A Novel Combination of Sotorasib and Metformin Enhances Cytotoxicity and Apoptosis in KRAS-Mutated Non-Small Cell Lung Cancer Cell Lines through MAPK and P70S6K Inhibition
Abstract
:1. Introduction
2. Results
2.1. Metformin Increases Sotorasib-Driven Cytotoxicity in KRAS-Mutated Lung Cancer Cell Lines
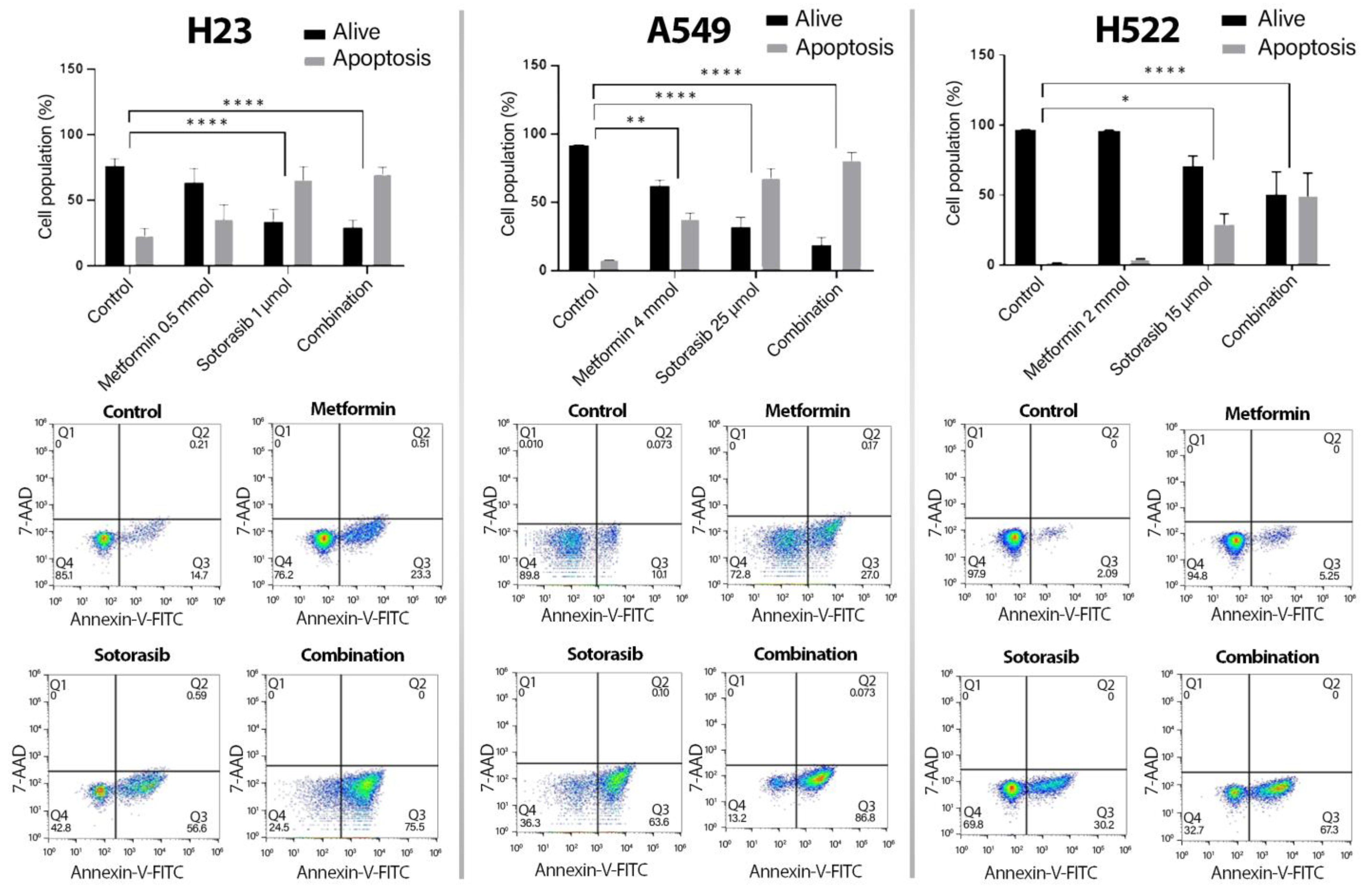
2.2. Increased Apoptosis Induction by the Addition of Metformin to Sotorasib, Regardless of KRAS Status
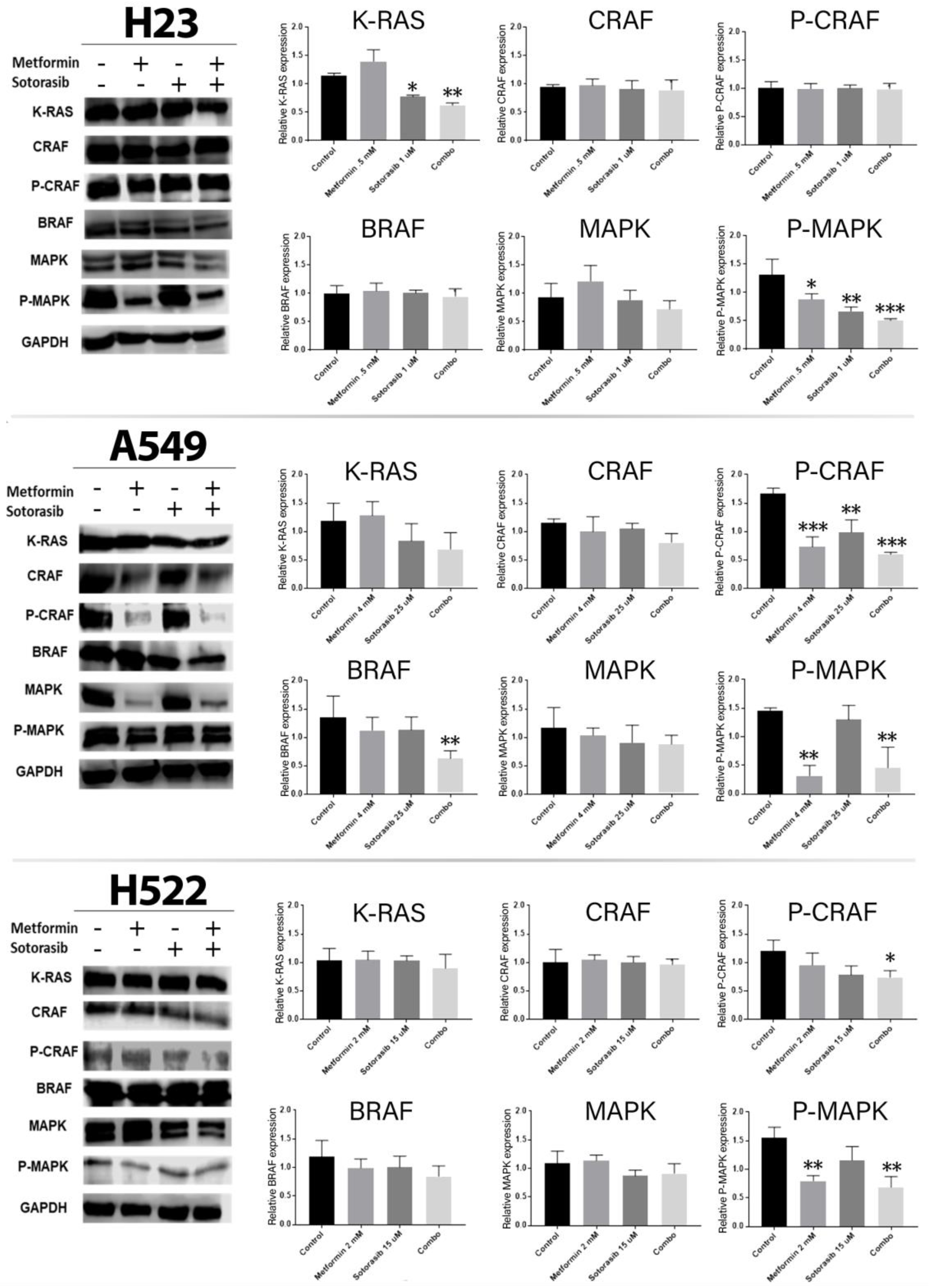
2.3. Combined Therapy Significantly Decreases MAPK Pathway Activity
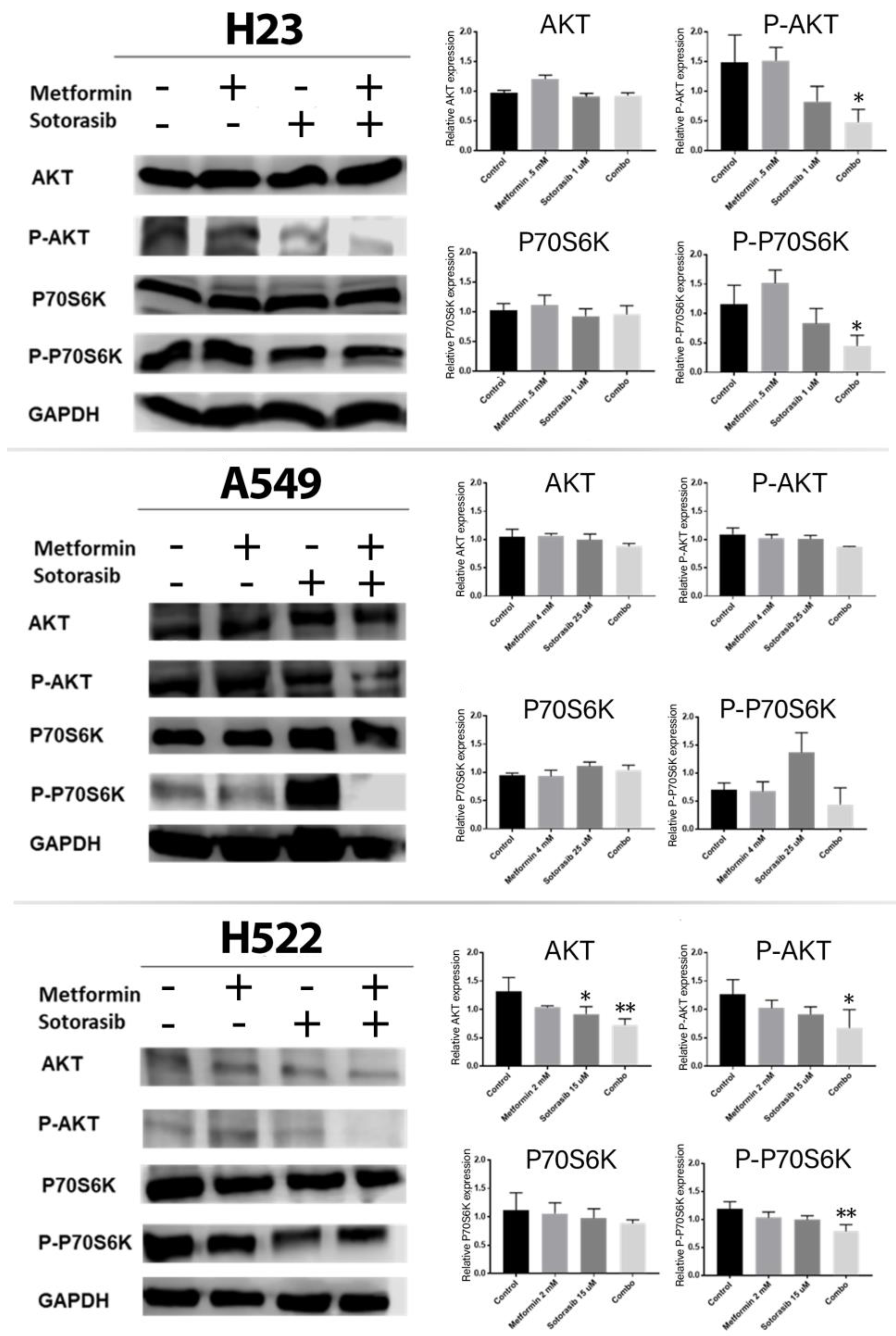
2.4. Combined Treatment of Metformin and Sotorasib Inhibits AKT and P70S6K Activation
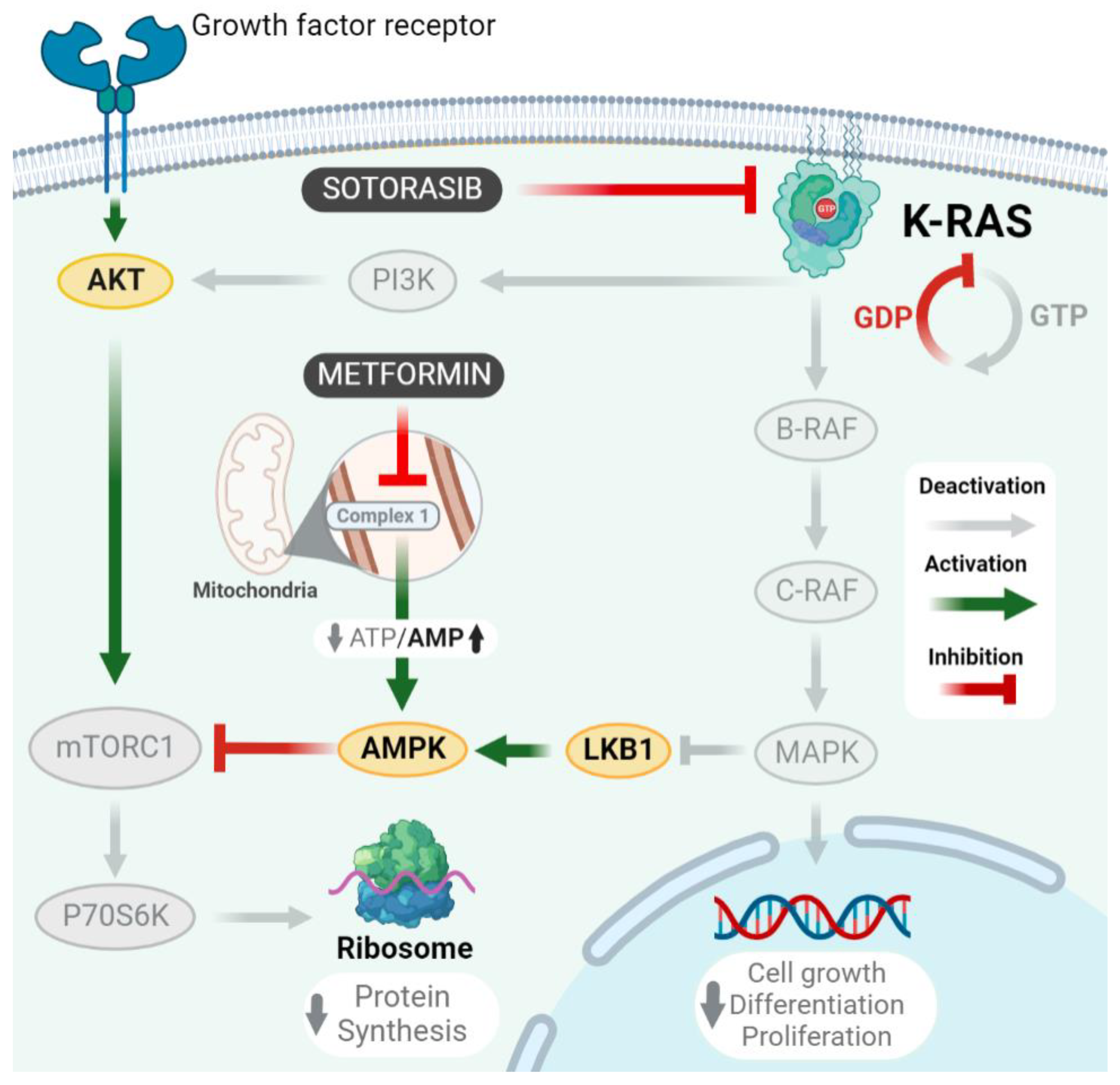
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Viability Assay
4.3. Analysis of Drug Combination Index
4.4. Apoptosis Assay
4.5. Western Blot Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reck, M.; Carbone, D.P.; Garassino, M.; Barlesi, F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pedro, N.; Soca-Chafre, G.; Alaez-Versón, C.; Carrillo-Sánchez, K.; Avilés-Salas, A.; Vergara, E.; Arrieta, O. Mutational profile by targeted next-generation sequencing of non-small cell lung cancer in the Mexican population. Salud Publica Mex. 2019, 61, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, J. KRAS oncogene may be another target conquered in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Goulding, R.E.; Chenoweth, M.; Carter, G.C.; Boye, M.E.; Sheffield, K.M.; John, W.J.; Leusch, M.S.; Muehlenbein, C.E.; Li, L.; Jen, M.H.; et al. KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: A systematic literature review and meta-analysis. Cancer Treat. Res. Commun. 2020, 24, 100200. [Google Scholar] [CrossRef] [PubMed]
- Lanman, B.A.; Allen, J.R.; Allen, J.G.; Amegadzie, A.K.; Ashton, K.S.; Booker, S.K.; Chen, J.J.; Chen, N.; Frohn, M.J.; Goodman, G.; et al. Discovery of a Covalent Inhibitor of KRASG12C (AMG 510) for the Treatment of Solid Tumors. J. Med. Chem. 2020, 63, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Kempf, E.; Rousseau, B.; Besse, B.; Paz-Ares, L. KRAS oncogene in lung cancer: Focus on molecularly driven clinical trials. Eur. Respir. Rev. 2016, 25, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Poh, A. AMG 510 Shows Activity beyond NSCLC. Cancer Discov. 2020, 10, 1084–1085. [Google Scholar]
- Helwick, C. Phase II CodeBreak 100 Validates Benefit of KRAS Inhibitor Sotorasib in Advanced Lung Cancer. The ASCO Post. 2021. Available online: https://ascopost.com/issues/february-25-2021/phase-ii-codebreak-100-validates-benefit-of-kras-inhibitor-sotorasib-in-advanced-lung-cancer/ (accessed on 21 May 2021).
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Li, B.T.; Velcheti, V.; Price, T.J.; Hong, D.S.; Fakih, M.; Kim, D.W.; Falchook, G.S.; Delord, J.P.; Dy, G.K.; Ramalingam, S.S.; et al. Largest evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated non-small cell lung cancer (NSCLC) and colorectal cancer (CRC): Plasma biomarker analysis of CodeBreaK100. J. Clin. Oncol. 2022, 40, 102. [Google Scholar] [CrossRef]
- Zhang, S.S.; Nagasaka, M. Spotlight on sotorasib (Amg 510) for KRASG12C positive non-small cell lung cancer. Lung Cancer Targets Ther. 2021, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Misale, S.; Fatherree, J.P.; Cortez, E.; Li, C.; Bilton, S.; Timonina, D.; Myers, D.T.; Lee, D.; Gomez-Caraballo, M.; Greenberg, M.; et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 2019, 25, 796–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Pedro, N.; Arrieta, O. The Combined Effect of Afatinib and Metformin on Glycolytic Regulation in EGFR-Mutant Non-Small Cell Lung Cancer. 2017. Available online: https://www.researchgate.net/publication/316319807 (accessed on 15 June 2022).
- Do, M.T.; Kim, H.G.; Khanal, T.; Choi, J.H.; Kim, D.H.; Jeong, T.C.; Jeong, H.G. Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol. Appl. Pharmacol. 2013, 271, 229–238. [Google Scholar] [CrossRef]
- Ko, J.C.; Huang, Y.C.; Chen, H.J.; Tseng, S.C.; Chiu, H.C.; Wo, T.Y.; Huang, Y.J.; Weng, S.H.; Chiou, R.Y.; Lin, Y.W. Metformin induces cytotoxicity by down-regulating thymidine phosphorylase and excision repair cross-complementation 1 expression in non-small cell lung cancer cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ashinuma, H.; Takiguchi, Y.; Kitazono, S.; Kitazono-Saitoh, M.; Kitamura, A.; Chiba, T.; Tada, Y.; Kurosu, K.; Sakaida, E.; Sekine, I.; et al. Antiproliferative action of metformin in human lung cancer cell lines. Oncol. Rep. 2012, 28, 8–14. [Google Scholar]
- Barrios-Bernal, P.; Hernandez-Pedro, N.; Orozco-Morales, M.; Viedma-Rodríguez, R.; Lucio-Lozada, J.; Avila-Moreno, F.; Cardona, A.F.; Rosell, R.; Arrieta, O. Metformin Enhances TKI-Afatinib Cytotoxic Effect, Causing Downregulation of Glycolysis, Epithelial–Mesenchymal Transition, and EGFR-Signaling Pathway Activation in Lung Cancer Cells. Pharmaceuticals 2022, 15, 381. [Google Scholar] [CrossRef]
- Yousef, M.; Tsiani, E. Metformin in lung cancer: Review of in vitro and in vivo animal studies. Cancers 2017, 9, 45. [Google Scholar] [CrossRef]
- Arrieta, O.; Barrón, F.; Padilla, M.Á.S.; Avilés-Salas, A.; Ramírez-Tirado, L.A.; Jiménez, M.J.A.; Vergara, E.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Cardona, A.F.; et al. Effect of Metformin Plus Tyrosine Kinase Inhibitors Compared with Tyrosine Kinase Inhibitors Alone in Patients with Epidermal Growth Factor Receptor–Mutated Lung Adenocarcinoma. JAMA Oncol. 2019, 5, e192553. [Google Scholar] [CrossRef]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Provinciali, N.; Lazzeroni, M.; Cazzaniga, M.; Gorlero, F.; Dunn, B.K.; Decensi, A. Metformin: Risk-benefit profile with a focus on cancer. Expert Opin. Drug Saf. 2015, 14, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, Z.; Jiang, L.; Liu, M.; Ma, J.; Yang, C.; Han, L.; Nan, K.; Liang, X. Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinase. Mol. Med. Rep. 2016, 13, 2590–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, O.; Varela-Santoyo, E.; Soto-Perez-de-Celis, E.; Sánchez-Reyes, R.; la Torre-Vallejo, D.; Muñiz-Hernández, S.; Cardona, A.F. Metformin use and its effect on survival in diabetic patients with advanced non-small cell lung cancer. BMC Cancer 2016, 16, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Corte, C.M.; Ciaramella, V.; Di Mauro, C.; Castellone, M.D.; Papaccio, F.; Fasano, M.; Sasso, F.C.; Martinelli, E.; Troiani, T.; De Vita, F.; et al. Metformin increases antitumor activity of MEK inhibitors through GLI1 downregulation in LKB1 positive human NSCLC cancer cells. Oncotarget 2016, 7, 4265. Available online: www.impactjournals.com/oncotarget (accessed on 20 August 2022). [CrossRef] [PubMed] [Green Version]
- Dillon, M.; Lopez, A.; Lin, E.; Sales, D.; Perets, R.; Jain, P. Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers 2021, 13, 5059. [Google Scholar] [CrossRef]
- Ma, Y.; Schulz, B.; Trakooljul, N.; Al Ammar, M.; Sekora, A.; Sender, S.; Hadlich, F.; Zechner, D.; Weiss, F.U.; Lerch, M.M.; et al. Inhibition of KRAS, MEK and PI3K Demonstrate Synergistic Anti-Tumor Effects in Pancreatic Ductal Adenocarcinoma Cell Lines. Cancers 2022, 14, 4467. [Google Scholar] [CrossRef]
- Khan, S.; Wiegand, J.; Zhang, P.; Hu, W.; Thummuri, D.; Budamagunta, V.; Hua, N.; Jin, L.; Allegra, C.J.; Kopetz, S.E.; et al. BCL-XL PROTAC degrader DT2216 synergizes with sotorasib in preclinical models of KRASG12C-mutated cancers. J. Hematol. Oncol. 2022, 15, 23. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, F.C.; Wang, W.; Shi, H.S.; Li, D.; Wang, Y.S. K-ras gene mutation as a predictor of cancer cell responsiveness to metformin. Mol. Med. Rep. 2013, 8, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, Q.; Wang, D.; Wang, Z.; Hu, C. Metformin inhibits growth of lung adenocarcinoma cells by inducing apoptosis via the mitochondria-mediated pathway. Oncol. Lett. 2015, 10, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Q.; Xie, X.F.; Li, G.M.; Chen, J.R.; Li, M.T.; Xu, X.; Xiong, Q.Y.; Chen, G.R.; Yin, Y.P.; Peng, F.; et al. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother. Res. 2021, 35, 4511–4525. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Lv, C.; Du, J.; Lian, F.; Zhang, S.; Wang, Z.; Zeng, Y. Gypenoside-Induced Apoptosis via the PI3K/AKT/mTOR Signaling Pathway in Bladder Cancer. Biomed. Res. Int. 2022, 2022, 9304552. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.S.; McDonald, P.C.; Nemirovsky, O.; Awrey, S.; Chafe, S.C.; Schaeffer, D.F.; Li, J.; Renouf, D.J.; Stanger, B.Z.; Dedhar, S. Overcoming Adaptive Resistance to KRAS and MEK Inhibitors by Co-targeting mTORC1/2 Complexes in Pancreatic Cancer. Cell Rep. Med. 2020, 1, 100131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, B.; Wu, J.; Zhang, H.; Wu, B. Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncol. Lett. 2014, 8, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Morgillo, F.; Sasso, F.C.; Della Corte, C.M.; Vitagliano, D.; D’aiuto, E.; Troiani, T.; Martinelli, E.; De Vita, F.; Orditura, M.; De Palma, R.; et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin. Cancer Res. 2013, 19, 3508–3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Kerk, S.A.; Papagiannakopoulos, T.; Shah, Y.M.; Lyssiotis, C.A. Metabolic networks in mutant KRAS-driven tumours: Tissue specificities and the microenvironment. Nat. Rev. Cancer 2021, 21, 510–525. [Google Scholar] [CrossRef]

| Cell Line | Metformin (IC 10) | Sotorasib (IC 50) |
|---|---|---|
| A549 | 4 mmol | 25 µmol |
| H522 | 2 mmol | 15 µmol |
| H23 | 0.5 mmol | 1 µmol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Bernal, P.; Lucio-Lozada, J.; Ramos-Ramírez, M.; Hernández-Pedro, N.; Arrieta, O. A Novel Combination of Sotorasib and Metformin Enhances Cytotoxicity and Apoptosis in KRAS-Mutated Non-Small Cell Lung Cancer Cell Lines through MAPK and P70S6K Inhibition. Int. J. Mol. Sci. 2023, 24, 4331. https://doi.org/10.3390/ijms24054331
Barrios-Bernal P, Lucio-Lozada J, Ramos-Ramírez M, Hernández-Pedro N, Arrieta O. A Novel Combination of Sotorasib and Metformin Enhances Cytotoxicity and Apoptosis in KRAS-Mutated Non-Small Cell Lung Cancer Cell Lines through MAPK and P70S6K Inhibition. International Journal of Molecular Sciences. 2023; 24(5):4331. https://doi.org/10.3390/ijms24054331
Chicago/Turabian StyleBarrios-Bernal, Pedro, José Lucio-Lozada, Maritza Ramos-Ramírez, Norma Hernández-Pedro, and Oscar Arrieta. 2023. "A Novel Combination of Sotorasib and Metformin Enhances Cytotoxicity and Apoptosis in KRAS-Mutated Non-Small Cell Lung Cancer Cell Lines through MAPK and P70S6K Inhibition" International Journal of Molecular Sciences 24, no. 5: 4331. https://doi.org/10.3390/ijms24054331
APA StyleBarrios-Bernal, P., Lucio-Lozada, J., Ramos-Ramírez, M., Hernández-Pedro, N., & Arrieta, O. (2023). A Novel Combination of Sotorasib and Metformin Enhances Cytotoxicity and Apoptosis in KRAS-Mutated Non-Small Cell Lung Cancer Cell Lines through MAPK and P70S6K Inhibition. International Journal of Molecular Sciences, 24(5), 4331. https://doi.org/10.3390/ijms24054331






