Critical Overview on Endocrine Disruptors in Diabetes Mellitus
Abstract
1. Introduction
2. Human Implications of Diabetogenic Pollutants
2.1. Persistent EDCs and Type 2 Diabetes
2.2. Non-Persistent EDCs and Type 2 Diabetes
2.3. EDCs and Type 1 Diabetes
2.4. Maternal Exposure to EDCs and Diabetes
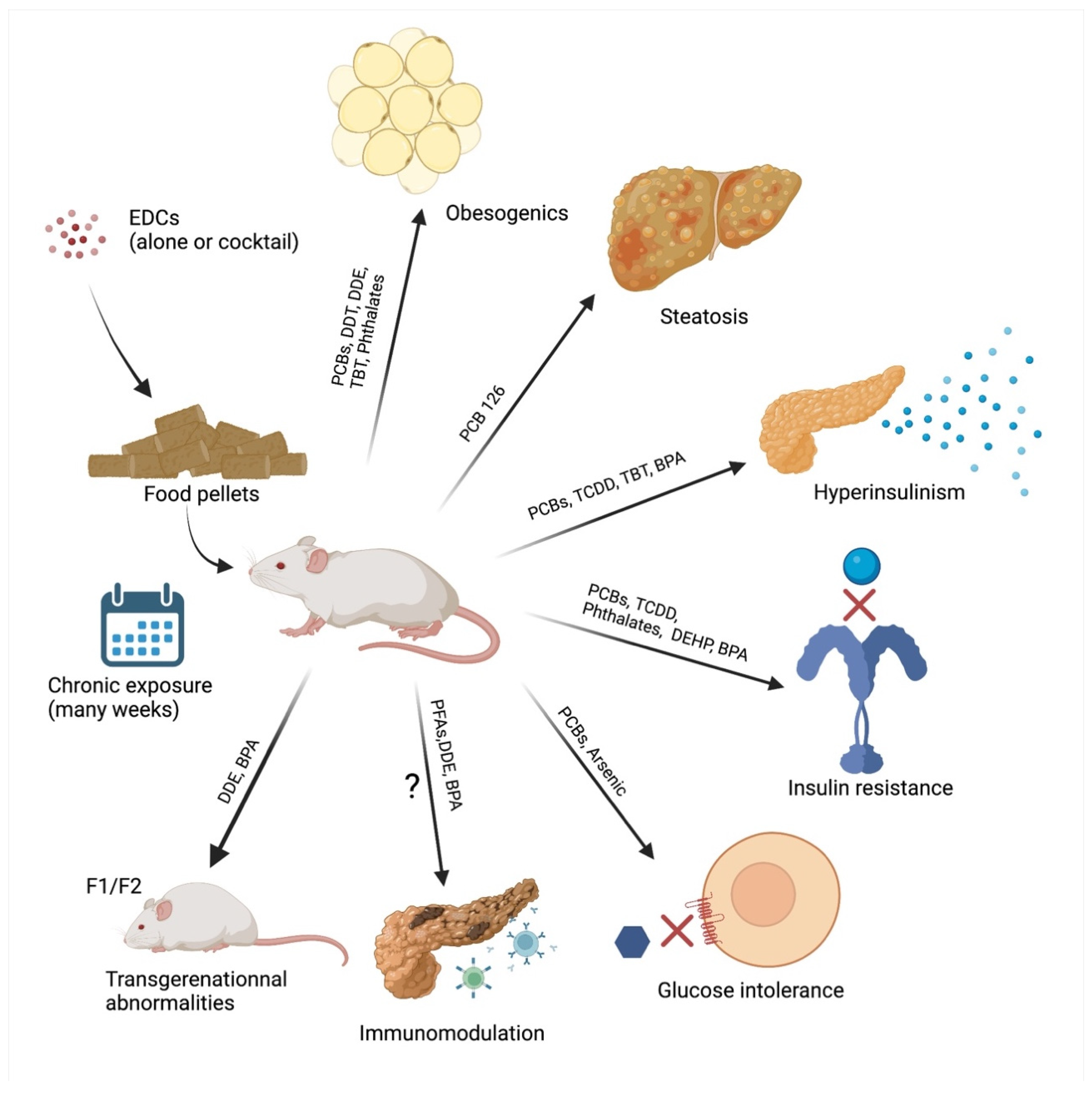
3. In Vivo Evidence of Diabetogenic Pollutants
3.1. Persistent EDCs and T2D
3.2. Non-Persistent EDCs and T2D
3.3. EDCs and T1D
3.4. Maternal-Fetal Exposure to EDCs and Diabetes
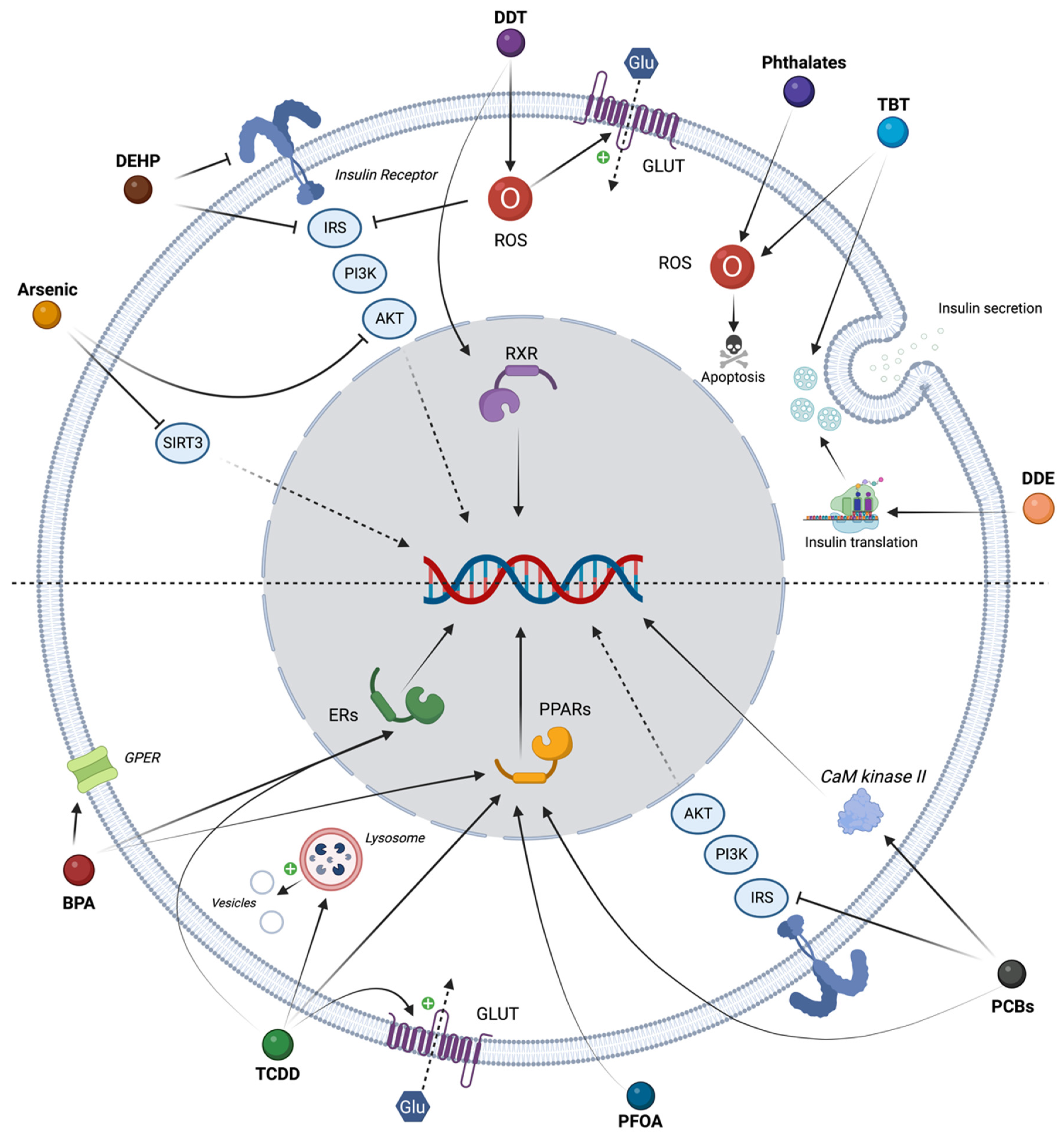
4. In Vitro Evidence of Diabetogenic Pollutants
4.1. Persistent EDCs and T2D
4.2. Non-Persistent EDCs and T2D
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Diabetes (Fact. Sheet N°312). Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 15 September 2022).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: http://www.idf.org/diabetesatlas (accessed on 15 January 2023).
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef]
- UNEP. Stockholm Convention On Persistent Organic Pollutants (POPs). Texts and Annexes. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 15 September 2022).
- Vasseghian, Y.; Hosseinzadeh, S.; Khataee, A.; Dragoi, E.N. The concentration of persistent organic pollutants in water resources: A global systematic review, meta-analysis and probabilistic risk assessment. Sci. Total Environ. 2021, 796, 149000. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. [Google Scholar] [CrossRef] [PubMed]
- Bertazzi, P.A.; Consonni, D.; Bachetti, S.; Rubagotti, M.; Baccarelli, A.; Zocchetti, C.; Pesatori, A.C. Health effects of dioxin exposure: A 20-year mortality study. Am. J. Epidemiol. 2001, 153, 1031–1044. [Google Scholar] [CrossRef]
- Warner, M.; Mocarelli, P.; Brambilla, P.; Wesselink, A.; Samuels, S.; Signorini, S.; Eskenazi, B. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: The Seveso women’s health study. Environ. Health Perspect. 2013, 121, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.E.; Pavuk, M. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med. 2008, 50, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Tsai, P.C.; Yang, C.Y.; Guo, Y.L. Increased risk of diabetes and polychlorinated biphenyls and dioxins: A 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008, 31, 1574–1579. [Google Scholar] [CrossRef]
- Chang, J.W.; Chen, H.L.; Su, H.J.; Lee, C.C. Abdominal Obesity and Insulin Resistance in People Exposed to Moderate-to-High Levels of Dioxin. PLoS ONE 2016, 11, e0145818. [Google Scholar] [CrossRef]
- Magliano, D.J.; Ranciere, F.; Slama, R.; Roussel, R.; Kiviranta, H.; Coumoul, X.; Balkau, B.; Botton, J.; the D.E.S.I.R. Study Group. Exposure to persistent organic pollutants and the risk of type 2 diabetes: A case-cohort study. Diabetes Metab. 2021, 47, 101234. [Google Scholar] [CrossRef]
- Magliano, D.J.; Loh, V.H.; Harding, J.L.; Botton, J.; Shaw, J.E. Persistent organic pollutants and diabetes: A review of the epidemiological evidence. Diabetes Metab. 2014, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, M.; Yu, L.; Liang, R.; Liu, W.; Dong, C.; Zhang, Y.; Li, M.; Ye, Z.; Wang, B.; et al. Associations of polychlorinated biphenyls exposure with plasma glucose and diabetes in general Chinese population: The mediating effect of lipid peroxidation. Environ. Pollut. 2022, 308, 119660. [Google Scholar] [CrossRef]
- Baumert, B.O.; Goodrich, J.A.; Hu, X.; Walker, D.I.; Alderete, T.L.; Chen, Z.; Valvi, D.; Rock, S.; Berhane, K.; Gilliland, F.D.; et al. Plasma concentrations of lipophilic persistent organic pollutants and glucose homeostasis in youth populations. Environ. Res. 2022, 212, 113296. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Gui, S.Y.; Qiao, J.C.; Xu, K.X.; Li, Z.L.; Chen, Y.N.; Wu, K.J.; Jiang, Z.X.; Hu, C.Y. Association between per- and polyfluoroalkyl substances exposure and risk of diabetes: A systematic review and meta-analysis. J. Expo. Sci. Environ. Epidemiol. 2022, 33, 40–55. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, H.; Yao, Y.; Li, Y.; Meng, Y.; Lu, Y.; Han, L.; Chen, L. Serum concentrations of per-/polyfluoroalkyl substances and risk of type 2 diabetes: A case-control study. Sci. Total Environ. 2021, 787, 147476. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Neel, B.A.; Sargis, R.M. The paradox of progress: Environmental disruption of metabolism and the diabetes epidemic. Diabetes 2011, 60, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Baillie-Hamilton, P.F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 2002, 8, 185–192. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharm. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef]
- Airaksinen, R.; Rantakokko, P.; Eriksson, J.G.; Blomstedt, P.; Kajantie, E.; Kiviranta, H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care 2011, 34, 1972–1979. [Google Scholar] [CrossRef]
- Ngwa, E.N.; Kengne, A.P.; Tiedeu-Atogho, B.; Mofo-Mato, E.P.; Sobngwi, E. Persistent organic pollutants as risk factors for type 2 diabetes. Diabetol. Metab. Syndr. 2015, 7, 41. [Google Scholar] [CrossRef]
- Jorgensen, M.E.; Borch-Johnsen, K.; Bjerregaard, P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia 2008, 51, 1416–1422. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, I.K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B.A.; Jacobs, D.R., Jr. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 2006, 29, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.J.; Frithsen, I.L.; Diaz, V.A.; Koopman, R.J.; Simpson, W.M., Jr.; Mainous, A.G., 3rd. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2007, 103, 413–418. [Google Scholar] [CrossRef]
- Hong, Y.C.; Park, E.Y.; Park, M.S.; Ko, J.A.; Oh, S.Y.; Kim, H.; Lee, K.H.; Leem, J.H.; Ha, E.H. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett. 2009, 184, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Bi, Y.; Wang, T.; Xu, M.; Xu, Y.; Huang, Y.; Li, M.; Li, X.; Wang, W.; Chen, Y.; et al. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: A cross-sectional analysis. Ann. Intern. Med. 2011, 155, 368–374. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cornelis, M.C.; Townsend, M.K.; Tobias, D.K.; Eliassen, A.H.; Franke, A.A.; Hauser, R.; Hu, F.B. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: A prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect. 2014, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Ranciere, F.; Botton, J.; Slama, R.; Lacroix, M.Z.; Debrauwer, L.; Charles, M.A.; Roussel, R.; Balkau, B.; Magliano, D.J.; the D.E.S.I.R. Study Group. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ. Health Perspect. 2019, 127, 107013. [Google Scholar] [CrossRef]
- Duan, Y.; Yao, Y.; Wang, B.; Han, L.; Wang, L.; Sun, H.; Chen, L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ. Pollut. 2018, 243, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Levine, D.; Wertenteil, S.; Vento, S.; Xue, J.; Rajendiran, K.; Kannan, K.; Thurman, J.M.; Morrison, D.; Brody, R.; et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res. 2017, 81, 857–864. [Google Scholar] [CrossRef]
- Song, S.; Duan, Y.; Zhang, T.; Zhang, B.; Zhao, Z.; Bai, X.; Xie, L.; He, Y.; Ouyang, J.P.; Huang, X.; et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose. Ecotoxicol. Environ. Saf. 2019, 169, 822–828. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Liu, W.; Li, X.; Hu, J.; Zhang, B.; Xu, S.; Zhou, Y.; Li, J.; Cai, Z.; et al. Exposure to Bisphenol a Substitutes and Gestational Diabetes Mellitus: A Prospective Cohort Study in China. Front. Endocrinol. 2019, 10, 262. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef]
- Shoshtari-Yeganeh, B.; Zarean, M.; Mansourian, M.; Riahi, R.; Poursafa, P.; Teiri, H.; Rafiei, N.; Dehdashti, B.; Kelishadi, R. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ. Sci. Pollut. Res. Int. 2019, 26, 9435–9442. [Google Scholar] [CrossRef]
- Zhang, H.; Ben, Y.; Han, Y.; Zhang, Y.; Li, Y.; Chen, X. Phthalate exposure and risk of diabetes mellitus: Implications from a systematic review and meta-analysis. Environ. Res. 2022, 204, 112109. [Google Scholar] [CrossRef]
- Xie, X.; Lu, C.; Wu, M.; Liang, J.; Ying, Y.; Liu, K.; Huang, X.; Zheng, S.; Du, X.; Liu, D.; et al. Association between triclocarban and triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ. Int. 2020, 136, 105445. [Google Scholar] [CrossRef]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef]
- Dai, L.; Lv, X.; Chen, Z.; Huang, Z.; Li, B.; Xie, Y.; Duan, Y.; Zhao, H.; Wang, Y.; Yu, Q.; et al. Elevated whole blood arsenic level is associated with type 2 diabetes in coal-burning areas in Guizhou. Toxicol. Appl. Pharmacol. 2020, 403, 115135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, Y.; Wang, D.; Xu, Y.; Wang, H.; Liu, J.; Xia, L.; Li, Y.; Tang, N.; Zheng, Q.; et al. Interactions of arsenic metabolism with arsenic exposure and individual factors on diabetes occurrence: Baseline findings from Arsenic and Non-Communicable disease cohort (AsNCD) in China. Environ. Pollut. 2020, 265, 114968. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liang, R.; Wang, B.; Yu, L.; Liu, W.; Wang, X.; Xiao, L.; Ma, J.; Zhou, M.; Chen, W. Cross-sectional and longitudinal associations of urinary zinc with glucose-insulin homeostasis traits and type 2 diabetes: Exploring the potential roles of systemic inflammation and oxidative damage in Chinese urban adults. Environ. Pollut. 2022, 314, 120331. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhou, J.; Guo, P.; Wang, J.; Wang, P.; Liu, L.; Wu, M.; Wang, P.; Liu, N. Association between environmental lead/cadmium co-exposure in drinking water and soil and type 2 diabetes mellitus/obesity in Southern China. Front. Public Health 2022, 10, 941922. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Mei, Y.; Zhao, M.; Xu, J.; Seery, S.; Li, R.; Zhao, J.; Zhou, Q.; Ge, X.; Xu, Q. The effect of ambient ozone on glucose-homoeostasis: A prospective study of non-diabetic older adults in Beijing. Sci. Total Environ. 2021, 761, 143308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Wang, Y.; Gong, J.; Wang, G.; Ge, W.; Chen, R.; Meng, X.; Zhao, Y.; Kan, H. Associations of long-term exposure to ambient nitrogen dioxide with indicators of diabetes and dyslipidemia in China: A nationwide analysis. Chemosphere 2021, 269, 128724. [Google Scholar] [CrossRef]
- Elbarbary, M.; Honda, T.; Morgan, G.; Kelly, P.; Guo, Y.; Negin, J. Ambient air pollution exposure association with diabetes prevalence and glycosylated hemoglobin (HbA1c) levels in China. Cross-sectional analysis from the WHO study of AGEing and adult health wave 1. J. Environ. Sci. Health Tox Hazard. Subst Environ. Eng. 2020, 55, 1149–1162. [Google Scholar] [CrossRef]
- Hwang, M.J.; Kim, J.H.; Koo, Y.S.; Yun, H.Y.; Cheong, H.K. Impacts of ambient air pollution on glucose metabolism in Korean adults: A Korea National Health and Nutrition Examination Survey study. Environ. Health 2020, 19, 70. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, S.; Liu, H.; Cui, Z.; Hou, F.; Feng, S.; Zhang, Y.; Liu, H.; Lu, C.; Yu, P. Risk Analysis of Air Pollution and Meteorological Factors Affecting the Incidence of Diabetes in the Elderly Population in Northern China. J. Diabetes Res. 2020, 2020, 3673980. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Bisphenol A: Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 2015, 16, 299–309. [Google Scholar] [CrossRef]
- Chevalier, N.; Fenichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef]
- Berg, V.; Charles, D.; Bergdahl, I.A.; Nost, T.H.; Sandanger, T.M.; Tornevi, A.; Huber, S.; Fuskevag, O.M.; Rylander, C. Pre- and post-diagnostic blood profiles of chlorinated persistent organic pollutants and metabolic markers in type 2 diabetes mellitus cases and controls; a pilot study. Environ. Res. 2021, 195, 110846. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.G. Developmental Exposure to Endocrine Disrupting Chemicals and Type 1 Diabetes Mellitus. Front. Endocrinol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Innes, K.E.; Long, D. Perfluoroalkyl substances and beta cell deficient diabetes. J. Diabetes Complicat. 2016, 30, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Malarvannan, G.; Dirinck, E.; Dirtu, A.C.; Pereira-Fernandes, A.; Neels, H.; Jorens, P.G.; Gaal, L.V.; Blust, R.; Covaci, A. Distribution of persistent organic pollutants in two different fat compartments from obese individuals. Environ. Int. 2013, 55, 33–42. [Google Scholar] [CrossRef]
- Elten, M.; Donelle, J.; Lima, I.; Burnett, R.T.; Weichenthal, S.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Chen, H.; Paul, L.A.; et al. Ambient air pollution and incidence of early-onset paediatric type 1 diabetes: A retrospective population-based cohort study. Environ. Res. 2020, 184, 109291. [Google Scholar] [CrossRef]
- Sheehan, A.; Freni Sterrantino, A.; Fecht, D.; Elliott, P.; Hodgson, S. Childhood type 1 diabetes: An environment-wide association study across England. Diabetologia 2020, 63, 964–976. [Google Scholar] [CrossRef]
- Sargis, R.M.; Heindel, J.J.; Padmanabhan, V. Interventions to Address Environmental Metabolism-Disrupting Chemicals: Changing the Narrative to Empower Action to Restore Metabolic Health. Front. Endocrinol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of adult disease. Eur. J. Epidemiol. 2003, 18, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Panimolle, F.; Lenzi, A.; Morano, S. Bisphenol A and Phthalates in Diet: An Emerging Link with Pregnancy Complications. Nutrients 2020, 12, 525. [Google Scholar] [CrossRef]
- Yan, D.; Jiao, Y.; Yan, H.; Liu, T.; Yan, H.; Yuan, J. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Environ. Health 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xia, W.; Zhang, B.; Zhou, A.; Huang, Z.; Zhang, H.; Liu, H.; Jiang, Y.; Hu, C.; Chen, X.; et al. Relation between cadmium exposure and gestational diabetes mellitus. Environ. Int. 2018, 113, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, N.; Villanacci, R.; Ottolina, J.; Bartiromo, L.; Cavoretto, P.; Dolci, C.; Lembo, R.; Schimberni, M.; Valsecchi, L.; Vigano, P.; et al. Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3094. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, J.; Xia, W.; Dai, J.; Liu, H.; Pan, Y.; Xu, S.; Lu, S.; Jin, S.; Li, Y.; et al. Association between exposure to per- and polyfluoroalkyl substances and blood glucose in pregnant women. Int J. Hyg. Environ. Health 2020, 230, 113596. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, Q.; Zhang, J.; Chen, X.; Zhao, H.; Lu, H.; Ma, B.; Wang, Z.; Wu, C.; Ying, C.; et al. Exposure to elevated per- and polyfluoroalkyl substances in early pregnancy is related to increased risk of gestational diabetes mellitus: A nested case-control study in Shanghai, China. Environ. Int. 2020, 143, 105952. [Google Scholar] [CrossRef]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Berger, K.; Hyland, C.; Ames, J.L.; Mora, A.M.; Huen, K.; Eskenazi, B.; Holland, N.; Harley, K.G. Prenatal Exposure to Mixtures of Phthalates, Parabens, and Other Phenols and Obesity in Five-Year-Olds in the CHAMACOS Cohort. Int. J. Environ. Res. Public Health 2021, 18, 1796. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Berger, K.; Rauch, S.; Kogut, K.; Claus Henn, B.; Calafat, A.M.; Huen, K.; Eskenazi, B.; Holland, N. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr. Res. 2017, 82, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Ye, M.; Harley, K.; Kogut, K.; Bradman, A.; Eskenazi, B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ. Res. 2017, 159, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Pereira, S.R.; DeBari, M.K.; Abbott, R.D. Mechanisms of action, chemical characteristics, and model systems of obesogens. BMC Biomed. Eng. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Bokobza, E.; Hinault, C.; Tiroille, V.; Clavel, S.; Bost, F.; Chevalier, N. The Adipose Tissue at the Crosstalk Between EDCs and Cancer Development. Front. Endocrinol. 2021, 12, 691658. [Google Scholar] [CrossRef]
- Misra, B.B.; Misra, A. The chemical exposome of type 2 diabetes mellitus: Opportunities and challenges in the omics era. Diabetes Metab. Syndr. 2020, 14, 23–38. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Egusquiza, R.J.; Blumberg, B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology 2020, 161, bqaa024. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N. Environmental endocrine disruptors: New diabetogens? C. R. Biol. 2017, 340, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Quesada, I.; Tuduri, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Mimoto, M.S.; Nadal, A.; Sargis, R.M. Polluted Pathways: Mechanisms of Metabolic Disruption by Endocrine Disrupting Chemicals. Curr. Environ. Health Rep. 2017, 4, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent Organic Pollutant-Mediated Insulin Resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef]
- Schulz, M.C.; Sargis, R.M. Inappropriately sweet: Environmental endocrine-disrupting chemicals and the diabetes pandemic. Adv. Pharm. 2021, 92, 419–456. [Google Scholar] [CrossRef]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Fjaere, E.; Lock, E.J.; Naville, D.; Amlund, H.; Meugnier, E.; Le Magueresse Battistoni, B.; Froyland, L.; Madsen, L.; Jessen, N.; et al. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS ONE 2011, 6, e25170. [Google Scholar] [CrossRef]
- Naville, D.; Pinteur, C.; Vega, N.; Menade, Y.; Vigier, M.; Le Bourdais, A.; Labaronne, E.; Debard, C.; Luquain-Costaz, C.; Begeot, M.; et al. Low-dose food contaminants trigger sex-specific, hepatic metabolic changes in the progeny of obese mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Shaw, A.C.; Gagne, A.X.; Chan, H.M. Chronic exposure to PCBs (Aroclor 1254) exacerbates obesity-induced insulin resistance and hyperinsulinemia in mice. J. Toxicol. Environ. Health A 2013, 76, 701–715. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, T.; Chen, M.; Guo, Z.; Yang, Z.; Zuo, Z.; Wang, C. Chronic Exposure to Aroclor 1254 Disrupts Glucose Homeostasis in Male Mice via Inhibition of the Insulin Receptor Signal Pathway. Environ. Sci Technol. 2015, 49, 10084–10092. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Karounos, M.; English, V.; Fang, J.; Wei, Y.; Stromberg, A.; Sunkara, M.; Morris, A.J.; Swanson, H.I.; Cassis, L.A. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ. Health Perspect. 2013, 121, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kwon, W.Y.; Kim, Y.A.; Oh, Y.J.; Yoo, S.H.; Lee, M.H.; Bae, J.Y.; Kim, J.M.; Yoo, Y.H. Polychlorinated biphenyls exposure-induced insulin resistance is mediated by lipid droplet enlargement through Fsp27. Arch. Toxicol. 2017, 91, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, W.; Meng, F.; Mi, J.; Peng, J.; Liu, J.; Zhang, X.; Hai, C.; Wang, X. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-kappaB signaling via downregulation of HNF1b. Redox Biol. 2017, 12, 300–310. [Google Scholar] [CrossRef]
- Shi, H.; Jan, J.; Hardesty, J.E.; Falkner, K.C.; Prough, R.A.; Balamurugan, A.N.; Mokshagundam, S.P.; Chari, S.T.; Cave, M.C. Polychlorinated biphenyl exposures differentially regulate hepatic metabolism and pancreatic function: Implications for nonalcoholic steatohepatitis and diabetes. Toxicol. Appl. Pharmacol. 2019, 363, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Yoshioka, W.; Nishimura, N.; Kubota, N.; Kadowaki, T.; Tohyama, C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J. Appl. Toxicol. 2009, 29, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Hoyeck, M.P.; Blair, H.; Ibrahim, M.; Solanki, S.; Elsawy, M.; Prakash, A.; Rick, K.R.C.; Matteo, G.; O’Dwyer, S.; Bruin, J.E. Long-term metabolic consequences of acute dioxin exposure differ between male and female mice. Sci. Rep. 2020, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Zhu, Y.; Yan, H.; Lu, Y. Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. Int. J. Environ. Res. Public Health 2021, 18, 13141. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lan, K.C.; Tsai, J.R.; Weng, T.I.; Yang, C.Y.; Liu, S.H. Tributyltin exposure at noncytotoxic doses dysregulates pancreatic beta-cell function in vitro and in vivo. Arch. Toxicol. 2017, 91, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.S.; Medina-Gali, R.M.; Babiloni-Chust, I.; Marroqui, L.; Nadal, A. In Vitro Assays to Identify Metabolism-Disrupting Chemicals with Diabetogenic Activity in a Human Pancreatic beta-Cell Model. Int. J. Mol. Sci. 2022, 23, 5040. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Sabir, S.; Rehman, K. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environ. Toxicol. Pharm. 2020, 77, 103373. [Google Scholar] [CrossRef]
- Abulehia, H.F.S.; Mohd Nor, N.S.; Sheikh Abdul Kadir, S.H. The Current Findings on the Impact of Prenatal BPA Exposure on Metabolic Parameters: In Vivo and Epidemiological Evidence. Nutrients 2022, 14, 2766. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, C.; Le Magueresse-Battistoni, B.; Viguie, C.; Babajko, S.; Canivenc-Lavier, M.C.; Chevalier, N.; Emond, C.; Habert, R.; Picard-Hagen, N.; Mhaouty-Kodja, S. Regulatory and academic studies to derive reference values for human health: The case of bisphenol S. Environ. Res. 2022, 204, 112233. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Algonaiman, R.; Alduwayghiri, R.; Aljutaily, T.; Algheshairy, R.M.; Almutairi, A.S.; Alharbi, R.M.; Alfurayh, L.A.; Alshahwan, A.A.; Alsadun, A.F.; et al. Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15918. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Munoz, M.; Dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic beta-cell ion channel expression and activity and insulin release through an estrogen receptor ERbeta mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, J.; Li, Y.; Chen, J.; Zhou, Z.; Song, L.; Wei, Z.; Lv, Z.; Chen, X.; Xia, W.; et al. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E527–E538. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Hesselbarth, N.; Gericke, M.; Kunath, A.; Biemann, R.; Chakaroun, R.; Kosacka, J.; Kovacs, P.; Kern, M.; Stumvoll, M.; et al. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PLoS ONE 2015, 10, e0143190. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hao, Q.; Chen, C.; Li, J.; Han, X.; Lei, Z.; Wang, T.; Wang, Y.; You, X.; Chen, X.; et al. Epigenetic repression of miR-17 contributed to di(2-ethylhexyl) phthalate-triggered insulin resistance by targeting Keap1-Nrf2/miR-200a axis in skeletal muscle. Theranostics 2020, 10, 9230–9248. [Google Scholar] [CrossRef]
- Baralic, K.; Zivancevic, K.; Jorgovanovic, D.; Javorac, D.; Radovanovic, J.; Gojkovic, T.; Buha Djordjevic, A.; Curcic, M.; Mandinic, Z.; Bulat, Z.; et al. Probiotic reduced the impact of phthalates and bisphenol A mixture on type 2 diabetes mellitus development: Merging bioinformatics with in vivo analysis. Food Chem. Toxicol. 2021, 154, 112325. [Google Scholar] [CrossRef]
- Castriota, F.; Rieswijk, L.; Dahlberg, S.; La Merrill, M.A.; Steinmaus, C.; Smith, M.T.; Wang, J.C. A State-of-the-Science Review of Arsenic’s Effects on Glucose Homeostasis in Experimental Models. Environ. Health Perspect. 2020, 128, 16001. [Google Scholar] [CrossRef]
- Kirkley, A.G.; Carmean, C.M.; Ruiz, D.; Ye, H.; Regnier, S.M.; Poudel, A.; Hara, M.; Kamau, W.; Johnson, D.N.; Roberts, A.A.; et al. Arsenic exposure induces glucose intolerance and alters global energy metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hodjat, M.; Rahimifard, M.; Nigjeh, M.N.; Azizi, M.; Baeeri, M.; Bayrami, Z.; Gholami, M.; Hassani, S.; Abdollahi, M. Assessment of arsenic-induced modifications in the DNA methylation of insulin-related genes in rat pancreatic islets. Ecotoxicol. Environ. Saf. 2020, 201, 110802. [Google Scholar] [CrossRef] [PubMed]
- Xenakis, J.G.; Douillet, C.; Bell, T.A.; Hock, P.; Farrington, J.; Liu, T.; Murphy, C.E.Y.; Saraswatula, A.; Shaw, G.D.; Nativio, G.; et al. An interaction of inorganic arsenic exposure with body weight and composition on type 2 diabetes indicators in Diversity Outbred mice. Mamm. Genome 2022, 33, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef]
- Kuiper, J.; Moran, M.; Cetkovic-Cvrlje, M. Exposure to polychlorinated biphenyl-153 decreases incidence of autoimmune Type 1 diabetes in non-obese diabetic mice. J. Immunotoxicol. 2016, 13, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Daniels, J.L. Environmental contaminants as etiologic factors for diabetes. Environ. Health Perspect. 2001, 109 (Suppl. S6), 871–876. [Google Scholar] [CrossRef] [PubMed]
- Langer, P.; Tajtakova, M.; Guretzki, H.J.; Kocan, A.; Petrik, J.; Chovancova, J.; Drobna, B.; Jursa, S.; Pavuk, M.; Trnovec, T.; et al. High prevalence of anti-glutamic acid decarboxylase (anti-GAD) antibodies in employees at a polychlorinated biphenyl production factory. Arch. Environ. Health 2002, 57, 412–415. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Elfving, M.; Ivarsson, S.A.; Lindh, C.; Jonsson, B.A.; Olofsson, P.; Rylander, L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants in relation to risk of type 1 diabetes. PLoS ONE 2010, 5, e11281. [Google Scholar] [CrossRef]
- Bodin, J.; Groeng, E.C.; Andreassen, M.; Dirven, H.; Nygaard, U.C. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicol. Rep. 2016, 3, 664–672. [Google Scholar] [CrossRef]
- Cetkovic-Cvrlje, M.; Olson, M.; Schindler, B.; Gong, H.K. Exposure to DDT metabolite p,p′-DDE increases autoimmune type 1 diabetes incidence in NOD mouse model. J. Immunotoxicol. 2016, 13, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.D.; Ramachandran, M.; Hussain, Q.Z. Sub-chronic effect of DDT on humoral immune response in mice. Bull. Environ. Contam Toxicol. 1986, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kerkvliet, N.I.; Steppan, L.B.; Vorachek, W.; Oda, S.; Farrer, D.; Wong, C.P.; Pham, D.; Mourich, D.V. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 2009, 1, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Bodin, J.; Kocbach Bolling, A.; Wendt, A.; Eliasson, L.; Becher, R.; Kuper, F.; Lovik, M.; Nygaard, U.C. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol. Rep. 2015, 2, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Guo, T.L. Bisphenol S Modulates Type 1 Diabetes Development in Non-Obese Diabetic (NOD) Mice with Diet- and Sex-Related Effects. Toxics 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Nagy, T.; Guo, T.L. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch. Toxicol. 2019, 93, 1083–1093. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L. Transgenerational pancreatic impairment with Igf2/H19 epigenetic alteration induced by p,p′-DDE exposure in early life. Toxicol. Lett. 2017, 280, 222–231. [Google Scholar] [CrossRef]
- Robles-Matos, N.; Artis, T.; Simmons, R.A.; Bartolomei, M.S. Environmental Exposure to Endocrine Disrupting Chemicals Influences Genomic Imprinting, Growth, and Metabolism. Genes 2021, 12, 1153. [Google Scholar] [CrossRef]
- Sargis, R.M.; Simmons, R.A. Environmental neglect: Endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 2019, 62, 1811–1822. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Rivera, F.J.; Guerrero-Bosagna, C. Bisphenol-A and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ. Epigenet 2016, 2, dvw022. [Google Scholar] [CrossRef]
- Bansal, A.; Rashid, C.; Xin, F.; Li, C.; Polyak, E.; Duemler, A.; van der Meer, T.; Stefaniak, M.; Wajid, S.; Doliba, N.; et al. Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring. Environ. Health Perspect. 2017, 125, 097022. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, L.; Wu, J.; Ke, S.; Morpurgo, B.; Golovko, A.; Ouyang, N.; Sun, Y.; Guo, S.; Tian, Y. A Murine Pancreatic Islet Cell-based Screening for Diabetogenic Environmental Chemicals. J. Vis. Exp. 2018, 136, e57327. [Google Scholar] [CrossRef]
- Chen, A.C.; Lee, K.F.; Yeung, W.S.B.; Lee, Y.L. Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes. World J. Stem Cells 2020, 12, 761–775. [Google Scholar] [CrossRef]
- Al-Abdulla, R.; Ferrero, H.; Soriano, S.; Boronat-Belda, T.; Alonso-Magdalena, P. Screening of Relevant Metabolism-Disrupting Chemicals on Pancreatic beta-Cells: Evaluation of Murine and Human In Vitro Models. Int. J. Mol. Sci. 2022, 23, 4182. [Google Scholar] [CrossRef]
- Fischer, L.J.; Wagner, M.A.; Madhukar, B.V. Potential involvement of calcium, CaM kinase II, and MAP kinases in PCB-stimulated insulin release from RINm5F cells. Toxicol. Appl. Pharmacol. 1999, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.J.; Zhou, H.R.; Wagner, M.A. Polychlorinated biphenyls release insulin from RINm5F cells. Life Sci. 1996, 59, 2041–2049. [Google Scholar] [CrossRef]
- Lee, Y.M.; Ha, C.M.; Kim, S.A.; Thoudam, T.; Yoon, Y.R.; Kim, D.J.; Kim, H.C.; Moon, H.B.; Park, S.; Lee, I.K.; et al. Low-Dose Persistent Organic Pollutants Impair Insulin Secretory Function of Pancreatic beta-Cells: Human and In Vitro Evidence. Diabetes 2017, 66, 2669–2680. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, K.T.; Rhyu, D.Y. Low-concentration exposure to organochlorine pesticides (OCPs) in L6 myotubes and RIN-m5F pancreatic beta cells induces disorders of glucose metabolism. Toxicol. In Vitro 2020, 65, 104767. [Google Scholar] [CrossRef]
- Singh, V.K.; Sarkar, S.K.; Saxena, A.; Koner, B.C. Effect of Subtoxic DDT Exposure on Glucose Uptake and Insulin Signaling in Rat L6 Myoblast-Derived Myotubes. Int. J. Toxicol. 2019, 38, 303–311. [Google Scholar] [CrossRef]
- Pavlikova, N.; Sramek, J.; Jelinek, M.; Halada, P.; Kovar, J. Markers of acute toxicity of DDT exposure in pancreatic beta-cells determined by a proteomic approach. PLoS ONE 2020, 15, e0229430. [Google Scholar] [CrossRef]
- Ward, A.B.; Dail, M.B.; Chambers, J.E. In vitro effect of DDE exposure on the regulation of B-TC-6 pancreatic beta cell insulin secretion: A potential role in beta cell dysfunction and type 2 diabetes mellitus. Toxicol. Mech. Methods 2021, 31, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; Piaggi, S.; De Tata, V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol. Lett. 2005, 156, 307–314. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Shim, Y.J.; Shin, Y.J.; Sul, D.; Lee, E.; Min, B.H. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces calcium influx through T-type calcium channel and enhances lysosomal exocytosis and insulin secretion in INS-1 cells. Int. J. Toxicol. 2009, 28, 151–161. [Google Scholar] [CrossRef]
- Novelli, M.; Beffy, P.; Masini, M.; Vantaggiato, C.; Martino, L.; Marselli, L.; Marchetti, P.; De Tata, V. Selective beta-cell toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin on isolated pancreatic islets. Chemosphere 2021, 265, 129103. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Matsumura, F. TCDD suppresses insulin-responsive glucose transporter (GLUT-4) gene expression through C/EBP nuclear transcription factors in 3T3-L1 adipocytes. J. Biochem. Mol. Toxicol. 2006, 20, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Vogel, C.F.; Matsumura, F. Studies on the cell treatment conditions to elicit lipolytic responses from 3T3-L1 adipocytes to TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Cell Biochem. 2007, 102, 389–402. [Google Scholar] [CrossRef]
- Nishiumi, S.; Yoshida, M.; Azuma, T.; Yoshida, K.; Ashida, H. 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs an insulin signaling pathway through the induction of tumor necrosis factor-alpha in adipocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 115, 482–491. [Google Scholar] [CrossRef]
- Kim, M.J.; Pelloux, V.; Guyot, E.; Tordjman, J.; Bui, L.C.; Chevallier, A.; Forest, C.; Benelli, C.; Clement, K.; Barouki, R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012, 120, 508–514. [Google Scholar] [CrossRef]
- Arsenescu, V.; Arsenescu, R.I.; King, V.; Swanson, H.; Cassis, L.A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008, 116, 761–768. [Google Scholar] [CrossRef]
- Qin, W.P.; Cao, L.Y.; Li, C.H.; Guo, L.H.; Colbourne, J.; Ren, X.M. Perfluoroalkyl Substances Stimulate Insulin Secretion by Islet beta Cells via G Protein-Coupled Receptor 40. Environ. Sci. Technol. 2020, 54, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, D.; Xu, Y.; Zhang, Y.; Sun, Y.; Chang, X.; Zhu, Y.; Tang, W. Perfluorooctanoic acid promotes pancreatic beta cell dysfunction and apoptosis through ER stress and the ATF4/CHOP/TRIB3 pathway. Environ. Sci. Pollut. Res. Int. 2022, 29, 84532–84545. [Google Scholar] [CrossRef] [PubMed]
- Bassler, J.; Ducatman, A.; Elliott, M.; Wen, S.; Wahlang, B.; Barnett, J.; Cave, M.C. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ. Pollut. 2019, 247, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.; Rock, S.; Stratakis, N.; Eckel, S.P.; Walker, D.I.; Valvi, D.; Cserbik, D.; Jenkins, T.; Xanthakos, S.A.; Kohli, R.; et al. Exposure to per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2022, 130, 46001. [Google Scholar] [CrossRef]
- Attema, B.; Janssen, A.W.F.; Rijkers, D.; van Schothorst, E.M.; Hooiveld, G.; Kersten, S. Exposure to low-dose perfluorooctanoic acid promotes hepatic steatosis and disrupts the hepatic transcriptome in mice. Mol. Metab. 2022, 66, 101602. [Google Scholar] [CrossRef]
- Qi, Q.; Niture, S.; Gadi, S.; Arthur, E.; Moore, J.; Levine, K.E.; Kumar, D. Per- and polyfluoroalkyl substances activate UPR pathway, induce steatosis and fibrosis in liver cells. Environ. Toxicol. 2023, 38, 225–242. [Google Scholar] [CrossRef]
- Park, C.; Song, H.; Choi, J.; Sim, S.; Kojima, H.; Park, J.; Iida, M.; Lee, Y. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020, 260, 114036. [Google Scholar] [CrossRef]
- Ferreira Azevedo, L.; Masiero, M.M.; Cherkaoui, S.; Hornos Carneiro, M.F.; Barbosa, F., Jr.; Zamboni, N. The alternative analog plasticizer BPS displays similar phenotypic and metabolomic responses to BPA in HepG2 and INS-1E cells. Food Chem. Toxicol. 2022, 167, 113266. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Zhang, J.; Wang, K.; De, X.; Li, L.; Zhang, Y. Typical phthalic acid esters induce apoptosis by regulating the PI3K/Akt/Bcl-2 signaling pathway in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2021, 208, 111461. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, C.; Yao, X.; Han, Q.; Wang, N.; Yuan, W.; Zhang, J.; Shi, Y.; Jiang, L.; Liu, X.; et al. AS3MT facilitates NLRP3 inflammasome activation by m(6)A modification during arsenic-induced hepatic insulin resistance. Cell Biol. Toxicol. 2022, 1–17. [Google Scholar] [CrossRef]
- Todero, J.E.; Koch-Laskowski, K.; Shi, Q.; Kanke, M.; Hung, Y.H.; Beck, R.; Styblo, M.; Sethupathy, P. Candidate master microRNA regulator of arsenic-induced pancreatic beta cell impairment revealed by multi-omics analysis. Arch. Toxicol. 2022, 96, 1685–1699. [Google Scholar] [CrossRef]
- Meleleo, D.; Gerbino, A.; Mastrodonato, M. Evidence of the different effect of mercury and cadmium on the hIAPP aggregation process. Biophys. Chem. 2022, 290, 106880. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Rood, B.; Ribble, A.; Haberzettl, P. Fine particulate matter (PM2.5) inhalation-induced alterations in the plasma lipidome as promoters of vascular inflammation and insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1836–H1850. [Google Scholar] [CrossRef] [PubMed]
| EDCs | In Humans | In Vivo | In Vitro | |
|---|---|---|---|---|
| Non persistent | BPA | Strong (T2D)/Low (T1D) | Strong (T2D)/Controversial (F1/F2) | Strong |
| BPA substitutes | Limited data | Limited data | Limited data | |
| Phthalates/DEHP | Controversial (T2D)/Strong (GDM)/Strong (Obesity) | Low (T2D)/Strong (Obesity) | Strong | |
| Arsenic | Low (T2D) | Strong (T2D) | Strong | |
| Persistent | PCBs | Strong (T2D)/Suggestive (T1D)/Strong (GDM) | Strong (T2D)/Strong (Obesity) | Strong |
| PFOA | Low (T2D)/Strong (GDM) | Low (T2D) | Low | |
| TCDD | Strong (T2D) | Strong (T2D) | Strong | |
| DDT/DDE | Strong (T2D)/Strong (Obesity) | Strong (F1/F2)/Strong (Obesity) | Strong | |
| TBT | Low (T2D, T1D)/Strong (Obesity) | Suggestive (T2D)/Strong (Obesity) | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinault, C.; Caroli-Bosc, P.; Bost, F.; Chevalier, N. Critical Overview on Endocrine Disruptors in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4537. https://doi.org/10.3390/ijms24054537
Hinault C, Caroli-Bosc P, Bost F, Chevalier N. Critical Overview on Endocrine Disruptors in Diabetes Mellitus. International Journal of Molecular Sciences. 2023; 24(5):4537. https://doi.org/10.3390/ijms24054537
Chicago/Turabian StyleHinault, Charlotte, Philippe Caroli-Bosc, Frédéric Bost, and Nicolas Chevalier. 2023. "Critical Overview on Endocrine Disruptors in Diabetes Mellitus" International Journal of Molecular Sciences 24, no. 5: 4537. https://doi.org/10.3390/ijms24054537
APA StyleHinault, C., Caroli-Bosc, P., Bost, F., & Chevalier, N. (2023). Critical Overview on Endocrine Disruptors in Diabetes Mellitus. International Journal of Molecular Sciences, 24(5), 4537. https://doi.org/10.3390/ijms24054537







