COL7A1 Editing via RNA Trans-Splicing in RDEB-Derived Skin Equivalents
Abstract
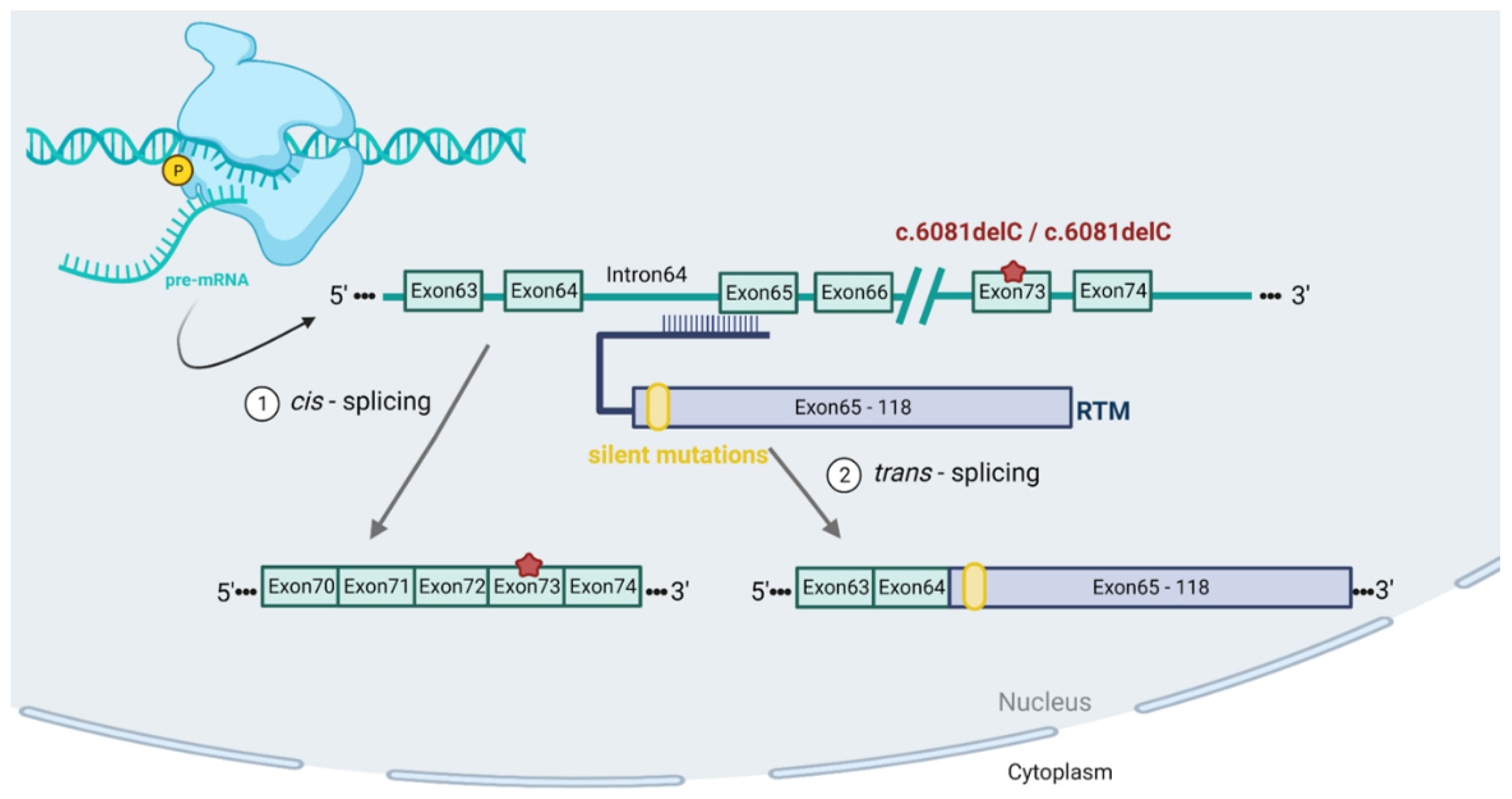
1. Introduction
2. Results
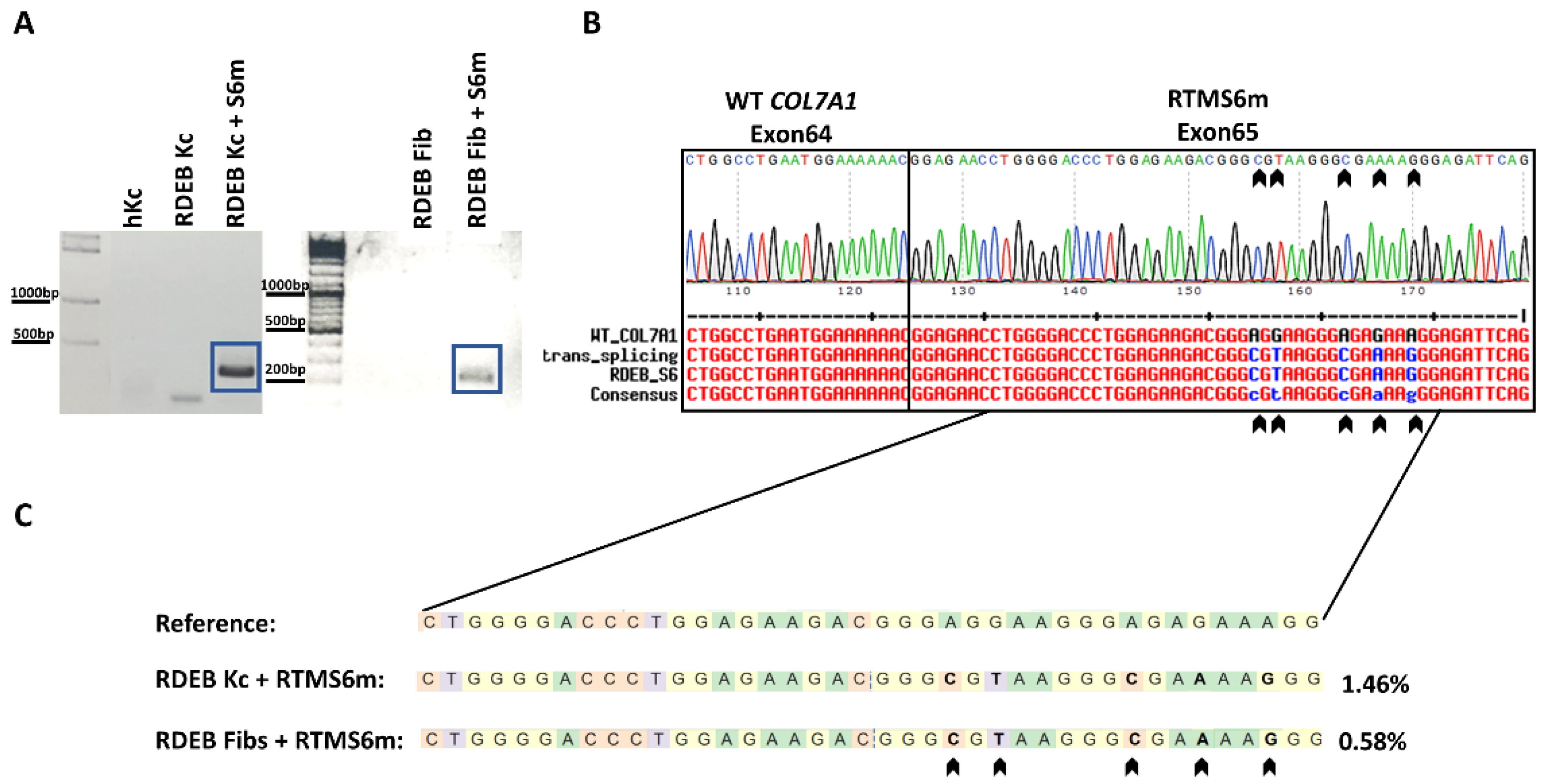
2.1. In Vitro 3′ Trans-Splicing in RDEB Keratinocytes and Fibroblasts
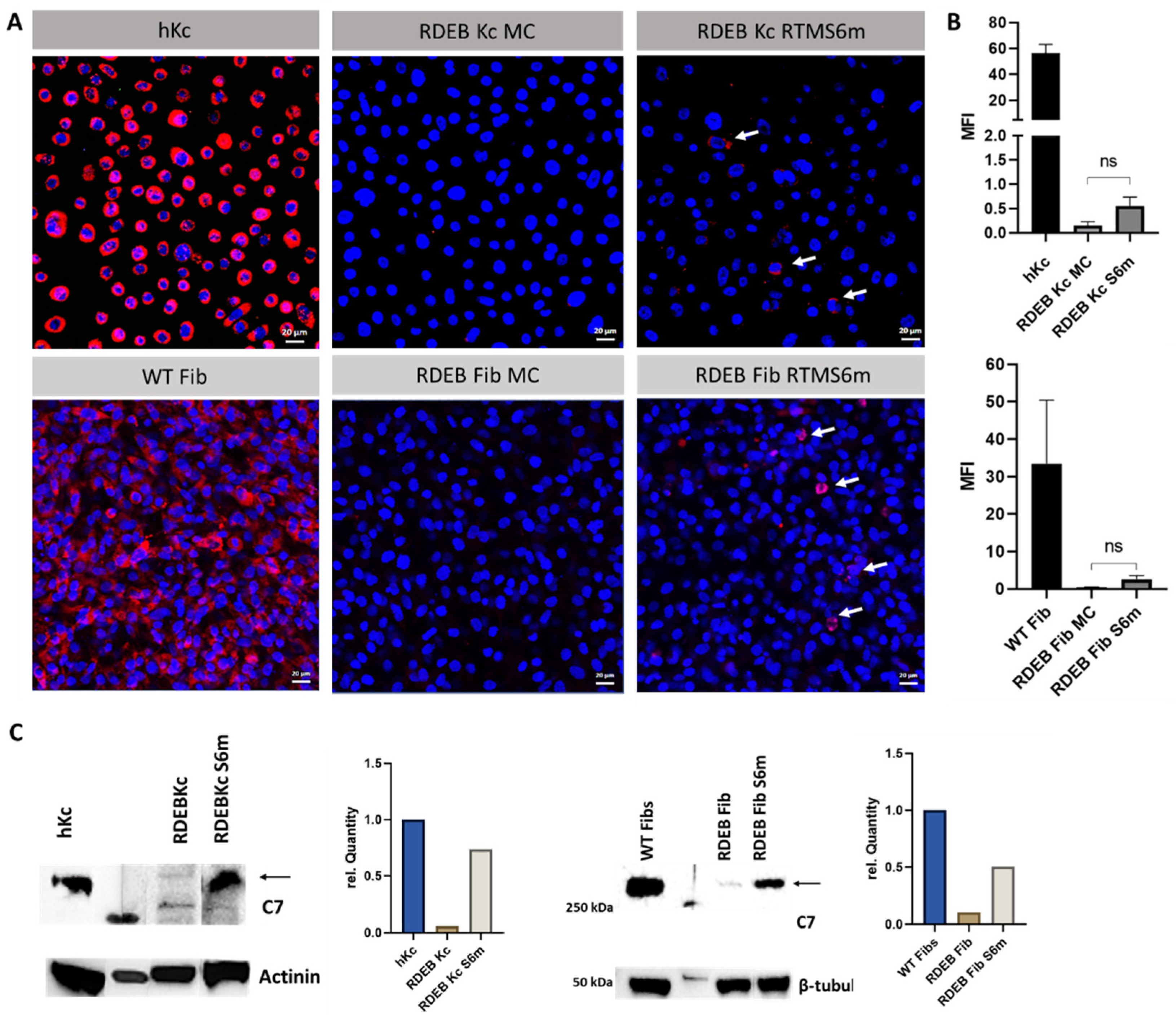
2.2. Minicircle RTMS6m Expression Induces C7 Restoration in RDEB Keratinocytes and Fibroblasts
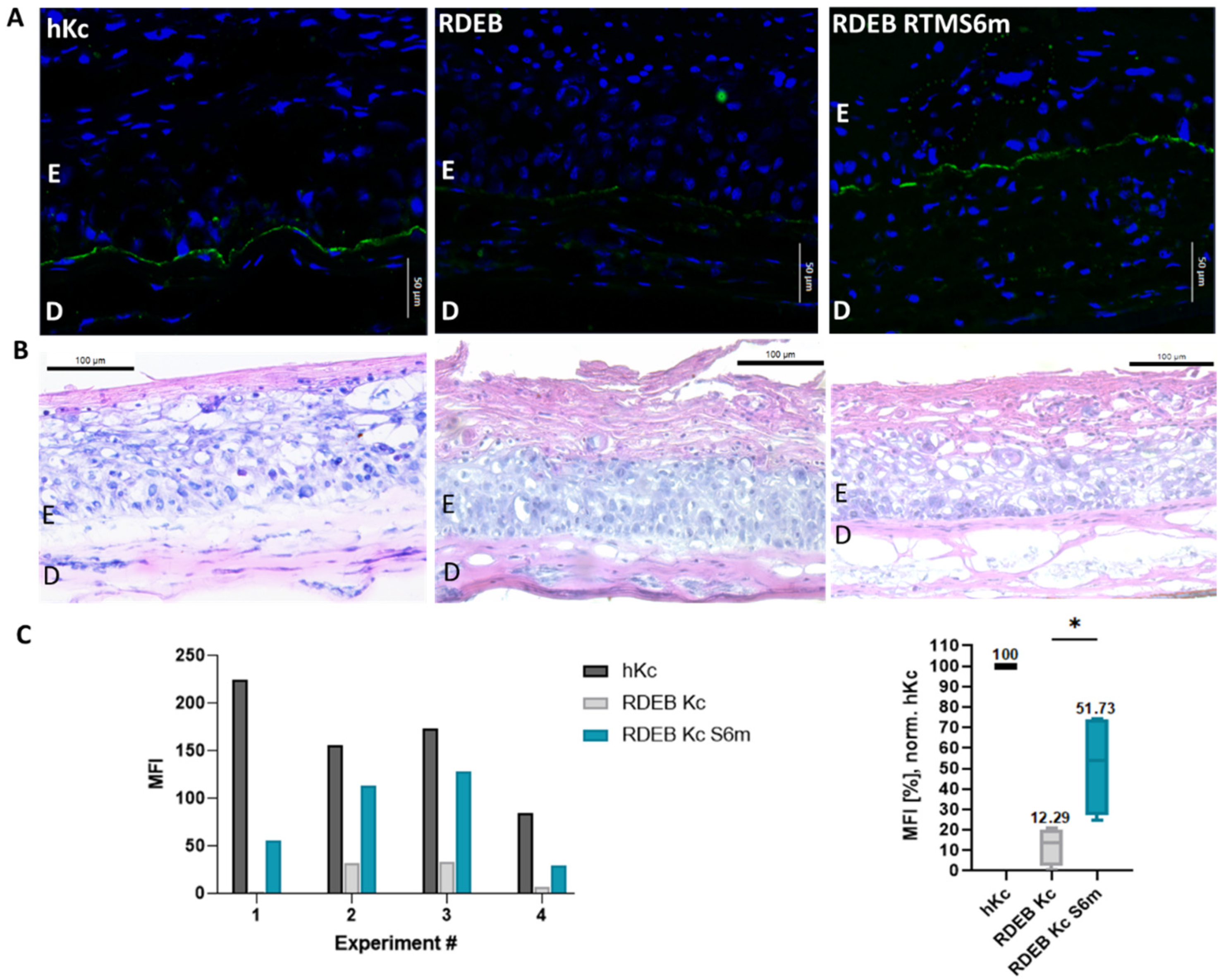
2.3. Accumulation of Restored C7 within Skin Equivalents
3. Discussion
4. Materials and Methods
4.1. Cell Culture & Cell Lines
4.2. Transfection and Electroporation of Keratinocytes and Fibroblasts
4.3. RNA Isolation and SqRT-PCR
4.4. Next-Generation Sequencing
4.5. Immunofluorescence Staining of C7 in Keratinocytes and Fibroblasts
4.6. Protein Isolation and Western Blot Analysis
4.7. Generation and Transfection of Skin Equivalents
4.8. Generation of DDC642 Liposomes
4.9. Immunofluorescence Staining of Skin Equivalents
4.10. Immunohistochemistry Staining of Skin Equivalents
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Has, C.; Bauer, J.W.; Bodemer, C.; Bolling, M.C.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.-D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.P.; et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Christiano, A.M. Molecular basis for the dystrophic forms of epidermolysis bullosa: Mutations in the type VII collagen gene. Arch. Dermatol. Res. 1994, 287, 16–22. [Google Scholar] [CrossRef]
- Fine, J.-D.; Bruckner-Tuderman, L.; Eady, R.A.; Bauer, E.A.; Bauer, J.W.; Has, C.; Heagerty, A.; Hintner, H.; Hovnanian, A.; Jonkman, M.F.; et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 2014, 70, 1103–1126. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, A.G.; Dellambra, E.; Logli, E.; Zambruno, G.; Castiglia, D. Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int. J. Mol. Sci. 2019, 20, 25707. [Google Scholar] [CrossRef]
- Wertheim-Tysarowska, K.; Sobczyńska-Tomaszewska, A.; Kowalewski, C.; Skroński, M.; Święćkowski, G.; Kutkowska-Kaźmierczak, A.; Bal, J. The COL7A1 mutation database. Hum. Mutat. 2012, 33, 327–331. [Google Scholar] [CrossRef]
- Dallinger, G.; Puttaraju, M.; Mitchell, L.G.; Yancey, K.B.; Yee, C.; Klausegger, A.; Hintner, H.; Bauer, J.W. Development of spliceosome-mediated RNA trans-splicing (SMaRT) for the correction of inherited skin diseases. Exp. Dermatol. 2003, 12, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.G.; Chao, H.; Walsh, C.E. RNA repair using spliceosome-mediated RNA trans-splicing. Trends Mol. Med. 2004, 10, 263–268. [Google Scholar] [CrossRef]
- Bauer, J.W.; Murauer, E.M.; Wally, V.; Koller, U. RNA trans-splicing for genodermatoses. Methods Mol. Biol. 2013, 961, 441–455. [Google Scholar] [PubMed]
- Wally, V.; Murauer, E.M.; Bauer, J.W. Spliceosome-mediated trans-splicing: The therapeutic cut and paste. J. Invest. Dermatol. 2012, 132, 1959–1966. [Google Scholar] [CrossRef]
- Koller, U.; Wally, V.; Bauer, J.W.; Murauer, E.M. Considerations for a Successful RNA Trans-splicing Repair of Genetic Disorders. Mol. Ther.-Nucleic Acids 2014, 3, e157. [Google Scholar] [CrossRef]
- Wally, V.; Brunner, M.; Lettner, T.; Wagner, M.; Koller, U.; Trost, A.; Murauer, E.M.; Hainzl, S.; Hintner, H.; Bauer, J.W. K14 mRNA reprogramming for dominant epidermolysis bullosa simplex. Hum. Mol. Genet. 2010, 19, 4715–4725. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Kathirvel, P.; Yee, W.C.; Lai, P.S. Correction of dystrophia myotonica type 1 pre-mRNA transcripts by artificial trans-splicing. Gene Ther. 2009, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Coady, T.H.; Lorson, C.L. Trans-splicing-mediated improvement in a severe mouse model of spinal muscular atrophy. J. Neurosci. 2010, 30, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Shababi, M.; Lorson, C.L. Optimization of SMN trans-splicing through the analysis of SMN introns. J. Mol. Neurosci. 2012, 46, 459–469. [Google Scholar] [CrossRef]
- Zayed, H.; Xia, L.; Yerich, A.; Yant, S.R.; A Kay, M.; Puttaraju, M.; McGarrity, G.J.; Wiest, D.L.; McIvor, R.S.; Tolar, J.; et al. Correction of DNA protein kinase deficiency by spliceosome-mediated RNA trans-splicing and sleeping beauty transposon delivery. Mol. Ther. 2007, 15, 1273–1279. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Zhang, L.N.; Yan, Z.; Zak, R.; Ding, W.; Mansfield, S.G.; Mitchell, L.G.; Engelhardt, J.F. Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells. Hum. Gene Ther. 2005, 16, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Wally, V.; Klausegger, A.; Koller, U.; Lochmüller, H.; Krause, S.; Wiche, G.; Mitchell, L.G.; Hintner, H.; Bauer, J.W. 5′ trans-splicing repair of the PLEC1 gene. J. Invest. Dermatol. 2008, 128, 568–574. [Google Scholar] [CrossRef]
- Liemberger, B.; Hofbauer, J.P.; Wally, V.; Arzt, C.; Hainzl, S.; Kocher, T.; Murauer, E.M.; Bauer, J.W.; Reichelt, J.; Koller, U. RNA Trans-Splicing Modulation via Antisense Molecule Interference. Int. J. Mol. Sci. 2018, 19, 762. [Google Scholar] [CrossRef]
- Peking, P.; Breitenbach, J.S.; Ablinger, M.; Muss, W.H.; Poetschke, F.J.; Kocher, T.; Koller, U.; Hainzl, S.; Kitzmueller, S.; Bauer, J.W.; et al. An ex vivo RNA trans-splicing strategy to correct human generalized severe epidermolysis bullosa simplex. Br. J. Dermatol. 2019, 180, 141–148. [Google Scholar] [CrossRef]
- Tockner, B.; Kocher, T.; Hainzl, S.; Reichelt, J.; Bauer, J.W.; Koller, U.; Murauer, E.M. Construction and validation of an RNA trans-splicing molecule suitable to repair a large number of COL7A1 mutations. Gene Ther. 2016, 23, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Peking, P.; Koller, U.; Duarte, B.; Murillas, R.; Wolf, S.; Maetzig, T.; Rothe, M.; Kocher, T.; García, M.; Brachtl, G.; et al. An RNA-targeted therapy for dystrophic epidermolysis bullosa. Nucleic Acids Res. 2017, 45, 10259–10269. [Google Scholar] [CrossRef]
- Mayr, E.; Ablinger, M.; Lettner, T.; Murauer, E.M.; Guttmann-Gruber, C.; Piñón Hofbauer, J.; Hainzl, S.; Kaiser, M.; Klausegger, A.; Bauer, J.W.; et al. 5′RNA Trans-Splicing Repair of COL7A1 Mutant Transcripts in Epidermolysis Bullosa. Int. J. Mol. Sci. 2022, 23, 1732. [Google Scholar] [CrossRef]
- Van Den Akker, P.C.; Jonkman, M.F.; Rengaw, T.; Bruckner-Tuderman, L.; Has, C.; Bauer, J.W.; Klausegger, A.; Zambruno, G.; Castiglia, D.; Mellerio, J.E.; et al. The international dystrophic epidermolysis bullosa patient registry: An online database of dystrophic epidermolysis bullosa patients and their COL7A1 mutations. Hum. Mutat. 2011, 32, 1100–1107. [Google Scholar] [CrossRef]
- Kocher, T.; March, O.P.; Bischof, J.; Liemberger, B.; Hainzl, S.; Klausegger, A.; Hoog, A.; Strunk, D.; Bauer, J.W.; Koller, U. Predictable CRISPR/Cas9-Mediated COL7A1 Reframing for Dystrophic Epidermolysis Bullosa. J. Invest. Dermatol. 2020, 140, 1985–1993.e5. [Google Scholar] [CrossRef]
- Clement, K.; Rees, H.; Canver, M.C.; Gehrke, J.M.; Farouni, R.; Hsu, J.Y.; Cole, M.A.; Liu, D.R.; Joung, J.K.; Bauer, D.E.; et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 2019, 37, 224–226. [Google Scholar] [CrossRef]
- Bornert, O.; Kocher, T.; Gretzmeier, C.; Liemberger, B.; Hainzl, S.; Koller, U.; Nyström, A. Generation of rabbit polyclonal human and murine collagen VII monospecific antibodies: A useful tool for dystrophic epidermolysis bullosa therapy studies. Matrix Biol. Plus 2019, 4, 100017. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. Characterization data on the topical carrier DDC642. Data Brief 2016, 7, 1204–1210. [Google Scholar]
- Hainzl, S.; Peking, P.; Kocher, T.; Murauer, E.M.; Larcher, F.; Del Rio, M.; Duarte, B.; Steiner, M.; Klausegger, A.; Bauer, J.W.; et al. COL7A1 Editing via CRISPR/Cas9 in Recessive Dystrophic Epidermolysis Bullosa. Mol. Ther. 2017, 25, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Jackow, J.; Guo, Z.; Hansen, C.; Abaci, H.E.; Doucet, Y.S.; Shin, J.U.; Hayashi, R.; DeLorenzo, D.; Kabata, Y.; Shinkuma, S.; et al. CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc. Natl. Acad. Sci. USA 2019, 116, 26846–26852. [Google Scholar] [CrossRef]
- Kocher, T.; Bischof, J.; Haas, S.A.; March, O.P.; Liemberger, B.; Hainzl, S.; Illmer, J.; Hoog, A.; Muigg, K.; Binder, H.-M.; et al. A non-viral and selection-free COL7A1 HDR approach with improved safety profile for dystrophic epidermolysis bullosa. Mol. Ther. Nucleic Acids 2021, 25, 237–250. [Google Scholar] [CrossRef]
- Bonafont, J.; Mencía, Á.; García, M.; Torres, R.; Rodríguez, S.; Carretero, M.; Chacón-Solano, E.; Modamio-Høybjør, S.; Marinas, L.; León, C.; et al. Clinically Relevant Correction of Recessive Dystrophic Epidermolysis Bullosa by Dual sgRNA CRISPR/Cas9-Mediated Gene Editing. Mol. Ther. 2019, 27, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Bonafont, J.; Mencía, A.; Chacón-Solano, E.; Srifa, W.; Vaidyanathan, S.; Romano, R.; Garcia, M.; Hervás-Salcedo, R.; Ugalde, L.; Duarte, B.; et al. Correction of recessive dystrophic epidermolysis bullosa by homology-directed repair-mediated genome editing. Mol. Ther. 2021, 29, 2008–2018. [Google Scholar] [CrossRef]
- Petković, I.; Bischof, J.; Kocher, T.; March, O.P.; Liemberger, B.; Hainzl, S.; Strunk, D.; Raninger, A.M.; Binder, H.-M.; Reichelt, J.; et al. COL17A1 editing via homology-directed repair in junctional epidermolysis bullosa. Front. Med. 2022, 9, 976604. [Google Scholar] [CrossRef]
- Bischof, J.; March, O.P.; Liemberger, B.; Haas, S.A.; Hainzl, S.; Petković, I.; Leb-Reichl, V.; Illmer, J.; Korotchenko, E.; Klausegger, A.; et al. Paired nicking-mediated COL17A1 reframing for junctional epidermolysis bullosa. Mol. Ther. 2022, 30, 2680–2692. [Google Scholar] [CrossRef]
- Aushev, M.; Koller, U.; Mussolino, C.; Cathomen, T.; Reichelt, J. Traceless Targeting and Isolation of Gene-Edited Immortalized Keratinocytes from Epidermolysis Bullosa Simplex Patients. Mol. Ther.-Methods Clin. Dev. 2017, 6, 112–123. [Google Scholar] [CrossRef]
- Kocher, T.; Peking, P.; Klausegger, A.; Murauer, E.M.; Hofbauer, J.P.; Wally, V.; Lettner, T.; Hainzl, S.; Ablinger, M.; Bauer, J.W.; et al. Cut and Paste: Efficient Homology-Directed Repair of a Dominant Negative KRT14 Mutation via CRISPR/Cas9 Nickases. Mol. Ther. 2017, 25, 2585–2598. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, L.; Zhu, D.; Ding, W.; Wang, X.; Zhang, C.; Wang, L.; Jiang, X.; Shen, H.; He, D.; et al. Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Titeux, M.; Pendaries, V.; Zanta-Boussif, M.A.; Décha, A.; Pironon, N.; Tonasso, L.; Mejia, J.E.; Brice, A.; Danos, O.; Hovnanian, A. SIN retroviral vectors expressing COL7A1 under human promoters for ex vivo gene therapy of recessive dystrophic epidermolysis bullosa. Mol. Ther. 2010, 18, 1509–1518. [Google Scholar] [CrossRef]
- Eichstadt, S.; Barriga, M.; Ponakala, A.; Teng, C.; Nguyen, N.T.; Siprashvili, Z.; Nazaroff, J.; Gorell, E.S.; Chiou, A.S.; Taylor, L.; et al. Phase 1/2a clinical trial of gene-corrected autologous cell therapy for recessive dystrophic epidermolysis bullosa. JCI Insight 2019, 4, e130554. [Google Scholar] [CrossRef]
- Guide, S.V.; Gonzalez, M.E.; Bağcı, I.S.; Agostini, B.; Chen, H.; Feeney, G.; Steimer, M.; Kapadia, B.; Sridhar, K.; Sanchez, L.Q.; et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N. Engl. J. Med. 2022, 387, 2211–2219. [Google Scholar] [CrossRef]
- Wang, X.; Alshehri, F.; Manzanares, D.; Li, Y.; He, Z.; Qiu, B.; Zeng, M.; A, S.; Lara-Sáez, I.; Wang, W. Development of Minicircle Vectors Encoding COL7A1 Gene with Human Promoters for Non-Viral Gene Therapy for Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2021, 22, 12774. [Google Scholar] [CrossRef]
- Murauer, E.M.; Gache, Y.; Gratz, I.K.; Klausegger, A.; Muss, W.; Gruber, C.; Meneguzzi, G.; Hintner, H.; Bauer, J.W. Functional correction of type VII collagen expression in dystrophic epidermolysis bullosa. J. Invest. Dermatol. 2011, 131, 74–83. [Google Scholar] [CrossRef]
- Peking, P.; Koller, U.; Hainzl, S.; Kitzmueller, S.; Kocher, T.; Mayr, E.; Nyström, A.; Lener, T.; Reichelt, J.; Bauer, J.W.; et al. A Gene Gun-mediated Nonviral RNA trans-splicing Strategy for COL7A1 Repair. Mol. Ther.-Nucleic Acids 2016, 5, e287. [Google Scholar] [CrossRef]
- Kühl, T.; Mezger, M.; Hausser, I.; Guey, L.T.; Handgretinger, R.; Bruckner-Tuderman, L.; Nyström, A. Collagen VII Half-Life at the Dermal-Epidermal Junction Zone: Implications for Mechanisms and Therapy of Genodermatoses. J. Invest. Dermatol. 2016, 136, 1116–1123. [Google Scholar] [CrossRef]
| Cell Line | Mutation | Exon | Cell Type |
|---|---|---|---|
| RDEB1 | c.6081delC/c.6081delC | exon 73 | keratinocytes |
| RDEB2 | c.8441-15del20/c.8505-8506dupCG | exon 115 | fibroblasts |
| RDEB3 | c.7795G>T/c.7795G>T | exon 105 | fibroblasts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liemberger, B.; Bischof, J.; Ablinger, M.; Hainzl, S.; Murauer, E.M.; Lackner, N.; Ebner, P.; Kocher, T.; Nyström, A.; Wally, V.; et al. COL7A1 Editing via RNA Trans-Splicing in RDEB-Derived Skin Equivalents. Int. J. Mol. Sci. 2023, 24, 4341. https://doi.org/10.3390/ijms24054341
Liemberger B, Bischof J, Ablinger M, Hainzl S, Murauer EM, Lackner N, Ebner P, Kocher T, Nyström A, Wally V, et al. COL7A1 Editing via RNA Trans-Splicing in RDEB-Derived Skin Equivalents. International Journal of Molecular Sciences. 2023; 24(5):4341. https://doi.org/10.3390/ijms24054341
Chicago/Turabian StyleLiemberger, Bernadette, Johannes Bischof, Michael Ablinger, Stefan Hainzl, Eva M. Murauer, Nina Lackner, Patricia Ebner, Thomas Kocher, Alexander Nyström, Verena Wally, and et al. 2023. "COL7A1 Editing via RNA Trans-Splicing in RDEB-Derived Skin Equivalents" International Journal of Molecular Sciences 24, no. 5: 4341. https://doi.org/10.3390/ijms24054341
APA StyleLiemberger, B., Bischof, J., Ablinger, M., Hainzl, S., Murauer, E. M., Lackner, N., Ebner, P., Kocher, T., Nyström, A., Wally, V., Mayr, E., Guttmann-Gruber, C., Hofbauer, J. P., Bauer, J. W., & Koller, U. (2023). COL7A1 Editing via RNA Trans-Splicing in RDEB-Derived Skin Equivalents. International Journal of Molecular Sciences, 24(5), 4341. https://doi.org/10.3390/ijms24054341






