Comparisons between Plant and Animal Stem Cells Regarding Regeneration Potential and Application
Abstract
:1. Introduction
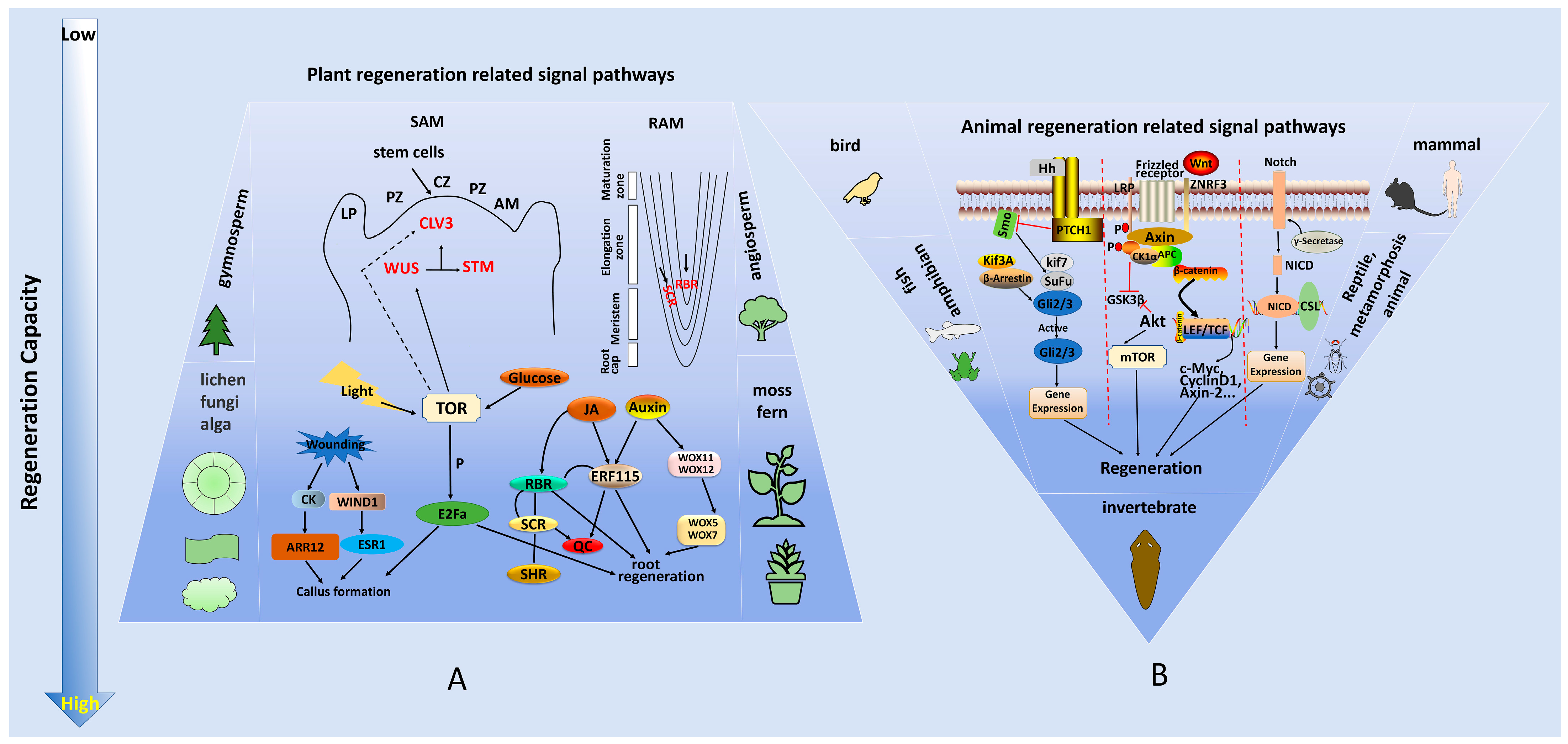
2. Similarities and Differences in Plant and Animal Regeneration
3. Molecular Mechanisms of Plant and Animal Regeneration
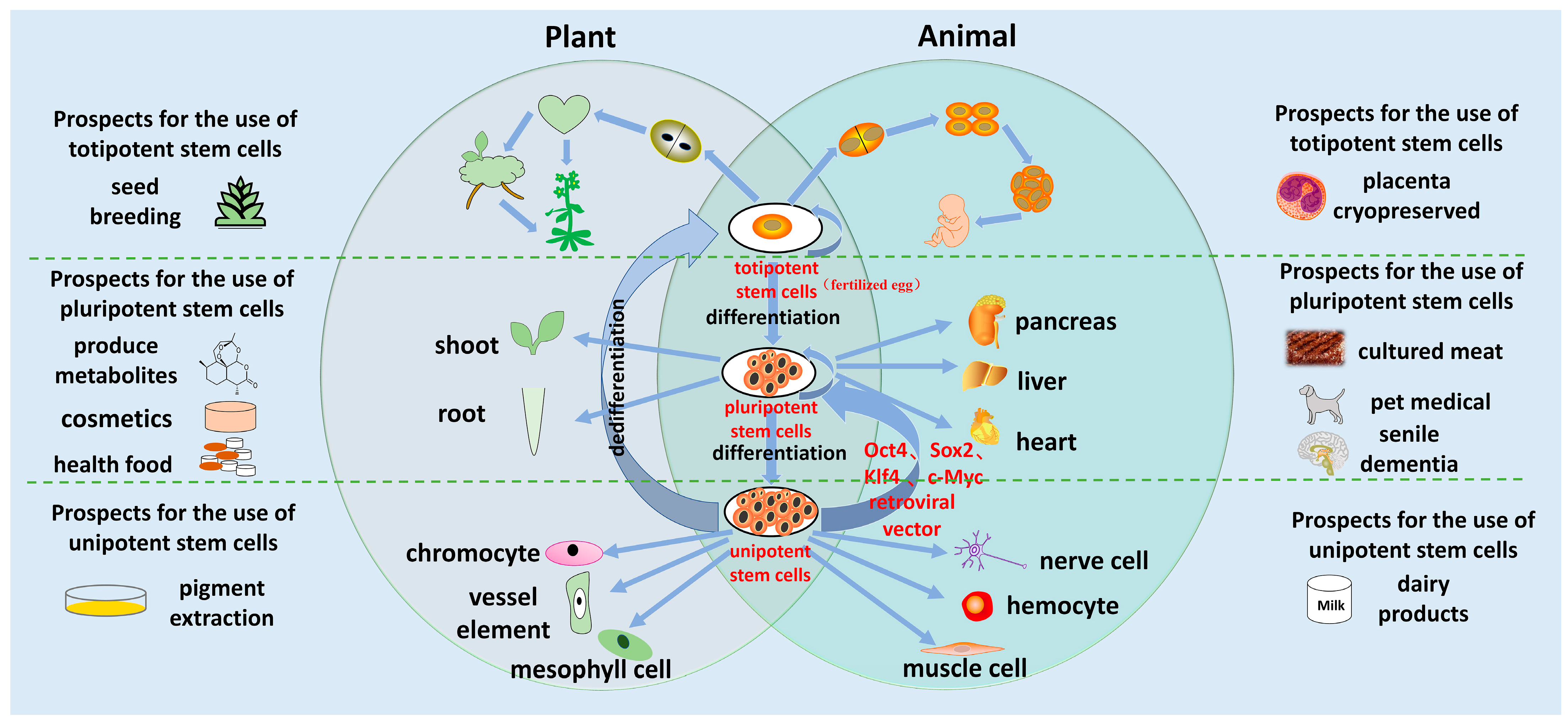
4. Applications of Regeneration Technology
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Heidstra, R.; Sabatini, S. Plant and animal stem cells: Similar yet different. Nat. Rev. Mol. Cell Biol. 2014, 15, 301–312. [Google Scholar] [CrossRef]
- Goldman, J.A.; Poss, K.D. Gene regulatory programmes of tissue regeneration. Nat. Rev. Genet. 2020, 21, 511–525. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, K.D.; Sanchez Alvarado, A. Slicing across kingdoms: Regeneration in plants and animals. Cell 2008, 132, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Sun, Y.; Gao, T.; Li, J.; Fang, H.; Zhang, S. Djnedd4L Is Required for Head Regeneration by Regulating Stem Cell Maintenance in Planarians. Int. J. Mol. Sci. 2021, 22, 11707. [Google Scholar] [CrossRef]
- Rossi, L.; Salvetti, A. Planarian stem cell niche, the challenge for understanding tissue regeneration. Semin. Cell Dev. Biol. 2019, 87, 30–36. [Google Scholar] [CrossRef]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Aichinger, E.; Kornet, N.; Friedrich, T.; Laux, T. Plant stem cell niches. Annu. Rev. Plant Biol. 2012, 63, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, Y. Axillary meristem initiation-a way to branch out. Curr. Opin. Plant Biol. 2018, 41, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tsata, V.; Mollmert, S.; Schweitzer, C.; Kolb, J.; Mockel, C.; Bohm, B.; Rosso, G.; Lange, C.; Lesche, M.; Hammer, J.; et al. A switch in pdgfrb(+) cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev. Cell 2021, 56, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Pedroni, A.; Bertuzzi, M.; Kizil, C.; Simon, A.; Ampatzis, K. Locomotion dependent neuron-glia interactions control neurogenesis and regeneration in the adult zebrafish spinal cord. Nat. Commun. 2021, 12, 4857. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Strand, N.S.; Yang, C.T.; Rabinowitz, J.S.; Moon, R.T. Macrophages modulate adult zebrafish tail fin regeneration. Development 2014, 141, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, D.C. Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2016, 12, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Barton, M.K. Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 2010, 341, 95–113. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, K.; Pajoro, A.; Angenent, G.C. Regulation of transcription in plants: Mechanisms controlling developmental switches. Nat. Rev. Genet. 2010, 11, 830–842. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, L.; Zhang, C. Regulation of Shoot Apical Meristem and Axillary Meristem Development in Plants. Int. J. Mol. Sci. 2020, 21, 2917. [Google Scholar] [CrossRef] [Green Version]
- Fouracre, J.P.; Poethig, R.S. Role for the shoot apical meristem in the specification of juvenile leaf identity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 10168–10177. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [Green Version]
- Shi, A.; Li, J.; Qiu, X.; Sabbah, M.; Boroumand, S.; Huang, T.C.; Zhao, C.; Terzic, A.; Behfar, A.; Moran, S.L. TGF-beta loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics 2021, 11, 6616–6631. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Seo, P.J. Arabidopsis TOR signaling is essential for sugar-regulated callus formation. J. Integr. Plant Biol. 2017, 59, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, A.; Janocha, D.; Dong, Y.; Medzihradszky, A.; Schone, S.; Daum, G.; Suzaki, T.; Forner, J.; Langenecker, T.; Rempel, E.; et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5, e17023. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Liu, Y.; Li, H.; Fu, L.; Liu, Z.; Xu, L.; Liu, H.; Xu, T.; Xiong, Y. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc. Natl. Acad. Sci. USA 2017, 114, 2765–2770. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; Dong, P.; Wang, W.; Feng, L.; Xiong, F.; Wang, K.; Zhang, S.; Feng, S.; Wang, B.; Zhang, J.; et al. The TOR Pathway Is Involved in Adventitious Root Formation in Arabidopsis and Potato. Front. Plant Sci. 2017, 8, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.; Venglat, P.; Qiu, S.; Feng, L.; Cao, Y.; Wang, E.; Xiang, D.; Wang, J.; Alexander, D.; Chalivendra, S.; et al. Target of rapamycin signaling regulates metabolism, growth, and life span in Arabidopsis. Plant Cell 2012, 24, 4850–4874. [Google Scholar] [CrossRef] [Green Version]
- Fingar, D.C.; Blenis, J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004, 23, 3151–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, S.A.M.; MHA, M.H.; Khairul Bariah, A.A.N.; Wan Kamarul Zaman, W.S.; Nordin, F. Regenerative Medicine Therapy in Malaysia: An Update. Front. Bioeng. Biotechnol. 2022, 10, 789644. [Google Scholar] [CrossRef]
- Guo, L.; Bloom, J.S.; Dols-Serrate, D.; Boocock, J.; Ben-David, E.; Schubert, O.T.; Kozuma, K.; Ho, K.; Warda, E.; Chui, C.; et al. Island-specific evolution of a sex-primed autosome in a sexual planarian. Nature 2022, 606, 329–334. [Google Scholar] [CrossRef]
- Chen, Y.; Lüttmann, F.F.; Schoger, E.; Schöler, H.R.; Zelarayán, L.C.; Kim, K.P.; Haigh, J.J.; Kim, J.; Braun, T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 2021, 373, 1537–1540. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, M.; Shah, A.M.; Ye, W.; Tan, W.; Min, Y.L.; Botten, G.A.; Shelton, J.M.; Liu, N.; Bassel-Duby, R.; et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc. Natl. Acad. Sci. USA 2019, 116, 18455–18465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, R.D.; Buckarma, E.H.; Tomlinson, J.L.; McCabe, C.E.; Yonkus, J.A.; Werneburg, N.W.; Bayer, R.L.; Starlinger, P.P.; Robertson, K.D.; Wang, C.; et al. SHP2 inhibition enhances YES-associated protein mediated liver regeneration in murine partial hepatectomy models. JCI Insight 2022, 7, e159930. [Google Scholar] [CrossRef] [PubMed]
- Bely, A.E.; Nyberg, K.G. Evolution of animal regeneration: Re-emergence of a field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef]
- Sánchez Alvarado, A.; Tsonis, P.A. Bridging the regeneration gap: Genetic insights from diverse animal models. Nat. Rev. Genet. 2006, 7, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. iPSCs: A Comparison between Animals and Plants. Trends Plant Sci. 2018, 23, 660–666. [Google Scholar] [CrossRef]
- Pulawska-Czub, A.; Pieczonka, T.D.; Mazurek, P.; Kobielak, K. The Potential of Nail Mini-Organ Stem Cells in Skin, Nail and Digit Tips Regeneration. Int. J. Mol. Sci. 2021, 22, 2864. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Hui, L. Cell Plasticity in Liver Regeneration. Trends Cell Biol. 2020, 30, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burns Trauma 2020, 8, tkaa002. [Google Scholar] [CrossRef]
- Perez-Garcia, P.; Moreno-Risueno, M.A. Stem cells and plant regeneration. Dev. Biol. 2018, 442, 3–12. [Google Scholar] [CrossRef]
- Slack, J.M.W. What is a stem cell? Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e323. [Google Scholar] [CrossRef]
- Pierre-Jerome, E.; Drapek, C.; Benfey, P.N. Regulation of Division and Differentiation of Plant Stem Cells. Annu. Rev. Cell Dev. Biol. 2018, 34, 289–310. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, A.J. The co-ordination of cell division, differentiation and morphogenesis in the shoot apical meristem: A perspective. J Exp. Bot. 2006, 57, 25–32. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef] [Green Version]
- Luchetti, F.; Carloni, S.; Nasoni, M.G.; Reiter, R.J.; Balduini, W. Melatonin, tunneling nanotubes, mesenchymal cells, and tissue regeneration. Neural. Regen. Res. 2023, 18, 760–762. [Google Scholar] [CrossRef]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Ramirez, A.; Diaz-Trivino, S.; Blilou, I.; Grieneisen, V.A.; Sozzani, R.; Zamioudis, C.; Miskolczi, P.; Nieuwland, J.; Benjamins, R.; Dhonukshe, P.; et al. A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell 2012, 150, 1002–1015. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Ramirez, A.; Diaz-Trivino, S.; Wachsman, G.; Du, Y.; Arteaga-Vazquez, M.; Zhang, H.; Benjamins, R.; Blilou, I.; Neef, A.B.; Chandler, V.; et al. A SCARECROW-RETINOBLASTOMA protein network controls protective quiescence in the Arabidopsis root stem cell organizer. PLoS Biol. 2013, 11, e1001724. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wu, H.; Zhu, G.; Yin, C.; Zhao, L.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Fu, T.; et al. Identification and Fine Mapping of the Candidate Gene Controlling Multi-Inflorescence in Brassica napus. Int. J. Mol. Sci. 2022, 23, 7244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Gaines, C.; Buck, A.; Galli, M.; Gallavotti, A. Structural variation at the maize WUSCHEL1 locus alters stem cell organization in inflorescences. Nat. Commun. 2021, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Miotk, A.; Šutiković, Z.; Ermakova, O.; Wenzl, C.; Medzihradszky, A.; Gaillochet, C.; Forner, J.; Utan, G.; Brackmann, K.; et al. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 2019, 10, 5093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Liu, L.; Crowell, O.; Zhao, H.; Brutnell, T.P.; Jackson, D.; Kellogg, E.A. The CLV3 Homolog in Setaria viridis Selectively Controls Inflorescence Meristem Size. Front. Plant Sci. 2021, 12, 636749. [Google Scholar] [CrossRef] [PubMed]
- Kuluev, B.; Avalbaev, A.; Nikonorov, Y.; Ermoshin, A.; Yuldashev, R.; Akhiarova, G.; Shakirova, F.; Chemeris, A. Effect of constitutive expression of Arabidopsis CLAVATA3 on cell growth and possible role of cytokinins in leaf size control in transgenic tobacco plants. J. Plant Physiol. 2018, 231, 244–250. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Laux, T.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. PNAS 2020, 117, 22561–22571. [Google Scholar] [CrossRef]
- Lenhard, M.; Jürgens, G.; Laux, T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 2002, 129, 3195–3206. [Google Scholar] [CrossRef]
- Kornet, N.; Scheres, B. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell 2009, 21, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Zhai, N.; Xu, L. Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat. Plants 2021, 7, 1453–1460. [Google Scholar] [CrossRef]
- Iwase, A.; Mita, K.; Nonaka, S.; Ikeuchi, M.; Koizuka, C.; Ohnuma, M.; Ezura, H.; Imamura, J.; Sugimoto, K. WIND1-based acquisition of regeneration competency in Arabidopsis and rapeseed. J. Plant Res. 2015, 128, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Iwase, A.; Harashima, H.; Ikeuchi, M.; Rymen, B.; Ohnuma, M.; Komaki, S.; Morohashi, K.; Kurata, T.; Nakata, M.; Ohme-Takagi, M.; et al. WIND1 Promotes Shoot Regeneration through Transcriptional Activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 2017, 29, 54–69. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Dai, X.; Li, J.; Liu, N.; Liu, X.; Li, S.; Xiang, F. The Type-B Cytokinin Response Regulator ARR1 Inhibits Shoot Regeneration in an ARR12-Dependent Manner in Arabidopsis. Plant Cell 2022, 32, 2271–2291. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Shibata, M.; Rymen, B.; Iwase, A.; Bågman, A.M.; Watt, L.; Coleman, D.; Favero, D.S.; Takahashi, T.; Ahnert, S.E.; et al. A Gene Regulatory Network for Cellular Reprogramming in Plant Regeneration. Plant Cell Physiol. 2018, 59, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.M.; Zhang, H.K.; Zhai, J.F.; Zhang, X.S.; Sang, Y.L.; Cheng, Z.J. ARF4 regulates shoot regeneration through coordination with ARF5 and IAA12. Plant Cell Rep. 2021, 40, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aros, C.J.; Pantoja, C.J.; Gomperts, B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021, 4, 601. [Google Scholar] [CrossRef]
- Leucht, P.; Lee, S.; Yim, N. Wnt signaling and bone regeneration: Can’t have one without the other. Biomaterials 2019, 196, 46–50. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Song, P.; Fang, Z.Q.; Yue, Z.S.; Liu, J.J.; Dou, G.R.; Han, H.; Wang, L. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Nomura, K.; Tanimoto, Y.; Hayashi, F.; Harada, E.; Shan, X.Y.; Shionyu, M.; Hijikata, A.; Shirai, T.; Morigaki, K.; Shimamoto, K. The Role of the Prod1 Membrane Anchor in Newt Limb Regeneration. Angew. Chem. Int. Ed. Engl. 2017, 56, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zha, L.; Li, H.; Liao, G.; Huang, Z.; Peng, X.; Wang, Z. Upregulation of GNL3 expression promotes colon cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Oncol. Rep. 2017, 38, 2023–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentinmikko, N.; Iqbal, S.; Mana, M.; Andersson, S.; Cognetta III, A.B.; Suciu, R.M.; Roper, J.; Luopajärvi, K.; Markelin, E.; Gopalakrishnan, S.; et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Rabadan Ros, R.; Martinez-Redondo, P.; Ma, Z.; Shi, L.; Xue, Y.; Guillen-Guillen, I.; Huang, L.; Hishida, T.; Liao, H.K.; et al. In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nat. Commun. 2021, 12, 3094. [Google Scholar] [CrossRef]
- Gehrke, A.R.; Neverett, E.; Luo, Y.J.; Brandt, A.; Ricci, L.; Hulett, R.E.; Gompers, A.; Ruby, J.G.; Rokhsar, D.S.; Reddien, P.W.; et al. Acoel genome reveals the regulatory landscape of whole-body regeneration. Science 2019, 363, eaau6173. [Google Scholar] [CrossRef]
- Scimone, M.L.; Cloutier, J.K.; Maybrun, C.L.; Reddien, P.W. The planarian wound epidermis gene equinox is required for blastema formation in regeneration. Nat. Commun. 2022, 13, 2726. [Google Scholar] [CrossRef]
- Novikova, E.L.; Bakalenko, N.I.; Nesterenko, A.Y.; Kulakova, M.A. Hox genes and animal regeneration. Russ. J. Dev. Biol. 2016, 47, 173–180. [Google Scholar] [CrossRef]
- Rossant, J. Genes for regeneration. eLife 2014, 3, e02517. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, L.; Lv, Y. Exosome derived from mesenchymal stem cells mediates hypoxia-specific BMP2 gene delivery and enhances bone regeneration. Chem. Eng. J. 2021, 422, 130084. [Google Scholar] [CrossRef]
- Uchida, N.; Torii, K.U. Stem cells within the shoot apical meristem: Identity, arrangement and communication. Cell Mol. Life Sci. 2019, 76, 1067–1080. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Kong, X.; Ding, Z.; Zhang, X.S. Initiation and maintenance of plant stem cells in root and shoot apical meristems. Abiotech 2020, 1, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Kiss, A.; Sassi, M.A.-O.; Chauvet, A.; Das, P.; Cortizo, M.; Laufs, P.; Takeda, S.; Aida, M.; Traas, J.; et al. Mechanical stress contributes to the expression of the STM homeobox gene in Arabidopsis shoot meristems. Elife 2015, 4, e07811. [Google Scholar] [CrossRef]
- Schuster, C.; Gaillochet, C.; Medzihradszky, A.; Busch, W.; Daum, G.; Krebs, M.; Kehle, A.; Lohmann, J.U. A regulatory framework for shoot stem cell control integrating metabolic, transcriptional, and phytohormone signals. Dev. Cell 2014, 28, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, H.; Matsubayashi, Y. Reevaluation of the CLV3-receptor interaction in the shoot apical meristem: Dissection of the CLV3 signaling pathway from a direct ligand-binding point of view. Plant J. 2015, 82, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Greb, T.; Lohmann, J.U. Plant Stem Cells. Curr. Biol. 2016, 26, 816–821. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal control of the shoot stem-cell niche. Nature 2010, 465, 1089–1092. [Google Scholar] [CrossRef]
- Besnard, F.; Refahi, Y.; Morin, V.; Marteaux, B.; Brunoud, G.; Chambrier, P.; Rozier, F.; Mirabet, V.; Legrand, J.; Lainé, S.; et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2014, 505, 417–421. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.T.; Benfey, P.N. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef] [Green Version]
- Smith, Z.R.; Long, J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 2010, 464, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Hofhuis, H.; Heidstra, R.; Sauer, M.; Friml, J.; Scheres, B. A molecular framework for plant regeneration. Science 2006, 311, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matosevich, R.A.-O.; Efroni, I.A.-O. The quiescent center and root regeneration. J. Exp. Bot. 2021, 72, 6739–6745. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Forzani, C.; Aichinger, E.; Sornay, E.; Willemsen, V.; Laux, T.; Dewitte, W.; Murray, J.A. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr. Biol. 2014, 24, 1939–1944. [Google Scholar] [CrossRef] [Green Version]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Scheres, B. Stem-cell niches: Nursery rhymes across kingdoms. Nat. Rev. Mol. Cell Biol. 2007, 8, 345–354. [Google Scholar] [CrossRef]
- Sage, J. The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 2012, 26, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, L.; Li, Y.; Zhao, D.; Liu, L.; Chang, W.; Zhang, K.; Zheng, Y.; Hou, J.; Fu, C.; et al. TOP1alpha fine-tunes TOR-PLT2 to maintain root tip homeostasis in response to sugars. Nat. Plants 2022, 8, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qu, Y.; Sheng, L.; Liu, J.; Huang, H.; Xu, L. A simple method suitable to study de novo root organogenesis. Front. Plant Sci. 2014, 5, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Lakehal, A.A.-O.; Dob, A.A.-O.; Novák, O.; Bellini, C.A.-O. A DAO1-Mediated Circuit Controls Auxin and Jasmonate Crosstalk Robustness during Adventitious Root Initiation in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 4428. [Google Scholar] [CrossRef] [Green Version]
- Umeda, M.; Ikeuchi, M.; Ishikawa, M.; Ito, T.; Nishihama, R.; Kyozuka, J.; Torii, K.U.; Satake, A.; Goshima, G.; Sakakibara, H. Plant stem cell research is uncovering the secrets of longevity and persistent growth. Plant J. 2021, 106, 326–335. [Google Scholar] [CrossRef]
- Che, P.; Lall, S.; Nettleton, D.; Howell, S.H. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006, 141, 620–637. [Google Scholar] [CrossRef] [Green Version]
- Iwase, A.; Mita, K.; Favero, D.S.; Mitsuda, N.; Sasaki, R.; Kobayshi, M.; Takebayashi, Y.; Kojima, M.; Kusano, M.; Oikawa, A.; et al. WIND1 induces dynamic metabolomic reprogramming during regeneration in Brassica napus. Dev. Biol. 2018, 442, 40–52. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H. Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 2014, 108, 1–33. [Google Scholar] [CrossRef]
- Salaün, C.; Lepiniec, L.; Dubreucq, B. Genetic and Molecular Control of Somatic Embryogenesis. Plants 2021, 10, 1467. [Google Scholar] [CrossRef]
- Magnani, E.; Jiménez-Gómez, J.M.; Soubigou-Taconnat, L.; Lepiniec, L.; Fiume, E.A.-O. Profiling the onset of somatic embryogenesis in Arabidopsis. BMC Genom. 2017, 18, 998. [Google Scholar] [CrossRef] [Green Version]
- Elhiti, M.; Stasolla, C. Transduction of Signals during Somatic Embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jürgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef]
- Zhang, T.A.-O.; Lian, H.A.-O.; Zhou, C.M.; Xu, L.A.-O.; Jiao, Y.A.-O.; Wang, J.A.-O. A Two-Step Model for de Novo Activation of WUSCHEL during Plant Shoot Regeneration. Plant Cell 2017, 29, 1073–1087. [Google Scholar] [CrossRef] [Green Version]
- Lotan, T.; Ohto, M.A.; Yee, K.M.; West, M.A.; Lo, R.; Kwong, R.W.; Yamagishi, K.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 1998, 93, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.; Quimbaya, M.; Tohme, J.; Chavarriaga-Aguirre, P. Arabidopsis LEC1 and LEC2 Orthologous Genes Are Key Regulators of Somatic Embryogenesis in Cassava. Front. Plant Sci. 2019, 10, 673. [Google Scholar] [CrossRef]
- Bideau, L.; Kerner, P.; Hui, J.; Vervoort, M.; Gazave, E. Animal regeneration in the era of transcriptomics. Cell Mol. Life Sci. 2021, 78, 3941–3956. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef]
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and Regulation of Programmed Cell Death in Plant Development. Annu. Rev. Cell Dev. Biol. 2016, 32, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Desire, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Zhong, T.P. Wnt Signaling in Heart Development and Regeneration. Curr. Cardiol. Rep. 2022, 24, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Gruber, J.; Yee, Z.; Tolwinski, N.S. Developmental Drift and the Role of Wnt Signaling in Aging. Cancers 2016, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Ables, J.L.; Breunig, J.J.; Eisch, A.J.; Rakic, P. Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 2011, 12, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sato, C.; Cerletti, M.; Wagers, A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010, 92, 367–409. [Google Scholar] [CrossRef]
- Wehner, D.; Weidinger, G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015, 31, 336–343. [Google Scholar] [CrossRef]
- Li, H.; Chang, C.; Li, X.; Zhang, R. The roles and activation of endocardial Notch signaling in heart regeneration. Cell Regen. 2021, 10, 3. [Google Scholar] [CrossRef]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jang, Y.J.; Dabrowska, C.; Iich, E.; Evans, K.V.; Hall, H.; Janes, S.M.; Simons, B.D.; Koo, B.K.; Kim, J.; et al. Release of Notch activity coordinated by IL-1beta signalling confers differentiation plasticity of airway progenitors via Fosl2 during alveolar regeneration. Nat. Cell Biol. 2021, 23, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.M.; Giovannone, D.; Rydzik, R.; Balsbaugh, J.L.; Moss, I.L.; Schwedler, J.L.; Bertrand, J.Y.; Traver, D.; Hankenson, K.D.; Crump, J.G.; et al. Notch signaling enhances bone regeneration in the zebrafish mandible. Development 2022, 149, dev199995. [Google Scholar] [CrossRef] [PubMed]
- Beachy, P.A.; Hymowitz, S.G.; Lazarus, R.A.; Leahy, D.J.; Siebold, C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010, 24, 2001–2012. [Google Scholar] [CrossRef] [Green Version]
- Ingham, P.W. Hedgehog signaling. Curr. Top. Dev. Biol. 2022, 149, 1–58. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, J. Regulation of Smoothened Trafficking and Abundance in Hedgehog Signaling. Front. Cell Dev. Biol. 2022, 10, 847844. [Google Scholar] [CrossRef]
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011, 12, 393–406. [Google Scholar] [CrossRef]
- Falkenstein, K.N.; Vokes, S.A. Transcriptional regulation of graded Hedgehog signaling. Semin. Cell Dev. Biol. 2014, 33, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Chen, Y.; Zhuang, Y.; Wei, R.; Getachew, A.; Pan, T.; Yang, F.; Li, Y. Inhibition of Hedgehog Delays Liver Regeneration through Disrupting the Cell Cycle. Curr. Issues Mol. Biol. 2022, 44, 470–482. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, K.L. Hippo Signaling in Embryogenesis and Development. Trends Biochem. Sci. 2021, 46, 51–63. [Google Scholar] [CrossRef]
- Badouel, C.; McNeill, H. SnapShot: The hippo signaling pathway. Cell 2011, 145, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.M.N.; Deng, Y.; Wang, J.; Zhao, C.; Wang, J.; Rao, R.; Xu, L.; Zhou, W.; Choi, K.; Rizvi, T.A.; et al. Programming of Schwann Cells by Lats1/2-TAZ/YAP Signaling Drives Malignant Peripheral Nerve Sheath Tumorigenesis. Cancer Cell 2018, 33, 292–308.e297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, A.; Yu, F.X. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell 2017, 8, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, R.G.; Martin, J.F. The cell-autonomous and non-cell-autonomous roles of the Hippo pathway in heart regeneration. J. Mol. Cell Cardiol. 2022, 168, 98–106. [Google Scholar] [CrossRef]
- Kitisin, K.; Saha, T.; Blake, T.; Golestaneh, N.; Deng, M.; Kim, C.; Tang, Y.; Shetty, K.; Mishra, B.; Mishra, L.; et al. Tgf-Beta signaling in development. Sci. STKE 2007, 2007, cm1. [Google Scholar] [CrossRef]
- Schmierer, B.; Hill, C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Bensimon-Brito, A.; Ramkumar, S.; Boezio, G.L.M.; Guenther, S.; Kuenne, C.; Helker, C.S.M.; Sanchez-Iranzo, H.; Iloska, D.; Piesker, J.; Pullamsetti, S.; et al. TGF-beta Signaling Promotes Tissue Formation during Cardiac Valve Regeneration in Adult Zebrafish. Dev. Cell 2020, 52, 9–20. [Google Scholar] [CrossRef]
- Fink, M.; Wrana, J.L. Regulation of homeostasis and regeneration in the adult intestinal epithelium by the TGF-beta superfamily. Dev. Dyn. 2022, 1–18. [Google Scholar] [CrossRef]
- McCready, K.; Spencer, V.; Kim, M. The Importance of TOR Kinase in Plant Development. Front. Plant Sci. 2020, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Limon, J.J.; Fruman, D.A. Akt and mTOR in B Cell Activation and Differentiation. Front. Immunol. 2012, 3, 228. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Liu, L.; Hu, Y.; Li, J.; Li, J.; Cao, N.; Zhu, F.; Shi, C.; Zhang, L. Concentrated growth factor promotes gingival regeneration through the AKT/Wnt/beta-catenin and YAP signaling pathways. Artif. Cells Nanomed. Biotechnol. 2020, 48, 920–932. [Google Scholar] [CrossRef]
- Youn, J.H.; Kim, T.W. Functional insights of plant GSK3-like kinases: Multi-taskers in diverse cellular signal transduction pathways. Mol. Plant 2015, 8, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Zhang, R.; Meng, Z.; Deng, K.; Que, Y.; Zhuo, F.; Feng, L.; Guo, S.; Datla, R.; Ren, M. Brassinosteriod Insensitive 2 (BIN2) acts as a downstream effector of the Target of Rapamycin (TOR) signaling pathway to regulate photoautotrophic growth in Arabidopsis. New Phytol. 2017, 213, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.L.; Pera, M.F. Capturing Totipotent Stem Cells. Cell Stem Cell 2018, 22, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.K.; Zhao, F.Q. Stem Cells in Mammary Health and Milk Production. Curr. Stem Cell Res. Ther. 2022, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, L.; Liang, X.; Zhao, P.; Wang, W.; Liu, J.; Chang, Y.; Hiei, Y.; Yanagihara, C.; Du, L.; et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 2022, 8, 110–117. [Google Scholar] [CrossRef]
- Tomiczak, K.; Mikuła, A.; Niedziela, A.; Wójcik-Lewandowska, A.; Domżalska, L.; Rybczyński, J.J. Somatic Embryogenesis in the Family Gentianaceae and Its Biotechnological Application. Front. Plant Sci. 2019, 10, 762. [Google Scholar] [CrossRef]
- Wu, H.; Qu, X.; Dong, Z.; Luo, L.; Shao, C.; Forner, J.; Lohmann, J.U.; Su, M.; Xu, M.; Liu, X.; et al. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science 2020, 370, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22, 8. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Boonen, K.J.M.; Polak, R.B.; Baaijens, F.P.T.; Post, M.J.; van der Schaft, D.W.J. Meet the new meat: Tissue engineered skeletal muscle. Trends Food Sci. Technol. 2010, 21, 59–66. [Google Scholar] [CrossRef]
- Bryant, C.J. Culture, meat, and cultured meat. J. Anim. Sci. 2020, 98, skaa172. [Google Scholar] [CrossRef]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Marien, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K.; et al. Equine Allogeneic Chondrogenic Induced Mesenchymal Stem Cells Are an Effective Treatment for Degenerative Joint Disease in Horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, R.R.; Wang, M.Y.; Xiao, L.Y.; Liu, Y.Z.; Chen, S.L. Construction strategies and prospect of the Global Medicinal Plant Stem Cell Bank. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2022, 47, 2841–2851. [Google Scholar] [CrossRef]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sardana, C.; Ozturk, M.; Sarwat, M. Plant stem cells and their applications: Special emphasis on their marketed products. 3 Biotech 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Fornalè, S.; Esposti, D.D.; Navia-Osorio, A.; Cusidò, R.M.; Palazòn, J.; Teresa Piñol, M.; Bagni, N. Taxol transport in Taxus baccata cell suspension cultures. Plant Physiol. Biochem. 2002, 40, 81–88. [Google Scholar] [CrossRef]
- Siddiqui, Z.H.; Mujib, A.; Abbas, Z.K.; Noorani, M.S.; Khan, S. Vinblastine synthesis under the influence of CaCl2 elicitation in embryogenic cell suspension culture of Catharanthus roseus. South Afr. J. Bot. 2023, 154, 319–329. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Atala, A. Regenerative medicine. Methods 2016, 99, 1–2. [Google Scholar] [CrossRef]
- Tavakoli, S.; Ghaderi Jafarbeigloo, H.R.; Shariati, A.; Jahangiryan, A.; Jadidi, F.; Jadidi Kouhbanani, M.A.; Hassanzadeh, A.A.-O.; Zamani, M.; Javidi, K.; Naimi, A.A.-O. Mesenchymal stromal cells; a new horizon in regenerative medicine. J. Cell Physiol. 2020, 235, 9185–9210. [Google Scholar] [CrossRef]
- Gan, E.H.; Pearce, S.H. MANAGEMENT OF ENDOCRINE DISEASE: Regenerative therapies in autoimmune Addison’s disease. Eur. J. Endocrinol. 2017, 176, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Chen, X.; Feng, S. Autologous Hematopoietic Stem Cell Transplantation in Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. 2019, 25, 285–292. [Google Scholar] [CrossRef]
- Chingale, M.; Zhu, D.; Cheng, K.; Huang, K. Bioengineering Technologies for Cardiac Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 681705. [Google Scholar] [CrossRef]
- Williams, J.K.; Dean, A.; Badlani, G.; Andersson, K.E. Regenerative Medicine Therapies for Stress Urinary Incontinence. J. Urol. 2016, 196, 1619–1626. [Google Scholar] [CrossRef]
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.-O.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Visintini, S.; Quay, T. An Overview of Clinical Applications of 3-D Printing and Bioprinting. In CADTH Issues in Emerging Health Technologies; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542711/ (accessed on 10 February 2023).
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S.; Lerman, A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015, 33, 1503–1521. [Google Scholar] [CrossRef]
- Bouma, M.J.; van Iterson, M.; Janssen, B.; Mummery, C.L.; Salvatori, D.C.; Freund, C. Differentiation-Defective Human Induced Pluripotent Stem Cells Reveal Strengths and Limitations of the Teratoma Assay and In Vitro Pluripotency Assays. Stem Cell Reports 2017, 8, 1340–1353. [Google Scholar] [CrossRef] [Green Version]
- Everson, E.M.; Trobridge, G.D. Retroviral vector interactions with hematopoietic cells. Curr. Opin. Virol. 2016, 21, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Glinsky, G.V. Regenerative medicine: Clinical relevance, implications and limitations of the stem cell-based therapies. Cell Cycle 2008, 7, 3292–3293. [Google Scholar] [CrossRef]
- Afshar, L.; Aghayan, H.R.; Sadighi, J.; Arjmand, B.; Hashemi, S.M.; Basiri, M.; Samani, R.O.; Ashtiani, M.K.; Azin, S.A.; Hajizadeh-Saffar, E.; et al. Ethics of research on stem cells and regenerative medicine: Ethical guidelines in the Islamic Republic of Iran. Stem Cell Res. Ther. 2020, 11, 396. [Google Scholar] [CrossRef]
- Zhang, S.; Lachance, B.B.; Moiz, B.; Jia, X. Optimizing Stem Cell Therapy after Ischemic Brain Injury. J. Stroke 2020, 22, 286–305. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front. Neurol. 2022, 8, 1048113. [Google Scholar] [CrossRef]
- Wei, X.; Fu, S.; Li, H.; Liu, Y.; Wang, S.; Feng, W.; Yang, Y.; Liu, X.; Zeng, Y.Y.; Cheng, M.; et al. Single-cell Stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science 2022, 377, eabp9444. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Buck, M.D.; Chakravarty, P.; Carvalho, J.; Frederico, B.; Cardoso, A.; Healy, L.; Ulferts, R.; Beale, R.; Reis e Sousa, C. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 2021, 373, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef]
- Baek, S.; Tran, N.T.; Diaz, D.C.; Tsai, Y.Y.; Acedo, J.N.; Lush, M.E.; Piotrowski, T. Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev. Cell 2022, 57, 799–819.e796. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Artegiani, B.; Hu, H.; Chuva de Sousa Lopes, S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2021, 16, 182–217. [Google Scholar] [CrossRef]
- Garreta, E.; Kamm, R.D.; Chuva de Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Bozorov, T.A.; Li, D.X.; Zhou, P.; Wen, X.J.; Ding, Y.; Zhang, D.Y. An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods 2020, 16, 56. [Google Scholar] [CrossRef] [Green Version]
| Regeneration-Related Genes | Related Signaling Pathways | Promote or Inhibit Regeneration | Functions | References |
|---|---|---|---|---|
| WUS | Auxin signal, CLV3-WUS feedback pathway | promote | Stem cell maintenance (SAM) | [51,52,53] |
| CLV3 | Cytokinin signal, CLV3-WUS feedback pathway | inhibit | Maintains stem cell balance (SAM) | [51,54,55] |
| SHOOTMERISTEMLESS (STM) | Cytokinin signal | promote | Establishment and maintenance of stem end meristem | [56,57] |
| PLETHORAs (PLTs) | JA signal, PLT pathway | promote | Maintains the niche of root stem cells | [58,59] |
| WOUND INDUCED DEDIFFERENTIATION 1 (WIND1) | Cytokinin signal | promote | Promotes callus formation | [60,61] |
| Type-A ARRs (RRAs) | Cytokinin signal | inhibit | Inhibits callus formation and bud regeneration | [62] |
| Type-B ARRs (RRBs) | Cytokinin signal | promote | Promotes callus formation and bud regeneration | [62] |
| ENHANCER OF SHOOT REGENERATION 1 (ESR1) | Auxin signal Cytokinin signal | promote | Promotes callus formation and bud regeneration | [61,63] |
| MYC2 | JA signal | promote | Promotes callus formation | [64] |
| LATERAL ORGAN BOUNDARY DOMAINs (LBDs) | Auxin signal | promote | Promotes callus formation | [63] |
| WOX5/7 | Auxin signal Cytokinin signal | promote | Stem cell maintenance (RAM) | [65] |
| SHR/SCR | Auxin signal | promote | Maintains the niche of root stem cells | [49] |
| ETHYLENE RESPONSE FACTOR 115 (ERF115) | JA signal | promote | Activates the root stem cell and promotes regeneration | [64] |
| ERF109 | JA signal | promote | Activates the root stem cell and promotes regeneration | [64] |
| Auxin Response Factors (ARFs) | Auxin signal | promote | Regulates development and shoot regeneration | [66,67] |
| Regeneration-Related Genes | Related Signaling Pathways | Promote or Inhibit Regeneration | Functions | References |
|---|---|---|---|---|
| Wnt1 | Wnt signaling | promote | Promotes regeneration of organs and tissues | [68,69] |
| P21 | Notch signaling | inhibit | Inhibits the regeneration of the liver and other organs | [70] |
| Prod1 (Salamander) | unsure | promote | Promotes salamander limb regeneration | [71] |
| G protein nucleolar 3 (GNL3) | unsure | promote | Conservative stem cell gene, promotes regeneration | [72] |
| Notum | Wnt signaling | promote | Promotes the regeneration of aging tissues | [73] |
| Oct-3/4, Sox2, Klf4 and c-Myc (OSKM) | unsure | promote | Short-term induction of OSKM in muscle fibers can promote tissue regeneration by changing the niche of stem cells | [74] |
| Early growth response (EGR) | Jun N-terminal kinase (JNK) signaling | promote | Whole-body regeneration “switch” | [75] |
| Equinox | BMP signaling | promote | Promotes the formation blastema formation in regeneration | [76] |
| HOX | Wnt signaling | promote | Specifies the digestive system tissues | [77] |
| FoxA | Wnt signaling | promote | Specifies the tissues of the digestive system | [78] |
| Bone morphogenetic protein 2 (Bmp2) | BMP signaling | promote | Enhances bone regeneration | [79] |
| Bmp4 | BMP signaling | promote | Initiates regeneration | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Qiu, L.; Zhu, Y.; Luo, L.; Han, X.; Man, M.; Li, F.; Ren, M.; Xing, Y. Comparisons between Plant and Animal Stem Cells Regarding Regeneration Potential and Application. Int. J. Mol. Sci. 2023, 24, 4392. https://doi.org/10.3390/ijms24054392
Liu L, Qiu L, Zhu Y, Luo L, Han X, Man M, Li F, Ren M, Xing Y. Comparisons between Plant and Animal Stem Cells Regarding Regeneration Potential and Application. International Journal of Molecular Sciences. 2023; 24(5):4392. https://doi.org/10.3390/ijms24054392
Chicago/Turabian StyleLiu, Lulu, Lu Qiu, Yaqian Zhu, Lei Luo, Xinpei Han, Mingwu Man, Fuguang Li, Maozhi Ren, and Yadi Xing. 2023. "Comparisons between Plant and Animal Stem Cells Regarding Regeneration Potential and Application" International Journal of Molecular Sciences 24, no. 5: 4392. https://doi.org/10.3390/ijms24054392





