Experimental Models to Study Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy
Abstract
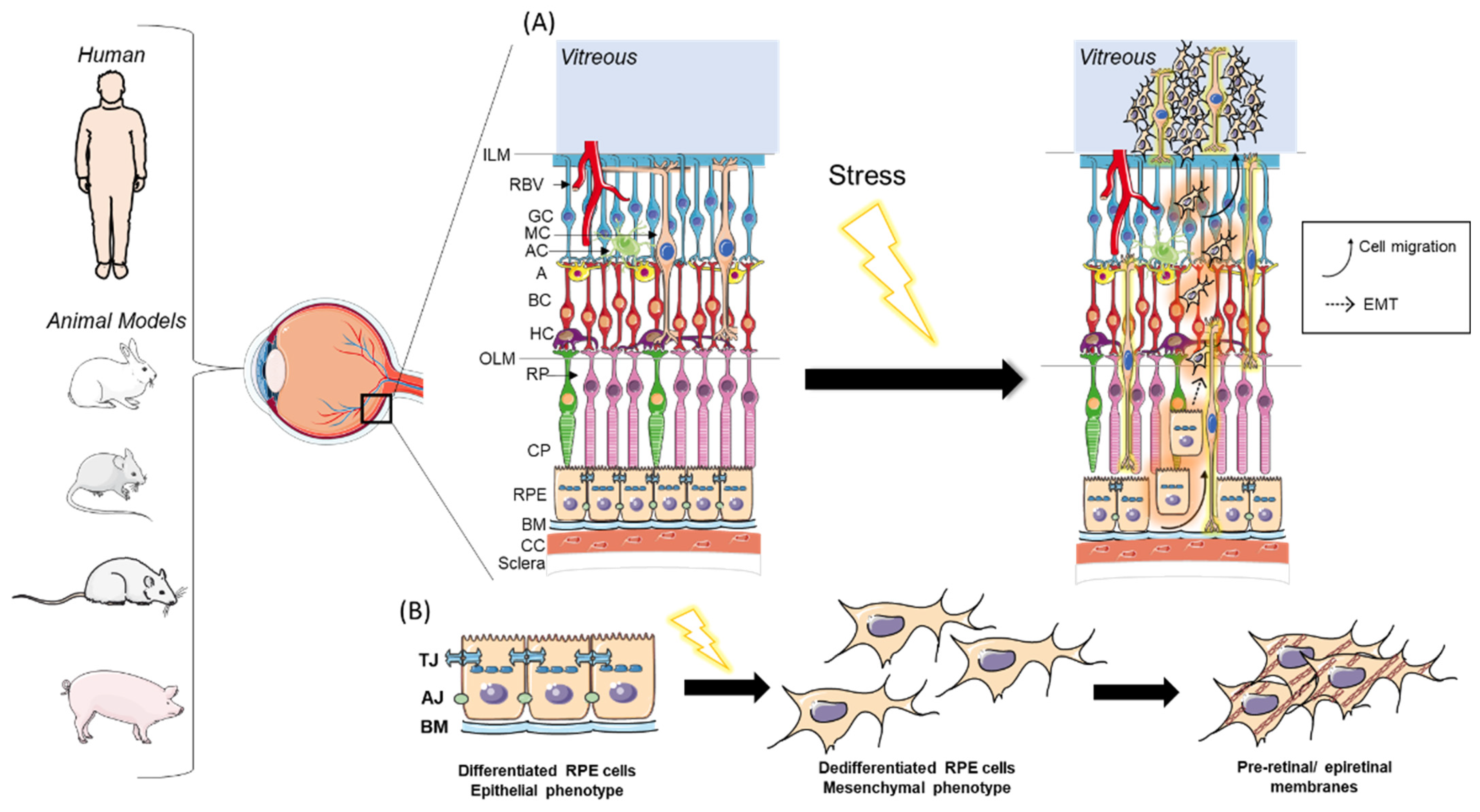
1. Introduction
2. In Vitro Models of PVR
2.1. EMT Induction by Growth Factors or Cytokines
2.2. EMT Induction through Mechanical Stimulation
2.3. Advantages and Limitations of In Vitro PVR Models
3. Animal Models
3.1. Rabbit PVR Models
3.1.1. Cell-Induced Rabbit PVR Models
3.1.2. Biologically Induced Rabbit PVR Models
3.1.3. Cell- and Biologically Induced Rabbit PVR Models
3.1.4. Surgically Induced Rabbit PVR Models
3.1.5. Cell- or Biologically Induced Rabbit PVR Models following Surgery
3.1.6. Advantages and Limitations of Rabbit PVR Models
3.2. Mouse PVR Models
3.2.1. Biologically Induced Mouse PVR Models
3.2.2. Cell-Induced Mouse PVR Models
3.2.3. Surgically Induced Mouse PVR Models
3.2.4. Cell-Induced Mouse PVR Models following Surgery
3.2.5. Transgenic Mouse PVR Models
3.2.6. Advantages and Limitations of Mouse PVR Models
3.3. Rat PVR Models
3.3.1. Cell-Induced Rat PVR Models
3.3.2. Biologically Induced Rat PVR Model
3.3.3. Advantages and Limitations of Rat PVR Models
3.4. Swine Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshida, S.; Nakama, T.; Ishikawa, K.; Nakao, S.; Sonoda, K.-H.; Ishibashi, T. Periostin in vitreoretinal diseases. Cell. Mol. Life Sci. 2017, 74, 4329–4337. [Google Scholar] [CrossRef]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kannan, R.; Hinton, D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016, 142, 19–25. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.-Y.; Klein, B.E.K.; et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sardarinia, M.; Asgari, S.; Hizomi Arani, R.; Eskandari, F.; Azizi, F.; Khalili, D.; Hadaegh, F. Incidence and risk factors of severe non-proliferative/proliferative diabetic retinopathy: More than a decade follow up in the Tehran Lipids and Glucose Study. J. Diabetes Investig. 2022, 13, 317–327. [Google Scholar] [CrossRef]
- van Leeuwen, R.; Haarman, A.E.G.; van de Put, M.A.J.; Klaver, C.C.W.; Los, L.I. Dutch Rhegmatogenous Retinal Detachment Study Group Association of Rhegmatogenous Retinal Detachment Incidence With Myopia Prevalence in The Netherlands. JAMA Ophthalmol. 2021, 139, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.R.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Naduvilath, T.J.; Ho, S.M.; Wong, T.Y.; Resnikoff, S. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: Systematic review, meta-analysis and modelling. Br. J. Ophthalmol. 2018, 102, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Lazzara, F.; Conti, F.; Ferrara, M.; Lippera, M.; Coppola, M.; Rossi, S.; Drago, F.; Bucolo, C.; Romano, M.R. Safety Profile of Lutein- Versus Triamcinolone Acetonide-Based Vitreous Staining. Transl. Vis. Sci. Technol. 2023, 12, 5. [Google Scholar] [CrossRef]
- Spadaro, A.; Rao, M.; Lorenti, M.; Romano, M.R.; Augello, A.; Eandi, C.M.; Platania, C.B.M.; Drago, F.; Bucolo, C. New Brilliant Blue G Derivative as Pharmacological Tool in Retinal Surgery. Front. Pharmacol. 2020, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Little, K.; Ma, J.H.; Yang, N.; Chen, M.; Xu, H. Myofibroblasts in macular fibrosis secondary to neovascular age-related macular degeneration—The potential sources and molecular cues for their recruitment and activation. EBioMedicine 2018, 38, 283–291. [Google Scholar] [CrossRef]
- Tenbrock, L.; Wolf, J.; Boneva, S.; Schlecht, A.; Agostini, H.; Wieghofer, P.; Schlunck, G.; Lange, C. Subretinal fibrosis in neovascular age-related macular degeneration: Current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022, 387, 361–375. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Li, M.; Wang, F. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov. Med. 2015, 20, 207–217. [Google Scholar] [PubMed]
- Tamiya, S.; Kaplan, H.J. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp. Eye Res. 2016, 142, 26–31. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Nudleman, E.; Weinreb, R.N. Animal Models of Proliferative Vitreoretinopathy and Their Use in Pharmaceutical Investigations. Ophthalmic Res. 2018, 60, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, S.H.; Koh, H.J.; Kwon, O.W. TGF-betas synthesized by RPE cells have autocrine activity on mesenchymal transformation and cell proliferation. Yonsei Med. J. 2001, 42, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Kono-Saika, S.; Tanaka, T.; Yamanaka, O.; Ohnishi, Y.; Sato, M.; Muragaki, Y.; Ooshima, A.; Yoo, J.; Flanders, K.C.; et al. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab. Investig. 2004, 84, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Por, E.D.; Greene, W.A.; Burke, T.A.; Wang, H.-C. Trichostatin A Inhibits Retinal Pigmented Epithelium Activation in an In Vitro Model of Proliferative Vitreoretinopathy. J. Ocul. Pharmacol. Ther. 2016, 32, 415–424. [Google Scholar] [CrossRef]
- Wei, J.; Wu, L.; Yang, S.; Zhang, C.; Feng, L.; Wang, M.; Li, H.; Wang, F. E-cadherin to N-cadherin switching in the TGF-β1 mediated retinal pigment epithelial to mesenchymal transition. Exp. Eye Res. 2022, 220, 109085. [Google Scholar] [CrossRef]
- Lee, H.; O’Meara, S.J.; O’Brien, C.; Kane, R. The role of gremlin, a BMP antagonist, and epithelial-to-mesenchymal transition in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4291–4299. [Google Scholar] [CrossRef]
- He, H.; Kuriyan, A.E.; Su, C.-W.; Mahabole, M.; Zhang, Y.; Zhu, Y.-T.; Flynn, H.W.; Parel, J.-M.; Tseng, S.C.G. Inhibition of Proliferation and Epithelial Mesenchymal Transition in Retinal Pigment Epithelial Cells by Heavy Chain-Hyaluronan/Pentraxin 3. Sci. Rep. 2017, 7, 43736. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Pan, J.; Yang, S.; Yao, H.; Li, M.; Li, H.; Lei, H.; Jin, H.; Wang, F. Exosomes mediate an epithelial-mesenchymal transition cascade in retinal pigment epithelial cells: Implications for proliferative vitreoretinopathy. J. Cell. Mol. Med. 2020, 24, 13324–13335. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, S.; Liu, L.; Kaplan, H.J. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2755–2763. [Google Scholar] [CrossRef]
- Takahashi, E.; Nagano, O.; Ishimoto, T.; Yae, T.; Suzuki, Y.; Shinoda, T.; Nakamura, S.; Niwa, S.; Ikeda, S.; Koga, H.; et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J. Biol. Chem. 2010, 285, 4060–4073. [Google Scholar] [CrossRef] [PubMed]
- Matoba, R.; Morizane, Y.; Shiode, Y.; Hirano, M.; Doi, S.; Toshima, S.; Araki, R.; Hosogi, M.; Yonezawa, T.; Shiraga, F. Suppressive effect of AMP-activated protein kinase on the epithelial-mesenchymal transition in retinal pigment epithelial cells. PLoS ONE 2017, 12, e0181481. [Google Scholar] [CrossRef] [PubMed]
- Schiff, L.; Boles, N.C.; Fernandes, M.; Nachmani, B.; Gentile, R.; Blenkinsop, T.A. P38 inhibition reverses TGFβ1 and TNFα-induced contraction in a model of proliferative vitreoretinopathy. Commun. Biol. 2019, 2, 162. [Google Scholar] [CrossRef] [PubMed]
- Boles, N.C.; Fernandes, M.; Swigut, T.; Srinivasan, R.; Schiff, L.; Rada-Iglesias, A.; Wang, Q.; Saini, J.S.; Kiehl, T.; Stern, J.H.; et al. Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Rep. 2020, 14, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhao, X.; Liao, M.; Song, Y.; You, C.; Dong, X.; Yang, X.; Wang, X.; Huang, B.; Du, M.; et al. Activated Blood Coagulation Factor X (FXa) Contributes to the Development of Traumatic PVR Through Promoting RPE Epithelial-Mesenchymal Transition. Investig. Ophthalmol. Vis. Sci. 2021, 62, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, W.; Deng, X.; Ye, S.; Xiao, W. Interleukin-6 promotes proliferative vitreoretinopathy by inducing epithelial-mesenchymal transition via the JAK1/STAT3 signaling pathway. Mol. Vis. 2020, 26, 517–529. [Google Scholar]
- Zhang, W.; Han, H. Targeting matrix stiffness-induced activation of retinal pigment epithelial cells through the RhoA/YAP pathway ameliorates proliferative vitreoretinopathy. Exp. Eye Res. 2021, 209, 108677. [Google Scholar] [CrossRef]
- Greene, W.A.; Kaini, R.R.; Wang, H.-C. Utility of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for an In Vitro Model of Proliferative Vitreoretinopathy. Adv. Exp. Med. Biol. 2019, 1186, 33–53. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, B.; He, J.F.; Chen, J.; Kang, Z.W.; Liu, D.; Luo, J.; Fang, K.; Leng, X.; Tian, H.; et al. Effectively Intervening Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells With a Combination of ROCK and TGF-β Signaling Inhibitors. Investig. Ophthalmol. Vis. Sci. 2021, 62, 21. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Miyata, Y.; Shimada, A.; Nagase, T.; Katsura, Y.; Kosano, H. Cell-mediated contraction of vitreous explants from chicken embryo: Possibility of screening for therapeutic agents against proliferative vitreoretinal diseases. Mol. Vis. 2013, 19, 2374–2384. [Google Scholar] [PubMed]
- Chen, H.; Wang, H.; An, J.; Shang, Q.; Ma, J. Inhibitory Effects of Plumbagin on Retinal Pigment Epithelial Cell Epithelial-Mesenchymal Transition In Vitro and In Vivo. Med. Sci. Monit. 2018, 24, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Domínguez, R.L.; Palma-Nicolas, J.P.; López, E.; López-Colomé, A.M. The activation of MEK-ERK1/2 by glutamate receptor-stimulation is involved in the regulation of RPE proliferation and morphologic transformation. Exp. Eye Res. 2008, 86, 207–219. [Google Scholar] [CrossRef]
- Davis, A.A.; Bernstein, P.S.; Bok, D.; Turner, J.; Nachtigal, M.; Hunt, R.C. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Investig. Ophthalmol. Vis. Sci. 1995, 36, 955–964. [Google Scholar]
- Kozlowski, M.R. The ARPE-19 cell line: Mortality status and utility in macular degeneration research. Curr. Eye Res. 2015, 40, 501–509. [Google Scholar] [CrossRef]
- Fasler-Kan, E.; Aliu, N.; Wunderlich, K.; Ketterer, S.; Ruggiero, S.; Berger, S.; Meyer, P. The Retinal Pigment Epithelial Cell Line (ARPE-19) Displays Mosaic Structural Chromosomal Aberrations. Methods Mol. Biol. 2018, 1745, 305–314. [Google Scholar] [CrossRef]
- Lehmann, G.L.; Benedicto, I.; Philp, N.J.; Rodriguez-Boulan, E. Plasma membrane protein polarity and trafficking in RPE cells: Past, present and future. Exp. Eye Res. 2014, 126, 5–15. [Google Scholar] [CrossRef]
- Bharti, K.; den Hollander, A.I.; Lakkaraju, A.; Sinha, D.; Williams, D.S.; Finnemann, S.C.; Bowes-Rickman, C.; Malek, G.; D’Amore, P.A. Cell culture models to study retinal pigment epithelium-related pathogenesis in age-related macular degeneration. Exp. Eye Res. 2022, 222, 109170. [Google Scholar] [CrossRef]
- Ahmado, A.; Carr, A.-J.; Vugler, A.A.; Semo, M.; Gias, C.; Lawrence, J.M.; Chen, L.L.; Chen, F.K.; Turowski, P.; da Cruz, L.; et al. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7148–7159. [Google Scholar] [CrossRef]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Z.; You, C.; Han, J.; Li, H.; Zhang, Z.; Yan, H. Overexpression p21WAF1/CIP1 in suppressing retinal pigment epithelial cells and progression of proliferative vitreoretinopathy via inhibition CDK2 and cyclin E. BMC Ophthalmol. 2014, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Hui, Y.; Li, Y.; Guo, C.; Meng, H. Epidermal growth factor receptor in cultured human retinal pigment epithelial cells. Ophthalmologica 2007, 221, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Umazume, K.; Liu, L.; Scott, P.A.; de Castro, J.P.F.; McDonald, K.; Kaplan, H.J.; Tamiya, S. Inhibition of PVR with a tyrosine kinase inhibitor, dasatinib, in the swine. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1150–1159. [Google Scholar] [CrossRef]
- Wu, W.-C.; Hu, D.-N.; Mehta, S.; Chang, Y.-C. Effects of retinoic acid on retinal pigment epithelium from excised membranes from proliferative vitreoretinopathy. J. Ocul. Pharmacol. Ther. 2005, 21, 44–54. [Google Scholar] [CrossRef]
- Amarnani, D.; Machuca-Parra, A.I.; Wong, L.L.; Marko, C.K.; Stefater, J.A.; Stryjewski, T.P.; Eliott, D.; Arboleda-Velasquez, J.F.; Kim, L.A. Effect of Methotrexate on an In Vitro Patient-Derived Model of Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3940–3949. [Google Scholar] [CrossRef]
- Delgado-Tirado, S.; Amarnani, D.; Zhao, G.; Rossin, E.J.; Eliott, D.; Miller, J.B.; Greene, W.A.; Ramos, L.; Arevalo-Alquichire, S.; Leyton-Cifuentes, D.; et al. Topical delivery of a small molecule RUNX1 transcription factor inhibitor for the treatment of proliferative vitreoretinopathy. Sci. Rep. 2020, 10, 20554. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.A.; Wong, L.L.; Amarnani, D.S.; Bigger-Allen, A.A.; Hu, Y.; Marko, C.K.; Eliott, D.; Shah, V.A.; McGuone, D.; Stemmer-Rachamimov, A.O.; et al. Characterization of cells from patient-derived fibrovascular membranes in proliferative diabetic retinopathy. Mol. Vis. 2015, 21, 673–687. [Google Scholar]
- Oshima, Y.; Sakamoto, T.; Hisatomi, T.; Tsutsumi, C.; Ueno, H.; Ishibashi, T. Gene transfer of soluble TGF-beta type II receptor inhibits experimental proliferative vitreoretinopathy. Gene Ther. 2002, 9, 1214–1220. [Google Scholar] [CrossRef]
- Dong, X.; Chen, N.; Xie, L.; Wang, S. Prevention of experimental proliferative vitreoretinopathy with a biodegradable intravitreal drug delivery system of all-trans retinoic acid. Retina 2006, 26, 210–213. [Google Scholar] [CrossRef]
- Daftarian, N.; Baigy, O.; Suri, F.; Kanavi, M.R.; Balagholi, S.; Afsar Aski, S.; Moghaddasi, A.; Nourinia, R.; Abtahi, S.-H.; Ahmadieh, H. Intravitreal connective tissue growth factor neutralizing antibody or bevacizumab alone or in combination for prevention of proliferative vitreoretinopathy in an experimental model. Exp. Eye Res. 2021, 208, 108622. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, M.-W.; Li, X.-X.; Yu, W.-Z.; Bian, Z.-M. siRNA targeting mammalian target of rapamycin (mTOR) attenuates experimental proliferative vitreoretinopathy. Curr. Eye Res. 2007, 32, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Zhao, M.; He, P.; Yu, W.; Dong, J.; Liu, G.; Li, C.; Shi, X. Antisense oligonucleotide targeting c-fos mRNA limits retinal pigment epithelial cell proliferation: A key step in the progression of proliferative vitreoretinopathy. Exp. Eye Res. 2006, 83, 1405–1411. [Google Scholar] [CrossRef]
- Pao, S.-I.; Lin, L.-T.; Chen, Y.-H.; Chen, C.-L.; Chen, J.-T. MicroRNA-4516 suppresses proliferative vitreoretinopathy development via negatively regulating OTX1. PLoS ONE 2022, 17, e0270526. [Google Scholar] [CrossRef]
- Chen, M.; Hou, J.; Tan, G.; Xie, P.; Freeman, W.R.; Beadle, J.R.; Hostetler, K.Y.; Cheng, L. A novel lipid prodrug strategy for sustained delivery of hexadecyloxypropyl 9-[2-(phosphonomethoxy)ethyl]guanine (HDP-PMEG) on unwanted ocular proliferation. Drug Deliv. 2017, 24, 1703–1712. [Google Scholar] [CrossRef]
- McGillem, G.S.; Dacheux, R.F. Rabbit retinal Müller cells undergo antigenic changes in response to experimentally induced proliferative vitreoretinopathy. Exp. Eye Res. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Ma, P.; Mu, H.; Wang, A.; Zhang, L.; Wu, Z.; Sun, K. Preparation and evaluation of a novel biodegradable long-acting intravitreal implant containing ligustrazine for the treatment of proliferative vitreoretinopathy. J. Pharm. Pharmacol. 2015, 67, 160–169. [Google Scholar] [CrossRef]
- Hou, H.; Huffman, K.; Rios, S.; Freeman, W.R.; Sailor, M.J.; Cheng, L. A Novel Approach of Daunorubicin Application on Formation of Proliferative Retinopathy Using a Porous Silicon Controlled Delivery System: Pharmacodynamics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2755–2763. [Google Scholar] [CrossRef]
- Wong, C.A.; Potter, M.J.; Cui, J.Z.; Chang, T.S.; Ma, P.; Maberley, A.L.; Ross, W.H.; White, V.A.; Samad, A.; Jia, W.; et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can. J. Ophthalmol. 2002, 37, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.-I.; Lin, L.-T.; Chen, Y.-H.; Chen, C.-L.; Chen, J.-T. Repression of Smad4 by MicroRNA-1285 moderates TGF-β-induced epithelial-mesenchymal transition in proliferative vitreoretinopathy. PLoS ONE 2021, 16, e0254873. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, X.; Lu, B. [Intravitreally injectable poly (D, L-Lactide) microspheres containing dexamethasone acetate for sustained release]. Yao Xue Xue Bao 2001, 36, 766–770. [Google Scholar] [PubMed]
- Lei, H.; Rheaume, M.-A.; Kazlauskas, A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp. Eye Res. 2010, 90, 376–381. [Google Scholar] [CrossRef]
- Baudouin, C.; Khosravi, E.; Pisella, P.J.; Ettaiche, M.; Elena, P.P. Inflammation measurement and immunocharacterization of cell proliferation in an experimental model of proliferative vitreoretinopathy. Ophthalmic Res. 1998, 30, 340–350. [Google Scholar] [CrossRef]
- Ozer, M.A.; Polat, N.; Ozen, S.; Ogurel, T.; Parlakpinar, H.; Vardi, N. Histopathological and ophthalmoscopic evaluation of apocynin on experimental proliferative vitreoretinopathy in rabbit eyes. Int. Ophthalmol. 2017, 37, 599–605. [Google Scholar] [CrossRef]
- Cheng, L.; Hostetler, K.; Valiaeva, N.; Tammewar, A.; Freeman, W.R.; Beadle, J.; Bartsch, D.-U.; Aldern, K.; Falkenstein, I. Intravitreal crystalline drug delivery for intraocular proliferation diseases. Investig. Ophthalmol. Vis. Sci. 2010, 51, 474–481. [Google Scholar] [CrossRef]
- Kralinger, M.T.; Kieselbach, G.F.; Voigt, M.; Hayden, B.; Hernandez, E.; Fernandez, V.; Parel, J.-M. Experimental model for proliferative vitreoretinopathy by intravitreal dispase: Limited by zonulolysis and cataract. Ophthalmologica 2006, 220, 211–216. [Google Scholar] [CrossRef]
- Goczalik, I.; Ulbricht, E.; Hollborn, M.; Raap, M.; Uhlmann, S.; Weick, M.; Pannicke, T.; Wiedemann, P.; Bringmann, A.; Reichenbach, A.; et al. Expression of CXCL8, CXCR1, and CXCR2 in neurons and glial cells of the human and rabbit retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4578–4589. [Google Scholar] [CrossRef]
- Mandava, N.; Blackburn, P.; Paul, D.B.; Wilson, M.W.; Read, S.B.; Alspaugh, E.; Tritz, R.; Barber, J.R.; Robbins, J.M.; Kruse, C.A. Ribozyme to proliferating cell nuclear antigen to treat proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3338–3348. [Google Scholar]
- Isiksoy, S.; Basmak, H.; Kasapoglu Dundar, E.; Ozer, A. Expression of proteins associated with cell-matrix adhesion in proliferative vitreoretinopathy designed by Dispase model. Eur. J. Ophthalmol. 2007, 17, 89–103. [Google Scholar] [CrossRef]
- Moon, S.W.; Sun, Y.; Warther, D.; Huffman, K.; Freeman, W.R.; Sailor, M.J.; Cheng, L. New model of proliferative vitreoretinopathy in rabbit for drug delivery and pharmacodynamic studies. Drug Deliv. 2018, 25, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Badaro, E.; Novais, E.A.; Abdala, K.; Chun, M.; Urias, M.; de Arruda Melo Filho, P.A.; Farah, M.E.; Rodrigues, E.B. Development of an experimental model of proliferative retinopathy by intravitreal injection of VEGF165. J. Ocul. Pharmacol. Ther. 2014, 30, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Pisella, P.J.; Ettaiche, M.; Goldschild, M.; Becquet, F.; Gastaud, P.; Droy-Lefaix, M.T. Effects of EGb761 and superoxide dismutase in an experimental model of retinopathy generated by intravitreal production of superoxide anion radical. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.; Lüke, J.; Lüke, M.; Kamal, M.; Abd El-Nabi, E.; Soliman, M.; Rohrbach, M.; Grisanti, S. The novel use of decorin in prevention of the development of proliferative vitreoretinopathy (PVR). Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1649–1660. [Google Scholar] [CrossRef]
- Hoerster, R.; Muether, P.S.; Vierkotten, S.; Hermann, M.M.; Kirchhof, B.; Fauser, S. Upregulation of TGF-ß1 in experimental proliferative vitreoretinopathy is accompanied by epithelial to mesenchymal transition. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.R.; Sakamoto, T.R.; Oshima, Y.R.; Ishibashi, T.R.; Inomata, H.R.; Murata, T.R.; Hinton, D.R.; Ryan, S.J. The antidepressant hypericin inhibits progression of experimental proliferative vitreoretinopathy. Curr. Eye Res. 1999, 19, 323–329. [Google Scholar] [CrossRef]
- Pastor, J.C.; Rodríguez, E.; Marcos, M.A.; Lopez, M.I. Combined pharmacologic therapy in a rabbit model of proliferative vitreoretinopathy (PVR). Ophthalmic Res. 2000, 32, 25–29. [Google Scholar] [CrossRef]
- Liou, G.I.; Pakalnis, V.A.; Matragoon, S.; Samuel, S.; Behzadian, M.A.; Baker, J.; Khalil, I.E.; Roon, P.; Caldwell, R.B.; Hunt, R.C.; et al. HGF regulation of RPE proliferation in an IL-1beta/retinal hole-induced rabbit model of PVR. Mol. Vis. 2002, 8, 494–501. [Google Scholar]
- Kosnosky, W.; Li, T.H.; Pakalnis, V.A.; Fox, A.; Hunt, R.C. Interleukin-1-beta changes the expression of metalloproteinases in the vitreous humor and induces membrane formation in eyes containing preexisting retinal holes. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4260–4267. [Google Scholar]
- Zhou, G.; Duan, Y.; Ma, G.; Wu, W.; Hu, Z.; Chen, N.; Chee, Y.; Cui, J.; Samad, A.; Matsubara, J.A.; et al. Introduction of the MDM2 T309G Mutation in Primary Human Retinal Epithelial Cells Enhances Experimental Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5361–5367. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Park, J.K.; Kim, Y.-T.; Kwon, B.-M.; Kang, S.G.; Yoo, Y.D.; Yu, Y.S.; Chung, H. Effect of 2’-benzoyl-oxycinnamaldehyde on RPE cells in vitro and in an experimental proliferative vitreoretinopathy model. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3117–3124. [Google Scholar]
- Lei, H.; Velez, G.; Cui, J.; Samad, A.; Maberley, D.; Matsubara, J.; Kazlauskas, A. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am. J. Pathol. 2010, 177, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Lin, Y.-J.; Wang, L.-C.; Tsai, H.-Y.; Yang, C.-H.; Teng, Y.-T.; Hsu, S.-M. Doxycycline Ameliorates the Severity of Experimental Proliferative Vitreoretinopathy in Mice. Int. J. Mol. Sci. 2021, 22, 11670. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Yuan, G.; Dong, M.; Shi, W. Notch signaling regulates M2 type macrophage polarization during the development of proliferative vitreoretinopathy. Cell. Immunol. 2015, 298, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.-M.; Porter, R.F.; Toguri, J.T.; Borowska-Fielding, J.; Gebremeskel, S.; Siwakoti, A.; Johnston, B.; Lehmann, C.; Kelly, M.E.M. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology 2017, 113, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M.; Ogawa, L.; Torbidoni, V.; Dodds, C.M.; Dodds, R.A.; Suburo, A.M. Blockade of endothelinergic receptors prevents development of proliferative vitreoretinopathy in mice. Am. J. Pathol. 2008, 172, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, W.; Lan, Y.; Chen, X.; Yang, W.; Yuan, Y.; Tan, J.; Zong, Y.; Jiang, Z. The inhibitory effect of small interference RNA protein kinase C-alpha on the experimental proliferative vitreoretinopathy induced by dispase in mice. Int. J. Nanomed. 2013, 8, 1563–1572. [Google Scholar] [CrossRef]
- Yoo, K.; Son, B.K.; Kim, S.; Son, Y.; Yu, S.-Y.; Hong, H.S. Substance P prevents development of proliferative vitreoretinopathy in mice by modulating TNF-α. Mol. Vis. 2017, 23, 933–943. [Google Scholar] [PubMed]
- Zhang, W.; Tan, J.; Liu, Y.; Li, W.; Gao, Q.; Lehmann, P.V. Assessment of the innate and adaptive immune system in proliferative vitreoretinopathy. Eye (Lond.) 2012, 26, 872–881. [Google Scholar] [CrossRef]
- Cantó Soler, M.V.; Gallo, J.E.; Dodds, R.A.; Suburo, A.M. A mouse model of proliferative vitreoretinopathy induced by dispase. Exp. Eye Res. 2002, 75, 491–504. [Google Scholar] [CrossRef]
- Liang, C.-M.; Tai, M.-C.; Chang, Y.-H.; Chen, Y.-H.; Chen, C.-L.; Lu, D.-W.; Chen, J.-T. Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol. 2011, 89, e505–e514. [Google Scholar] [CrossRef]
- Saika, S.; Yamanaka, O.; Ikeda, K.; Kim-Mitsuyama, S.; Flanders, K.C.; Yoo, J.; Roberts, A.B.; Nishikawa-Ishida, I.; Ohnishi, Y.; Muragaki, Y.; et al. Inhibition of p38MAP kinase suppresses fibrotic reaction of retinal pigment epithelial cells. Lab. Investig. 2005, 85, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Yamanaka, O.; Nishikawa-Ishida, I.; Kitano, A.; Flanders, K.C.; Okada, Y.; Ohnishi, Y.; Nakajima, Y.; Ikeda, K. Effect of Smad7 gene overexpression on transforming growth factor beta-induced retinal pigment fibrosis in a proliferative vitreoretinopathy mouse model. Arch. Ophthalmol. 2007, 125, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Heffer, A.; Wang, V.; Sridhar, J.; Feldon, S.E.; Libby, R.T.; Woeller, C.F.; Kuriyan, A.E. A Mouse Model of Proliferative Vitreoretinopathy Induced by Intravitreal Injection of Gas and RPE Cells. Transl. Vis. Sci. Technol. 2020, 9, 9. [Google Scholar] [CrossRef]
- Mori, K.; Gehlbach, P.; Ando, A.; Dyer, G.; Lipinsky, E.; Chaudhry, A.G.; Hackett, S.F.; Campochiaro, P.A. Retina-specific expression of PDGF-B versus PDGF-A: Vascular versus nonvascular proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2001–2006. [Google Scholar]
- Akiyama, H.; Kachi, S.; Silva, R.L.E.; Umeda, N.; Hackett, S.F.; McCauley, D.; McCauley, T.; Zoltoski, A.; Epstein, D.M.; Campochiaro, P.A. Intraocular injection of an aptamer that binds PDGF-B: A potential treatment for proliferative retinopathies. J. Cell. Physiol. 2006, 207, 407–412. [Google Scholar] [CrossRef]
- Edwards, M.M.; McLeod, D.S.; Grebe, R.; Heng, C.; Lefebvre, O.; Lutty, G.A. Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of fetal vasculature, and epiretinal membrane formation in mice. BMC Dev. Biol. 2011, 11, 60. [Google Scholar] [CrossRef]
- Chen, X.-L.; Bai, Y.-J.; Hu, Q.-R.; Li, S.-S.; Huang, L.-Z.; Li, X.-X. Small Interfering RNA Targeted to ASPP2 Promotes Progression of Experimental Proliferative Vitreoretinopathy. Mediators Inflamm. 2016, 2016, 7920631. [Google Scholar] [CrossRef]
- Zheng, X.-Z.; Du, L.-F.; Wang, H.-P. An immunohistochemical analysis of a rat model of proliferative vitreoretinopathy and a comparison of the expression of TGF-β and PDGF among the induction methods. Bosn. J. Basic Med. Sci. 2010, 10, 204–209. [Google Scholar] [CrossRef]
- Zheng, X.; Du, L.; Wang, H.; Gu, Q. A novel approach to attenuate proliferative vitreoretinopathy using ultrasound-targeted microbubble destruction and recombinant adeno-associated virus-mediated RNA interference targeting transforming growth factor-β2 and platelet-derived growth factor-B. J. Gene Med. 2012, 14, 339–347. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, W.; Zhang, J.; Li, M.; Xiang, Q.; Li, Y.; Tan, T.; Ou, Q.; Zhang, J.; Tian, H.; et al. Protein Kinase A Inhibitor H89 Attenuates Experimental Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Lin, M.; Li, Y.; Li, Z.; Lin, J.; Zhou, X.; Liang, D. Macrophages acquire fibroblast characteristics in a rat model of proliferative vitreoretinopathy. Ophthalmic Res. 2011, 45, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Uslubas, I.; Kanli, A.; Kasap, M.; Akpinar, G.; Karabas, L. Effect of aflibercept on proliferative vitreoretinopathy: Proteomic analysis in an experimental animal model. Exp. Eye Res. 2021, 203, 108425. [Google Scholar] [CrossRef]
- Wong, C.W.; Busoy, J.M.F.; Cheung, N.; Barathi, V.A.; Storm, G.; Wong, T.T. Endogenous or Exogenous Retinal Pigment Epithelial Cells: A Comparison of Two Experimental Animal Models of Proliferative Vitreoretinopathy. Transl. Vis. Sci. Technol. 2020, 9, 46. [Google Scholar] [CrossRef]
- Umazume, K.; Barak, Y.; McDonald, K.; Liu, L.; Kaplan, H.J.; Tamiya, S. Proliferative vitreoretinopathy in the Swine-a new model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4910–4916. [Google Scholar] [CrossRef]
- Velikay, M.; Stolba, U.; Wedrich, A.; Datlinger, P.; Akramian, J.; Binder, S. The antiproliferative effect of fractionized radiation therapy: Optimization of dosage. Doc. Ophthalmol. 1994, 87, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Velez, G.; Hovland, P.; Hirose, T.; Gilbertson, D.; Kazlauskas, A. Growth factors outside the PDGF family drive experimental PVR. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Cheung, N.; Ho, C.; Barathi, V.; Storm, G.; Wong, T.T. Characterisation of the inflammatory cytokine and growth factor profile in a rabbit model of proliferative vitreoretinopathy. Sci. Rep. 2019, 9, 15419. [Google Scholar] [CrossRef]
- Khoroshilova-Maslova, I.P.; Leparskaya, N.L.; Nabieva, M.M.; Andreeva, L.D. Experimental Modeling of Proliferative Vitreoretinopathy. An Experimental Morphological Study. Bull. Exp. Biol. Med. 2015, 159, 100–102. [Google Scholar] [CrossRef]
- Wiedemann, P.; Sorgente, N.; Ryan, S.J. Proliferative vitreoretinopathy: The rabbit cell injection model for screening of antiproliferative drugs. J. Pharmacol. Methods 1984, 12, 69–78. [Google Scholar] [CrossRef]
- Kawahara, S.; Hata, Y.; Kita, T.; Arita, R.; Miura, M.; Nakao, S.; Mochizuki, Y.; Enaida, H.; Kagimoto, T.; Goto, Y.; et al. Potent inhibition of cicatricial contraction in proliferative vitreoretinal diseases by statins. Diabetes 2008, 57, 2784–2793. [Google Scholar] [CrossRef]
- Guo, L.; Yu, W.; Li, X.; Zhao, G.; Liang, J.; He, P.; Wang, K.; Zhou, P.; Jiang, Y.; Zhao, M. Targeting of integrin-linked kinase with a small interfering RNA suppresses progression of experimental proliferative vitreoretinopathy. Exp. Eye Res. 2008, 87, 551–560. [Google Scholar] [CrossRef]
- Agrawal, R.N.; He, S.; Spee, C.; Cui, J.Z.; Ryan, S.J.; Hinton, D.R. In vivo models of proliferative vitreoretinopathy. Nat. Protoc. 2007, 2, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bali, E.; Willermain, F.; Caspers-Velu, L.; Dubois, C.; Dehou, M.-F.; Velu, T.; Libert, J.; Bruyns, C. IL-10 in vivo gene expression in a cell-induced animal model of proliferative vitreoretinopathy. Int. J. Mol. Med. 2003, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Khoroshilova-Maslova, I.P.; Leparskaya, N.L.; Vorotelyak, E.A.; Vasiliev, A.V. The significance of fibroblasts in experimental modeling of proliferative vitreoretinopathy. Vestn. Oftalmol. 2017, 133, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pennock, S.; Rheaume, M.-A.; Mukai, S.; Kazlauskas, A. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am. J. Pathol. 2011, 179, 2931–2940. [Google Scholar] [CrossRef]
- Ramirez, M.; Davidson, E.A.; Luttenauer, L.; Elena, P.P.; Cumin, F.; Mathis, G.A.; De Gasparo, M. The renin-angiotensin system in the rabbit eye. J. Ocul. Pharmacol. Ther. 1996, 12, 299–312. [Google Scholar] [CrossRef]
- Nakagawa, M.; Refojo, M.F.; Marin, J.F.; Doi, M.; Tolentino, F.I. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2388–2395. [Google Scholar]
- Zheng, Y.; Ikuno, Y.; Ohj, M.; Kusaka, S.; Jiang, R.; Cekiç, O.; Sawa, M.; Tano, Y. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn. J. Ophthalmol. 2003, 47, 158–165. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, S.; Wu, M.; Cui, H.; Wu, R.; Chen, S.; Xu, C.; Lu, X.; Feng, S. Self-assembling hydrogel loaded with 5-FU PLGA microspheres as a novel vitreous substitute for proliferative vitreoretinopathy. J. Biomed. Mater. Res. A 2020, 108, 2435–2446. [Google Scholar] [CrossRef]
- Frenzel, E.M.; Neely, K.A.; Walsh, A.W.; Cameron, J.D.; Gregerson, D.S. A new model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2157–2164. [Google Scholar]
- Zhao, X.; Han, H.; Song, Y.; Du, M.; Liao, M.; Dong, X.; Wang, X.; Kuhn, F.; Hoskin, A.; Xu, H.; et al. The Role of Intravitreal Anti-VEGF Agents in Rabbit Eye Model of Open-Globe Injury. J. Ophthalmol. 2021, 2021, 5565178. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Khawly, J.A.; Hainsworth, D.P.; Chen, S.N.; Ashton, P.; Guo, H.; Jaffe, G.J. An intravitreal sustained-release triamcinolone and 5-fluorouracil codrug in the treatment of experimental proliferative vitreoretinopathy. Arch. Ophthalmol. 1998, 116, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L.; D’Angelo, L.; De Girolamo, P.; Merighi, A. Anatomical features for an adequate choice of experimental animal model in biomedicine: II. Small laboratory rodents, rabbit, and pig. Ann. Anat. 2016, 204, 11–28. [Google Scholar] [CrossRef]
- Tosi, G.M.; Marigliani, D.; Romeo, N.; Toti, P. Disease pathways in proliferative vitreoretinopathy: An ongoing challenge. J. Cell. Physiol. 2014, 229, 1577–1583. [Google Scholar] [CrossRef]
- Khateb, S.; Aweidah, H.; Halpert, M.; Jaouni, T. Postoperative Macular Proliferative Vitreoretinopathy: A Case Series and Literature Review. Case Rep. Ophthalmol. 2021, 12, 464–472. [Google Scholar] [CrossRef]
- Men, G.; Peyman, G.A.; Kuo, P.-C.; Bezerra, Y.; Ghahramani, F.; Naaman, G.; Livir-Rallatos, C.; Lee, P.J. The role of scleral buckle in experimental posterior penetrating eye injury. Retina 2003, 23, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Heffer, A.M.; Wang, V.; Libby, R.T.; Feldon, S.E.; Woeller, C.F.; Kuriyan, A.E. Salinomycin inhibits proliferative vitreoretinopathy formation in a mouse model. PLoS ONE 2020, 15, e0243626. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J. Yes-associated protein is essential for proliferative vitreoretinopathy development via the epithelial-mesenchymal transition in retinal pigment epithelial fibrosis. J. Cell. Mol. Med. 2021, 25, 10213–10223. [Google Scholar] [CrossRef]
- Peirson, S.N.; Brown, L.A.; Pothecary, C.A.; Benson, L.A.; Fisk, A.S. Light and the laboratory mouse. J. Neurosci. Methods 2018, 300, 26–36. [Google Scholar] [CrossRef]
- Chang, B. Mouse models for studies of retinal degeneration and diseases. Methods Mol. Biol. 2013, 935, 27–39. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Cui, C.; Zhao, H.-M.; Ba, J.; Chen, H.; Yu, J. Expression of IGFBP-6 in proliferative vitreoretinopathy rat models and its effects on retinal pigment epithelial-J cells. Mol. Med. Rep. 2014, 9, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-K.; Chen, X.-L.; Liu, S.; Liu, W. Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model. Int. J. Ophthalmol. 2021, 14, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
| Model Type | Methodology | Strengths | Limitations | Therapeutic Investigations | Pathogenesis Studies |
|---|---|---|---|---|---|
| Rabbit Models | |||||
| Cell-induced models | Intravitreal injection of: |
|
| [59,64,65,68,69,70,71] | [66,72] |
|
| [74] | [75] | ||
| Biologically induced models |
|
| [78] | [79] | |
|
| [80] | |||
|
|
| [82] | ||
| Surgically induced models |
|
| [83] | [84] | |
|
|
| [37] | ||
| Association of cell- and biologically induced models |
|
| [52,70] | ||
| Association of cell-, biologically and Surgically induced models |
|
| [85,86,88] | ||
|
| [61,91] | |||
| Mouse Models | |||||
| Cell-induced models |
|
| [92] | ||
| Biologicallyinduced models |
|
| [38,94,98] | [37,39,95,97,99] | |
| Surgically induced models |
|
| [26,100,101,102,103] | ||
| Transgenic models |
|
|
| [105] | |
| Rat Models | |||||
| Cell-inducedmodels |
|
| [108,109,110] | [108,109,110,111] | |
| Biologically induced models |
|
| [41,112] | ||
| Swine Models | |||||
| Association of cell- and surgically induced models |
|
| [54] | [114] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datlibagi, A.; Zein-El-Din, A.; Frohly, M.; Willermain, F.; Delporte, C.; Motulsky, E. Experimental Models to Study Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy. Int. J. Mol. Sci. 2023, 24, 4509. https://doi.org/10.3390/ijms24054509
Datlibagi A, Zein-El-Din A, Frohly M, Willermain F, Delporte C, Motulsky E. Experimental Models to Study Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy. International Journal of Molecular Sciences. 2023; 24(5):4509. https://doi.org/10.3390/ijms24054509
Chicago/Turabian StyleDatlibagi, Azine, Anna Zein-El-Din, Maxime Frohly, François Willermain, Christine Delporte, and Elie Motulsky. 2023. "Experimental Models to Study Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy" International Journal of Molecular Sciences 24, no. 5: 4509. https://doi.org/10.3390/ijms24054509
APA StyleDatlibagi, A., Zein-El-Din, A., Frohly, M., Willermain, F., Delporte, C., & Motulsky, E. (2023). Experimental Models to Study Epithelial-Mesenchymal Transition in Proliferative Vitreoretinopathy. International Journal of Molecular Sciences, 24(5), 4509. https://doi.org/10.3390/ijms24054509







