Kazmi, I. Europinidin Inhibits Rotenone-Activated Parkinson’s Disease in Rodents by Decreasing Lipid Peroxidation and Inflammatory Cytokines Pathways. Molecules 2022, 27, 7159. [Google Scholar] [CrossRef]
- Habib, C.N.; Mohamed, M.R.; Tadros, M.G.; Tolba, M.F.; Menze, E.T.; Masoud, S.I. The Potential Neuroprotective Effect of Diosmin in Rotenone-Induced Model of Parkinson’s Disease in Rats. Eur. J. Pharmacol. 2022, 914, 174573. [Google Scholar] [CrossRef] [PubMed]
- Coe, S.; Andreoli, D.; George, M.; Collett, J.; Reed, A.; Cossington, J.; Izadi, H.; Dixon, A.; Mansoubi, M.; Dawes, H. A Feasibility Study to Determine Whether the Daily Consumption of Flavonoid-Rich Pure Cocoa Has the Potential to Reduce Fatigue and Fatigability in People with Parkinson’s (PwP). Clin. Nutr. ESPEN 2022, 48, 68–73. [Google Scholar] [CrossRef]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.-P.; Sinaiko, A.R. Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef]
- Steptoe, A.; Gibson, E.L.; Vuononvirta, R.; Hamer, M.; Wardle, J.; Rycroft, J.A.; Martin, J.F.; Erusalimsky, J.D. The Effects of Chronic Tea Intake on Platelet Activation and Inflammation: A Double-Blind Placebo Controlled Trial. Atherosclerosis 2007, 193, 277–282. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Duffy, S.J.; Hamburg, N.M.; Gokce, N.; Warden, B.A.; Wiseman, S.; Keaney, J.F.; Frei, B.; Vita, J.A. Effects of Black Tea Consumption on Plasma Catechins and Markers of Oxidative Stress and Inflammation in Patients with Coronary Artery Disease. Free Radic. Biol. Med. 2005, 38, 499–506. [Google Scholar] [CrossRef]
- Costa, R.; Lima, S.A.C.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Hendawy, O.M. Nano-Delivery Systems for Improving Therapeutic Efficiency of Dietary Polyphenols. Altern. Ther. Health Med. 2021, 27, 162–177. [Google Scholar]

| Flavonoid | Effect | Model (In Vitro) | References |
| Myricetin | ↓ TNF-α, IL-1β, and IL-6 | OGD/R, endothelial cells | [92] |
| Quercetin | ↓ TLR4/MyD88/NF-κB signaling | BV2 microglial cells | [93] |
| Isoquercetin | ↓ TLR4, NF-κB, TNF-α, and IL-6 | OGD/R, neurons | [94,95] |
| Baicalin | ↓ TNFα and NOD2 receptor | OGD, BV2 microglial cells | [96] |
| Baicalin | ↓ NLRP3 inflammasome | OGD/R, cortical neurons | [97] |
| Baicalin | ↓ TNF-α, IL-1β, and IL-6 | OGD/R, endothelial cells | [98] |
| Baicalin | ↓ TNF-α, IL-1β, and IL-6 | OGD/R, BV2 microglia cell | [99] |
| Baicalin | ↓ TLR4/NF-κB pathway | microglia-neuron co-culture | [100] |
| Baicalin | ↓ TLR4, MYD88, p-NF-κB expression, ↓ IL-6, IL-1α, IL-1β, IL8, and TNF-α | OGD, endothelial cells | [101,102] |
| Icariin | ↓ IL-1β, IL-6, and TNF-α expression | OGD/R, microglia | [103] |
| Casticin | ↓ TLR4, NF-κB p65, NF-κB, and p50 expression | OGD/R, PC12 cells | [104] |
| Pratensein | ↓ NLRP3 inflammasome | OGD/R, HT22 cells | [105] |
| Tectorigenin | ↓ IL-1β, IL-6, and TNF-α | OGD/R, HT22 cells | [106] |
| Astilbin | ↓ NLRP3 inflammasome, IL-1β, and IL-18 | OGD, PC12 cells | [107] |
| Anthocyanin | ↓ TNF-α, IL-1β, and IL-6 | OGD, SH-SY5Y cells | [108] |
| Tricin | ↓ TNF-α and IL-6 and ↓ IL-1β expression | Neuroblastoma cells | [109] |
| Diosmetin | ↓ NLRP3 inflammasome | OGD/R, PC12 cells | [110] |
| Schaftoside | ↓ TLR4, IL-1β, IL-6, and ↓ TNFα expression | OGD, BV2 microglia | [111] |
| Flavonoid | Effect | Model (In Vivo) | References |
| Kaempferol-3-O-rutinoside and 3-O-glucoside | ↓ infarct volume, STAT3, NF-κB and IL-1β | MCAO/R, rat | [113] |
| Kaempferol | ↓ IL-5, TNF-α, IL-1β, and IL-6 | MCAO/R, rat | [114] |
| Fisetin | ↓ infarct size, TNFa | MCAO/R, mouse | [115] |
| Morin | ↓ neurological deficits, TNF-α, and IL-6 | MCAO/R, rat | [116] |
| Morin | ↓ pNF-κB, TNF-α, and IL-1β, TLR4 expression, ↑ occluding, claudin expression | MCAO/R, rat | [117] |
| EGCG | ↓ infarct volume, TNF-α, IL-1β, IL-6, and NF-κB/p65 | MCAO/R, rat | [118] |
| Luteolin | ↓ infarct volume, astrocytes and microglia activation | MCAO/R, rat | [119] |
| Luteoloside | ↓ infarct volume, TNF-α, and IL-1β | MCAO, rat | [120] |
| Nobiletin | ↓ infarct volume, brain swelling, and neurological deficits | MCOA/R, rat | [121] |
| Eriodictyol | ↓ infarct volume, neurological deficits, TNF-α, and GFAP expression | MCAO, mouse | [122] |
| Tricin | ↓ TNF-α, IL-6, and IL-1β in serum | MCAO, mouse | [[109] |
| Eupatilin | ↓ microglia activation and NF-κB pathway | MCAO, mouse | [123] |
| EGCG + rt-PA | ↓ neurobehavioral deficit, brain infarction, cerebral edema, and blood-brain barrier disruption | MCAO, rat | [124] |
| Flavonoid | Effect (Preventive Treatment) | Model (In Vivo) | References |
| Naringenin | ↓ infarct volume, NF-κB, TNF-α, IL-1β, GFAP, and Iba1 | MCOA/R, rat | [125] |
| Naringenin | ↓ infarct volume, neurologic deficits, and NF-κB | MCOA/R, rat | [126] |
| Hesperidin | ↓ IL-1β | MCOA, rat | [127] |
| Pinocembrin | ↓ infarct size and NF-κB, TNF-α, and IL-6 | Global stroke, rat | [128] |
| Genistein | ↓ infarct volume, neurological deficit, and NF-κB activation | MCAO, mouse | [129] |
| Sanggenon | ↓ TNF-α, IL-1β, and IL-6 | MCOA, rat | [130] |
| Astilbin | ↓ NLRP3 inflammasome, IL-1β, and IL-18 | MCOA, rat | [107] |
| Chrysin | ↓ IL-6, IL-1β, and TNF-α | MCOA, rat | [131] |
| Eupafolin | ↓ TLR-4, TNF-α, IL-1β, and IL-6 expression | MCOA/R, rat | [132] |
| Biochanin A | ↓ TNF-α and IL-1β expression | MCOA, rat | [133] |
| Flavonoid | Effect | Clinical Studies | References |
| fisetin + rt-PA | ↑ therapeutic window of rt-PA and ↓ neurological deficits, C-reactive protein | Double-blind randomized placebo-controlled | [134] |
| EGCG + rt-PA | ↑ therapeutic window of rt-PA, ↓ MMP 2, and MMP 9 | Double-blind randomized placebo-controlled | [135] |
| Flavonoid | Effect | Model (In Vitro) | References |
| EGCG | Prevents Aβ fibrillogenesis and ↓ cell toxicity | Protein aggregation, PC12 cells | [136,137] |
| EGCG | Protective against Aβ toxicity | Hippocampal neuronal cell culture | [138] |
| Quercetin | ↓ Aβ cytotoxicity, lipid peroxidation, protein oxidation, and apoptosis | Hippocampal neuronal cell culture | [139] |
| Luteolin | ↓ Aβ (1–40 and 1–42) | Neuronal cells and SweAPP N2a cells | [140] |
| Diosmetin | ↓ Aβ (1–40 and 1-42) | Neuronal cells and SweAPP N2a cells | [140] |
| Myricetin | Prevents Aβ fibrillogenesis | Cerebral cortices from Tg2576 mouse embryos | [141] |
| Cyanidin 3-O-β-glucopyranoside | ↓ Aβ (25–35) cytotoxicity | SH-SY5Y cells | [142] |
| Wogonin | ↓ Aβ aggregation and phosphorylated Tau | SH-SY5Y cells | [143] |
| Baicalein | Prevents tau protein aggregation | Several biochemical techniques | [144,145] |
| Quercetin | Prevent Aβ aggregation and ↑ disaggregate | Cell system overexpressing APP | [146] |
| Rutin | Prevent Aβ aggregation and ↑ disaggregate | Cell system overexpressing APP | [146] |
| Flavonoid | Effect | Model (In Vivo) | References |
| Isorhamnetin | ↓ learning and memory deficits and ↑ BDNF in prefrontal cortex and hippocampus | Chemical mouse model | [149] |
| Naringin | Improve memory | Chemical rat model | [150] |
| Rutin | Improve memory | Chemical rat model | [150] |
| kaempferol | Improve memory and ↑ density of neurons in hippocampus | Chemical rat model | [151] |
| Luteolin | Improve memory | Chemical rat model | [152] |
| Hesperidin | improves memory and ↓ NF κB, iNOS, COX-2, and astrogliosis | Chemical mouse model | [153] |
| Nobiletin | ↓ soluble Aβ (1–40 and 1–42) and Aβ plaques in the hippocampus | APP-SL 7-5 transgenic mouse | [154] |
| Diosmin and its bioactive metabolites | ↓ tau hyperphosphorylation and Aβ generation | 3xTg transgenic mouse | [155] |
| Hesperidin | ↓ Aβ plaque in cortex and hippocampus and ↓ astrocyte and microglial activation | Transgenic APP/PS1 mouse | [156] |
| Wogonin | Improve memory | Transgenic h-APPswe mouse | [143] |
| Diosmin | ↓ Aβ 1–40 and 1–42 | Tg2576 transgenic mouse | [140] |
| Luteolin | ↓ Aβ 1–40 and 1–42 | Tg2576 transgenic mouse | [140] |
| EGCG | ↓ soluble Aβ (1–40 and 1–42) and Aβ plaques in cortex and hippocampus | APPsw transgenic mouse | [157] |
| EGCG | ↓ Aβ (1–42) | Aβ infusion model, presenilin 2 mutant mouse | [158] |
| Diosmin | Improve memory | APPsw transgenic mouse | [159] |
| Nobiletin | ↓ Aβ plaques in the hippocampus and ↓ memory deficits | APP-SL 7-5 transgenic mouse | [154] |
| Nobiletin | ↓ memory impairment, ↓ the levels of Aβ 1–40 | 3XTg transgenic mouse | [160] |
| Nobiletin | ↓ memory impairment | Senescence-accelerated mice SAMP8 | [161] |
| Quercetin | ↑ memory and ↓ plaques of Aβ and hyperphosphorylated tau in hippocampus | 3XTg transgenic mouse | [162] |
| Cyanidin 3-O-glucoside | ↓ memory impairment, ↓ hyperphosphorylated tau in hippocampus | Aβ infusion rats | [163] |
| Fisetin | ↓ memory and learning problems | APP(swe)/PS1(ΔE9) mouse | [164] |
| Dihydromyricetin | ↑ exploratory and locomotor activity, and memory, ↓ anxiety and Aβ accumulation | TG2576 and TG-SwDI mouse | [165] |
| Flavonoid | Effect | Clinical Studies | References |
| Cocoa flavanol | Improves cognitive function | Patients with mild cognitive impairment | [166] |
| Cocoa flavanol | Improved cognitive function in aging subjects | Double-blind study | [167] |
| Cocoa flavanol | Improves dentate gyrus functions | fMRI in healthy 50–69-year-old subjects | [168] |
| Flavonoid | Effect | Model (In Vitro) | References |
| Apigenin | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| Baicalein | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| Myricetin | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [170] |
| Morin | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| Genistein | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| Quercetin | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| EGCG | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [171] |
| Scutellarein | Inhibit oligomer formation and aggregation of α-synuclein | Protein aggregation | [169] |
| Nobiletin | ↓ TNF-α and IL-1β | Activated microglia | [172] |
| Apigenin | ↓ TNF-α and IL-6 | Activated microglia | [173] |
| Luteolin | ↓ TNF-α and IL-6 | Activated microglia | [173] |
| Naringenin | ↓ NF-κB, iNOS, and COX-2 | Activated microglia | [174,175] |
| Diadzein | ↓ NF-κB and IL-6 | Activated microglia | [176] |
| Equol | ↓ TNF-α, IL-6, and NF-κB | Activated microglia | [177] |
| Morin | ↓ cell apoptosis and mortality | PC12 cells exposed to MPP+ | [178] |
| Morin | ↓ astrogliosis and NF-κB | Astrocytes exposed to MPP+ | [179] |
| Butein | ↓ toxicity | HT22 and Microglial BV2 cells exposed to glutamate | [180] |
| Butin | ↓ toxicity | HT22 and Microglial BV2 cells exposed to glutamate | [180] |
| Fisetin | ↓ toxicity | HT22 and Microglial BV2 cells exposed to glutamate | [180] |
| Fustin | ↓toxicity | HT22 and Microglial BV2 cells exposed to glutamate | [180] |
| Sulfuretin | ↓ toxicity | HT22 and Microglial BV2 cells exposed to glutamate | [180] |
| Genkwanin | ↓ TLR4/MyD88/NLRP3 inflammasome pathway | SH-SY5Y cells | [181] |
| Flavonoid | Effect | Model (In Vivo) | References |
| Baicalein | ↓ α-synuclein | Rotenone | [185] |
| Baicalein | ↓ α-synuclein and inflammasome | MPP+ rat | [186] |
| Baicalein | ↑ motor ability, ↓ activated microglia and astrocytes, and ↑ dopamine and serotonin in the striatum | MPP+ rat | [187,188,189] |
| Apigenin | ↓ α-synuclein and NF-κB | Rotenone rat | [190] |
| Nobiletin | ↓ microglial activation | MPP+ rat | [191] |
| Nobiletin | ↑ dopamine in the striatum and hippocampal region | MPP+ rat | [192] |
| Naringin | ↑ GDNF in the substantia nigra | MPP+ rat | [193] |
| Naringin | Protects the nigrostriatal DA projection | MPP+ rat | [194] |
| EGCG | ↓ dopamine neuronal loss | MPP+ mouse | [195] |
| EGCG | ↓ TNF-α, IL-1β, and IL-6 in the striatum | MPP+ rat | [196] |
| Quercetin | ↑ dopamine in the striatum, ↓ dopamine neuronal loss | 6-OHDA rat | [197] |
| Quercetin | ↓ dopaminergic neuronal loss and behavioral deficits | MitoPark transgenic mouse | [198] |
| Quercetin | ↑dopamine and motor coordination | MTPT mouse | [199,200] |
| Kaempferol | ↑dopamine and motor coordination | MTPT mouse | [199,200] |
| Hesperidin | ↑ motor coordination and ↓ TNF-α, IL-1β, and IL-6 | MTPT mouse | [201,202] |
| Tangeretin | Protects the striatal dopaminergic neurons | 6-OHDA rat | [203] |
| Rutin | ↑ motor coordination and dopaminergic neurons | 6-OHDA rat | [204] |
| Troxerutin | ↓ neuronal loss and astrogliosis | 6-Hydroxydopamine lesion, rat | [205] |
| Myricitrin | Protects the striatal dopaminergic neurons and ↓ TNF-α | 6-Hydroxydopamine lesion, rat | [206] |
| Morin | ↓ neuronal loss and behavioral deficits | MTPT mouse | [178] |
| Morin | ↑ motor coordination and ↓ dopamine neuronal loss | MTPT mouse | [179] |
| Icariin | ↓ NLRP3 inflammasome, IL-1β, and TNF-α in serum | MTPT mouse | [207] |
| Europinidin | ↓ IL-6, IL-1β, and TNF-α | Rotenone rat | [208] |
| Diosmin | ↓ TNF-α and NF-κB | Rotenone rat | [209] |
| Flavonoid | Effect | Clinical Studies | References |
| Cocoa flavanol | ↓ fatigue and fatigability | Double-blind placebo-controlled | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Coria, H.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; López-Valdés, H.E. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. Int. J. Mol. Sci. 2023, 24, 4297. https://doi.org/10.3390/ijms24054297
Martínez-Coria H, Arrieta-Cruz I, Gutiérrez-Juárez R, López-Valdés HE. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. International Journal of Molecular Sciences. 2023; 24(5):4297. https://doi.org/10.3390/ijms24054297
Chicago/Turabian StyleMartínez-Coria, Hilda, Isabel Arrieta-Cruz, Roger Gutiérrez-Juárez, and Héctor Eduardo López-Valdés. 2023. "Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging" International Journal of Molecular Sciences 24, no. 5: 4297. https://doi.org/10.3390/ijms24054297
APA StyleMartínez-Coria, H., Arrieta-Cruz, I., Gutiérrez-Juárez, R., & López-Valdés, H. E. (2023). Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. International Journal of Molecular Sciences, 24(5), 4297. https://doi.org/10.3390/ijms24054297




