Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents
Abstract
:1. Introduction
2. Mechanisms of Plant Innate Immunity-Mediated Antiviral Resistance
2.1. Disease Resistance Gene-Mediated Immunity
2.2. Cell Surface Receptor-Mediated Immunity
2.3. SAR-Mediated Immunity
3. Plant Immunity Agents
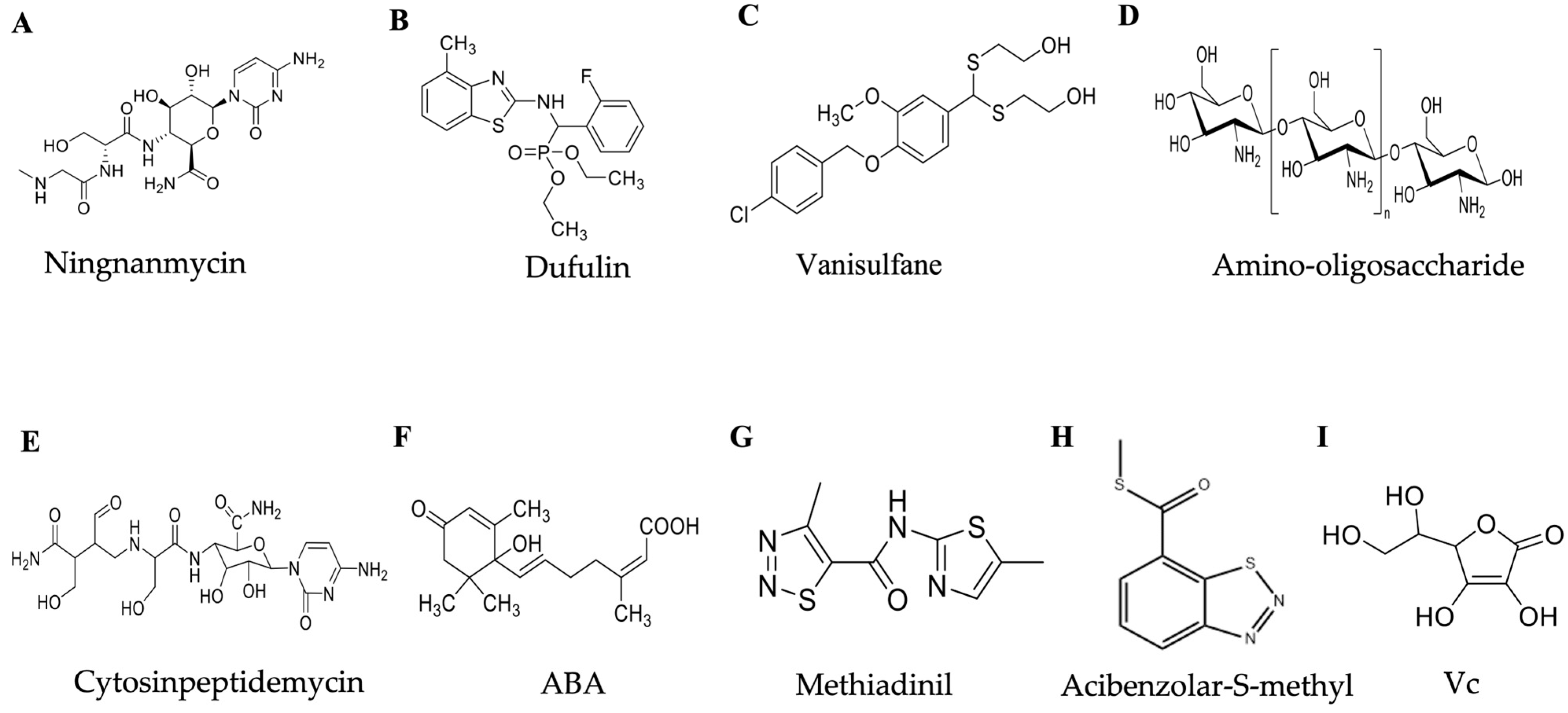
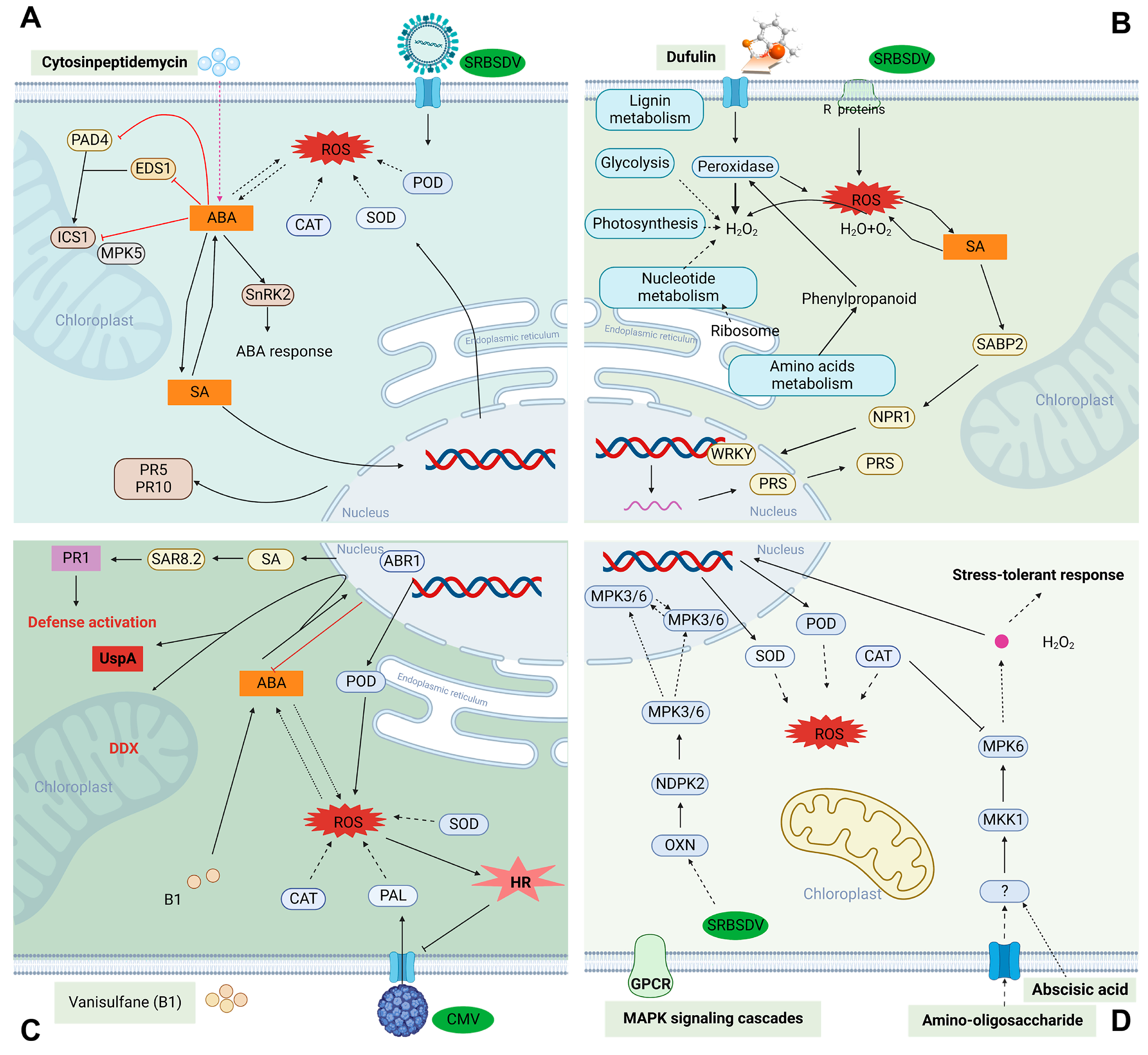
3.1. Ningnanmycin
3.2. Dufulin
3.3. Vanisulfane
3.4. Methiadinil
3.5. Cytosinpeptidemycin
3.6. Oligosaccharins
3.7. Acibenzolar-S-methyl
3.8. Phytohormone Abscisic Acid
3.9. Vitamin C
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, B.A.; Yang, S.; Jin, L.H.; Bhadury, S. Innovation and application of environment-friendly antiviral agents for plants. Springier. Berlin. 2009, 21, 207–300. [Google Scholar]
- Jones, J.D.; Dang, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ülker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 2007, 315, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Tsuda, K.; Igarashi, D.; Hillmer, R.A.; Sakakibara, H.; Myers, C.L.; Katagiri, F. Signaling mechanisms underlying robustness and tunability of the plant immune network. Cell Host Microbe 2014, 15, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.W. Progress and prospect of plant immunity inducer. J. Agric. Sci. Technol. 2014, 16, 39–45. [Google Scholar]
- Qiu, D.W.; Dong, Y.J.; Zhang, Y.; Li, S.P.; Shi, F.C. Plant immunity inducer development and application. Mol. Plant-Microbe Interact. 2017, 30, 355–360. [Google Scholar]
- He, S.; Krainer, K.M. Pandemics of people and plants: Which is the greater threat to food security? Mol. Plant 2020, 13, 933–934. [Google Scholar] [CrossRef]
- Chung, B.N.; Yoon, J.Y.; Palukaitis, P. Engineered resistance in potato against potato leaf roll virus, potato virus A and potato virus Y. Virus Genes 2013, 47, 86–92. [Google Scholar] [CrossRef]
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.L.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, T.; Ogata, Y.; Hirata, Y.; Ohki, S.T. Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with cucumber mosaic virus. Mol. Plant Pathol. 2014, 15, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, H.; Cui, X.; Wang, A. The altered photosyn thetic machinery during compatible virus infection. Curr. Opin. Virol. 2016, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, L.; Rajashekara, H.; Uppala, L.S.; Ambika, D.S.; Patil, B.; Shankarappa, K.S.; Nath, V.S.; Kavitha, T.R.; Mishra, A.K. Mechanisms of microbial plant protection and control of plant viruses. Plants 2022, 11, 3449. [Google Scholar] [CrossRef] [PubMed]
- Faoro, F.; Gozzo, F. Is modulating virus virulence by induced systemic resistance realistic? Plant Sci. 2015, 234, 1–13. [Google Scholar] [CrossRef]
- Chavhan, R.L.; Verma, M.K.; Hinge, V.R.; Kadam, U.S.; Kokane, S.B.; Chakrabarty Pranjib, K. Sequence analysis of coat protein and molecular profiling of sunflower necrosis virus (SNV) strains from Indian subcontinent. J. Plant Biochem. Biotechnol. 2018, 27, 28–35. [Google Scholar] [CrossRef]
- Hinge, R.; Vidya, C.L.; Rahul, K.P.; Sandeep, S.P.; Kadam, S.U. Engineering resistance against viruses in field crops using CRISPRCas9. Curr. Genom. 2021, 22, 214–231. [Google Scholar] [CrossRef]
- Balmer, D.; Planchamp, C.; Mauch-Mani, B. On the move: Induced resistance in monocots. J. Exp. Bot. 2013, 64, 1249–1261. [Google Scholar] [CrossRef]
- James, M.E.; Zuh-Jyh, D.L.; Gitta, C. Plant NB-LRR signaling: Upstreams and downstreams. Curr. Opin. Plant Biol. 2011, 14, 365–371. [Google Scholar]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Levy, M.; Edelbaum, O.; Sela, I. Tobacco mosaic virus regulates the expression of its own resistance gene N. Plant Physiol. 2004, 135, 2392–2397. [Google Scholar] [CrossRef] [Green Version]
- Roselló, S.; José Díez, M.; Nuez, F. Genetics of Tomato spotted wilt virus resistance coming from Lycopersicon peruvianum. Eur. J. Plant Pathol. 1998, 104, 499–509. [Google Scholar] [CrossRef]
- Dockter, K.G.; O’Neil, D.S.; Price, D.L.; Scott, J.; Stevens, M.R. Molecular mapping of the Tomato spotted wilt virus resistance gene Sw-7 in tomato. Hortscience 2009, 44, 1123. [Google Scholar]
- Jahn, M.; Paran, I.; Hoffmann, K.; Radwanski, E.R.; Livingstone, K.D.; Grube, R.C.; Aftergoot, E.; Lapidot, M.; Moyer, J. Genetic mapping of the Tsw locus for resistance to the tospovirus Tomato spotted wilt virus in Capsicum spp. and its relationship to the Sw-5 gene for resistance to the same pathogen in tomato. Mol. Plant-Microbe Interact. 2000, 13, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Jiang, L.; Bai, B.H.; Zhao, W.Y.; Chen, X.J.; Li, J.; Liu, Y.; Chen, Z.Q.; Wang, B.T.; Wang, C.L.; et al. The intracellular immune receptor Sw-5b confers broad-spectrum resistance to Tospoviruses through recognition of a conserved 21-amino acid viral effector epitope. Plant cell 2017, 29, 2214–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariana, H.; Silva, O.A.; Erico, C.D.; Dick, L.; Silva, B.L.; Kazuko, I.N.A.; Resende, R.O.; Kormelink, R. The Tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5-containing resistant tomato lines and in Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Mol. Plant Pathol. 2014, 15, 871–880. [Google Scholar]
- El-Sappah, A.H.; Qi, S.M.; Soaud, S.A.; Huang, Q.L.; Saleh, A.M.; Abourehab, M.A.; Wan, L.Y.; Cheng, G.T.; Liu, J.Y.; Ihtisham, M.; et al. Natural resistance of tomato plants to Tomato yellow leaf curl virus. Front. Plant Sci. 2022, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, A.; Querci, M.; Kanyuka, K.; Baulcombe, D.C. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: Application to the Rx2 locus in potato. Plant J. 2000, 21, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Mahajan, S.K.; Whitham, S.A.; Yamamoto, M.L.; Carrington, J.C. Cloning of the Arabidopsis RTM1 gene, which controls restriction of long-distance movement of tobacco etch virus. Proc. Natl. Acad. Sci. USA 2000, 97, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, K.; Ishikawa, M. The resistance protein Tm-1 inhibits formation of a Tomato mosaic virus replication protein-host membrane protein complex. J. Virol. 2013, 87, 7933–7939. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Liu, D.; Niu, X.; Wang, J.; Qian, L.; Han, L.; Liu, N.; Zhao, J.; Hong, Y.; Liu, Y. Antiviral resistance protein Tm-2(2) functions on the plasma membrane. Plant Physiol. 2017, 173, 2399–2410. [Google Scholar] [CrossRef] [Green Version]
- Kørner, C.J.; Klauser, D.; Niehl, A.; Domínguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant-Microbe Interact. 2013, 26, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Gou, X.P.; He, K.; Xi, D.H.; Du, J.B.; Lin, H.H.; Li, J. BAK1 and BKK1 in A rabidopsis thaliana confer reduced susceptibility to Turnip crinkle virus. Eur. J. Plant Pathol. 2010, 127, 149–156. [Google Scholar] [CrossRef]
- Zorzatto, C.; Machado, J.P.B.; Lopes, K.V.G.; Nascimento, K.J.T.; Pereira, W.A.; Brustolini, O.J.B.; Reis, P.A.B.; Calil, I.P.; Deguchi, M.; Sachetto-Martins, G.; et al. NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 2015, 520, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.R.; Jung, H.; Josephine, H.; Chellu, S.C. Domain-specific positive selection contributes to the evolution of A rabidopsis leucine-rich repeat receptor-like kinase (LRR-RLK) genes. J. Mol. Evol. 2006, 63, 612–621. [Google Scholar] [CrossRef]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Caarls, L.; Elberse, J.; Awwanah, M.; Ludwig, N.R.; De Vries, M.; Zeilmaker, T.; Van Wees, S.C.; Schuurink, R.C.; Van den Ackerveken, G. Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 6388–6393. [Google Scholar] [CrossRef] [Green Version]
- Motallebi, P.; Niknam, V.; Ebrahimzadeh, H. Central role of methyl jasmonate in resistance of wheat against crown and root rot caused by Fusarium culmorum. Physiol. Mol. Plant Pathol. 2022, 119, 101812. [Google Scholar] [CrossRef]
- Michael, H.; Jürgen, Z. N-hydroxypipecolic acid and salicylic acid: A metabolic duo for systemic acquired resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57. [Google Scholar]
- Jyoti, S.; Jürgen, Z. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 2013, 4, 30. [Google Scholar]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2020, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef]
- Chanda, B.; Xia, Y.; Mandal, M.K.; Yu, K.; Sekine, K.T.; Gao, Q.M.; Selote, D.; Hu, Y.; Stromberg, A.; Navarre, D.; et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 2011, 43, 421–427. [Google Scholar] [CrossRef]
- Bernsdorff, F.; Döring, A.C.; Gruner, K.; Schuck, S.; Bräutigam, A.; Zeier, J. Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and -independent pathways. Plant Cell 2016, 28, 102–129. [Google Scholar] [CrossRef] [Green Version]
- Návarová, H.; Bernsdorff, F.; Döring, A.C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.G.; Luo, Y.; Qin, S.R.; Lei, X.; Wan, B.; Du, L.F. Induction of systemic resistance against Tobacco mosaic virus by ningnanmycin in tobacco. Pestic. Biochem. Physiol. 2014, 111, 14–18. [Google Scholar]
- Katsir, L.; Chung, H.S.; Koo, A.J.; Howe, G.A. Jasmonate signaling: A conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 2008, 11, 428–435. [Google Scholar] [CrossRef] [Green Version]
- An, M.N.; Zhou, T.; Guo, Y.; Zhao, X.; Wu, Y. Molecular regulation of host defense responses mediated by biological anti-TMV agent Ningnanmycin. Viruses 2019, 11, 815. [Google Scholar] [CrossRef]
- Jin, L.H.; Song, B.A.; Zhang, G.P.; Xu, R.Q.; Zhang, S.M.; Gao, X.W.; Hu, D.Y.; Yang, S. Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O,O-dialkyl-alpha-aminophosphonates. Bioorg. Med. Chem. Lett. 2006, 16, 1537–1543. [Google Scholar] [CrossRef]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Hennig, J. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef]
- Abdul, B.W.; Hemlata, C.; Abdul, H.W.; Simranjeet, S.; Niraj, U. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar]
- Chen, Z.; Zeng, M.J.; Song, B.A.; Hou, C.R.; Hu, D.Y.; Li, X.Y.; Wang, Z.C.; Fan, H.T.; Bi, L.; Liu, J.J.; et al. Dufulin activates HrBP1 to produce antiviral responses in tobacco. PLoS ONE 2012, 7, e37944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.M.; Xie, X.; Gao, D.; Chen, K.; Chen, Z.; Jin, L.H.; Li, X.Y.; Song, B.A. Dufulin intervenes the viroplasmic proteins as the mechanism of action against Southern rice black-streaked dwarf virus. J. Agric. Food Chem. 2019, 67, 11380–11387. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, J.; Yang, X.; Ding, Y.; Wu, J.; Hu, D.Y.; Song, B.A. Studies of binding interactions between Dufulin and Southern rice black-streaked dwarf virus P9-1. Bioorg. Med. Chem. 2015, 23, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.M.; Zhang, Y.; Li, X.Y. Dufulin enhances salt resistance of rice. Pestic. Biochem. Physiol. 2022, 188, 105252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Zhu, C.; Wu, Z.; Zhang, G.; Gan, X.H.; Song, B.A. Facile synthesis of novel vanillin derivatives incorporating a bis(2-hydroxyethyl) dithhioacetal moiety as antiviral agents. J. Agric. Food Chem. 2017, 65, 4582–4588. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Song, B.A. Proteomics analysis of Xiangcaoliusuobingmi-treated Capsicum annuum L. infected with Cucumber mosaic virus. Pestic. Biochem. Physiol. 2018, 149, 113–122. [Google Scholar] [CrossRef]
- Shi, J.; He, H.F.; Hu, D.Y.; Song, B.A. Defense mechanism of Capsicum annuum L. infected with Pepper mild mottle virus induced by vanisulfane. J. Agric. Food Chem. 2022, 70, 3618–3632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; He, F.C.; Wu, S.K.; Luo, Y.Q.; Wu, R.; Hu, D.Y.; Song, B.A. Corrigendum to Design, synthesis, anti-TMV activity, and preliminary mechanism of cinnamic acid derivatives containing dithioacetal moiety. Pestic. Biochem. Physiol. 2020, 164, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.R.; Wei, C.L.; Wu, S.K.; Zhang, W.B.; Song, R.J.; Hu, D.Y. Synthesis, anti-Potato virus Y activities, and interaction mechanisms of novel quinoxaline derivatives bearing dithioacetal moiety. J. Agric. Food Chem. 2022, 70, 7029–7038. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. Nankai University found that plant activator candidate agents JYSA. Pestic. Market Inform. 2010, 8, 37. [Google Scholar]
- Yu, L.; Wang, W.L.; Zeng, S.; Chen, Z.; Yang, A.M.; Shi, J.; Zhao, X.Z.; Song, B.A. Label-free quantitative proteomics analysis of Cytosinpeptidemycin responses in Southern rice black-streaked dwarf virus-infected rice. Pestic. Biochem. Physiol. 2018, 147, 20–26. [Google Scholar] [CrossRef]
- An, M.N.; Zhao, X.X.; Zhou, T.; Wang, G.Z.; Xia, Z.H.; Wu, Y.H. A Novel biological agent Cytosinpeptidemycin inhibited the pathogenesis of Tobacco mosaic virus by inducing host resistance and stress response. J. Agric. Food Chem. 2019, 67, 7738–7747. [Google Scholar] [CrossRef]
- Kovtun, Y.; Chiu, W.L.; Zeng, W.; Sheen, J. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 1998, 395, 716–720. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, Y.Q.; Xu, C.T.; Xie, Q.; Xie, Y.B.; Xia, Z.H.; An, M.N.; Wu, Y.H. Novel combined biological antiviral agents Cytosinpeptidemycin and Chitosan oligosaccharide induced host resistance and changed movement protein subcellular localization of Tobacco mosaic virus. Pestic. Biochem. Physiol. 2020, 164, 40–46. [Google Scholar] [CrossRef]
- Yang, A.M.; Yu, L.; Chen, Z.; Zhang, S.X.; Shi, J.; Zhao, X.Z.; Yang, Y.Y.; Hu, D.Y.; Song, B.A.; Andrew White, K. Label-free quantitative proteomic analysis of chitosan oligosaccharide-treated rice infected with Southern rice black-streaked dwarf virus. Viruses 2017, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Kulye, M.; Liu, H.; Zhang, Y.L.; Zeng, H.M.; Yang, X.F.; Qiu, D.W. Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 2012, 35, 2104–2120. [Google Scholar] [CrossRef]
- Anand, A.; Uppalapati, S.R.; Ryu, C.; Allen, S.N.; Kang, L.; Tang, Y.; Mysore, K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008, 146, 703–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamir, J.; Darwiche, R.; van’t Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, M.; Maier, F.; Masroor, A.; Hirth, S.; Pfitzner, A.J.P.; Pfitzner, U.M. The Arabidopsis NIMIN proteins affect NPR1 differentially. Front. Plant Sci. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Kinkema, M.; Fan, W.; Dong, X. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 2000, 12, 2339–2350. [Google Scholar] [CrossRef] [Green Version]
- Lindermayr, C.; Sell, S.; Müller, B.; Leister, D.; Durner, J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 2010, 22, 2894–2907. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Birkenbihl, R.P.; Liu, S.; Somssich, I.E. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Priming agents of plant defence stimulate the accumulation of monoand diacylated quinic acids in cultured tobacco cells. Physiol. Mol. Plant Pathol. 2014, 88, 61–66. [Google Scholar] [CrossRef]
- Ncube, E.N.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Stimulatory effects of acibenzolar-S-methyl on chlorogenic acids biosynthesis in Centella asiatica cells. Front. Plant Sci. 2016, 7, 1469. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, P.; Vega-Arreguín, J.C.; Fry, W.E. Transcriptome profile of acibenzolar-S-methyl-induced genes in tomato suggests a complex polygenic effect on resistance to phytophthora infestans. Physiol. Mol. Plant Pathol. 2013, 81, 97–106. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.A.; Rodrigues, F.A.; Debona, D.; Resende, R.S.; Moreira, W.R.; Andrade, C.C.L. Induction of resistance to Pyricularia oryzae in wheat by acibenzolar-S-methyl, ethylene and jasmonic acid. Trop. Plant Pathol. 2014, 39, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, K.J.; Araujo, L.; Resende, R.S.; Schurt, D.A.; Da Silva, W.L.; De Ávila Rodrigues, F. Silicon, acibenzolar-S-methyl and potassium phosphite in the control of brown spot in rice. Bragantia 2016, 75, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Baysal, O.; Turgut, C.; Mao, G. Acibenzolar-S-methyl induced resistance to phytophthora capsici in pepper leaves. Biol. Plant. 2005, 49, 599–604. [Google Scholar] [CrossRef]
- Du, Q.S.; Shi, Y.X.; Li, P.F.; Zhao, Z.J.; Zhu, W.P.; Qian, X.H.; Li, B.J.; Xu, Y.F. Novel plant activators with thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate scaffold: Synthesis and bioactivity. Chin. Chem. Lett. 2013, 24, 967–969. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.G.; Zhang, L.P.; Li, D.B.; Wang, F.; Yu, D.Q. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global analysis of tomato gene expression during potato spindle tuber viroid infection reveals a complex array of changes affecting hormone signaling. Mol. Plant Microbe Interact. 2012, 25, 582–598. [Google Scholar] [CrossRef] [Green Version]
- Heinlein, M. Plasmodesmata: Channels for viruses on the move. Methods Mol. Biol. 2015, 1217, 25–52. [Google Scholar]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, M.; Bhattacharjee, S.; Mello, A.F.; Perry, K.L.; Moffett, P. ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol. 2011, 156, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alazem, M.; Lin, K.Y.; Lin, N.S. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol. Plant Microbe Interact. 2014, 27, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Luo, Z.L.; Hu, F.L.; Wu, T.H.; Tian, H.S.; Huang, R.M.; Yan, J.C. Ascorbic acid 6% AS inducing resistance against sphaerotheca sp. in Rosa roxburghii tratt. Agrochemicals 2017, 56, 528–530. [Google Scholar]
- Nguyen, A.D.; Vo, T.P.K.; Tran, T.D. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Abdelsalam, N.R.; El Maghraby, D.M.; Ghozlan, M.H.; EL Argawy, E.; Abou, S.R.A.I. Oscillatoria sp. as a potent anti-phytopathogenic agent and plant immune stimulator against root-knot nematode of Soybean cv. Giza 111. Front. Plant Sci. 2022, 13, 870518. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed suppresses powdery mildew of strawberry. J. Plant Pathol. 2019, 35, 406–416. [Google Scholar] [CrossRef]
- Hans, J.M.; Adrian, F.; Maher, A.R.; Neil, B.; Thierry, C. Application of HTS for routine plant virus diagnostics: State of the art and challenges. Front Plant Sci. 2018, 9, 1082. [Google Scholar]
- Marqués, M.C.; Sánchez, V.J.; Ruiz, R.; Montagud, M.R.; Márquez, C.R.; Gómez, G.; Carbonell, A.; Daròs, J.A.; Rodrigo, G. Diagnostics of infections produced by the plant viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13. ACS Synth. Biol. 2022, 11, 2384–2393. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Wu, Z.; Li, J.; Ding, Y.; Chen, S.; Li, X. Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents. Int. J. Mol. Sci. 2023, 24, 4453. https://doi.org/10.3390/ijms24054453
Huang M, Wu Z, Li J, Ding Y, Chen S, Li X. Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents. International Journal of Molecular Sciences. 2023; 24(5):4453. https://doi.org/10.3390/ijms24054453
Chicago/Turabian StyleHuang, Min, Zilin Wu, Jingxin Li, Yuyu Ding, Shilin Chen, and Xiangyang Li. 2023. "Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents" International Journal of Molecular Sciences 24, no. 5: 4453. https://doi.org/10.3390/ijms24054453
APA StyleHuang, M., Wu, Z., Li, J., Ding, Y., Chen, S., & Li, X. (2023). Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents. International Journal of Molecular Sciences, 24(5), 4453. https://doi.org/10.3390/ijms24054453




