Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts
Abstract
:1. Introduction
2. Results
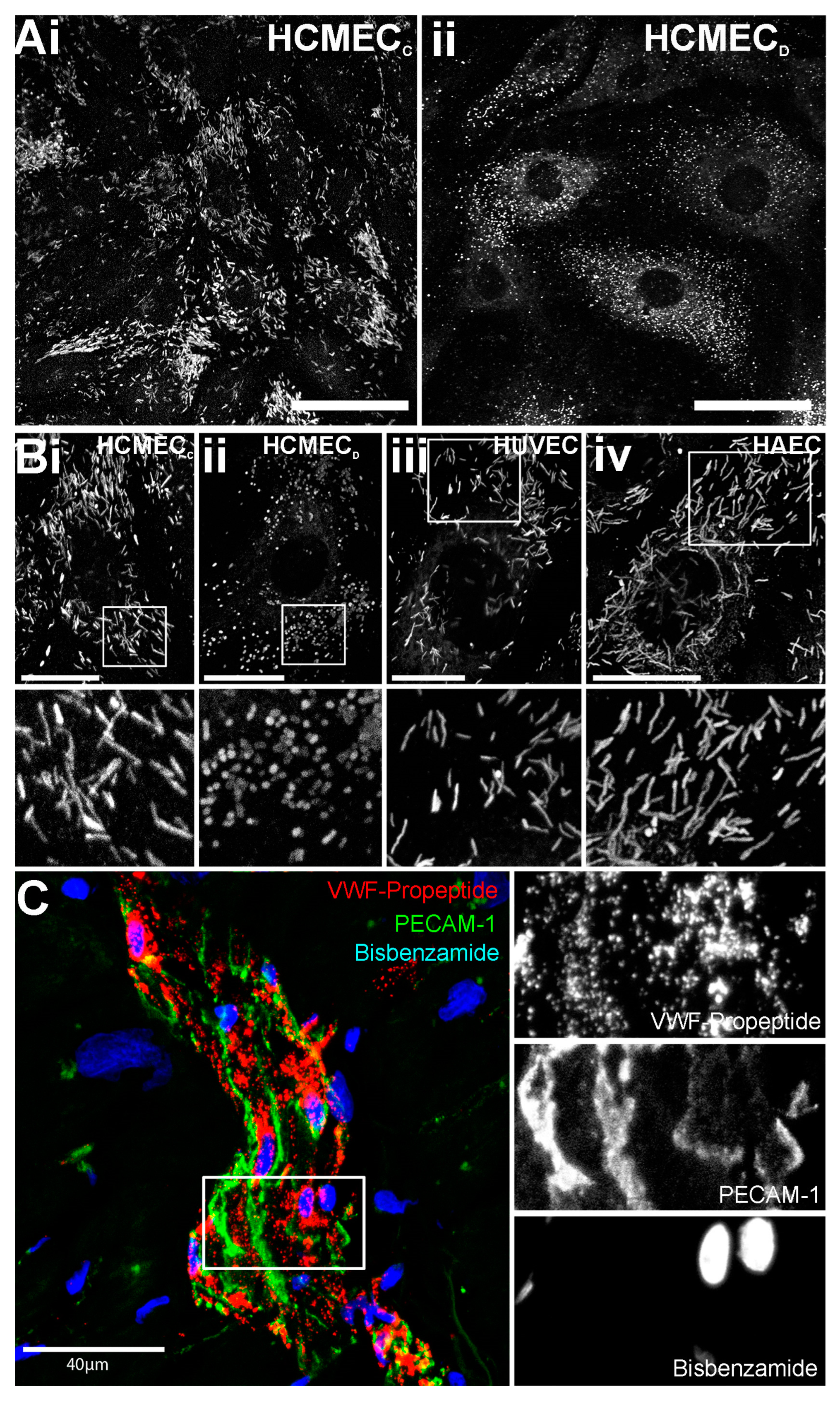
2.1. WPBs in HCMECD Are Rounded in Shape
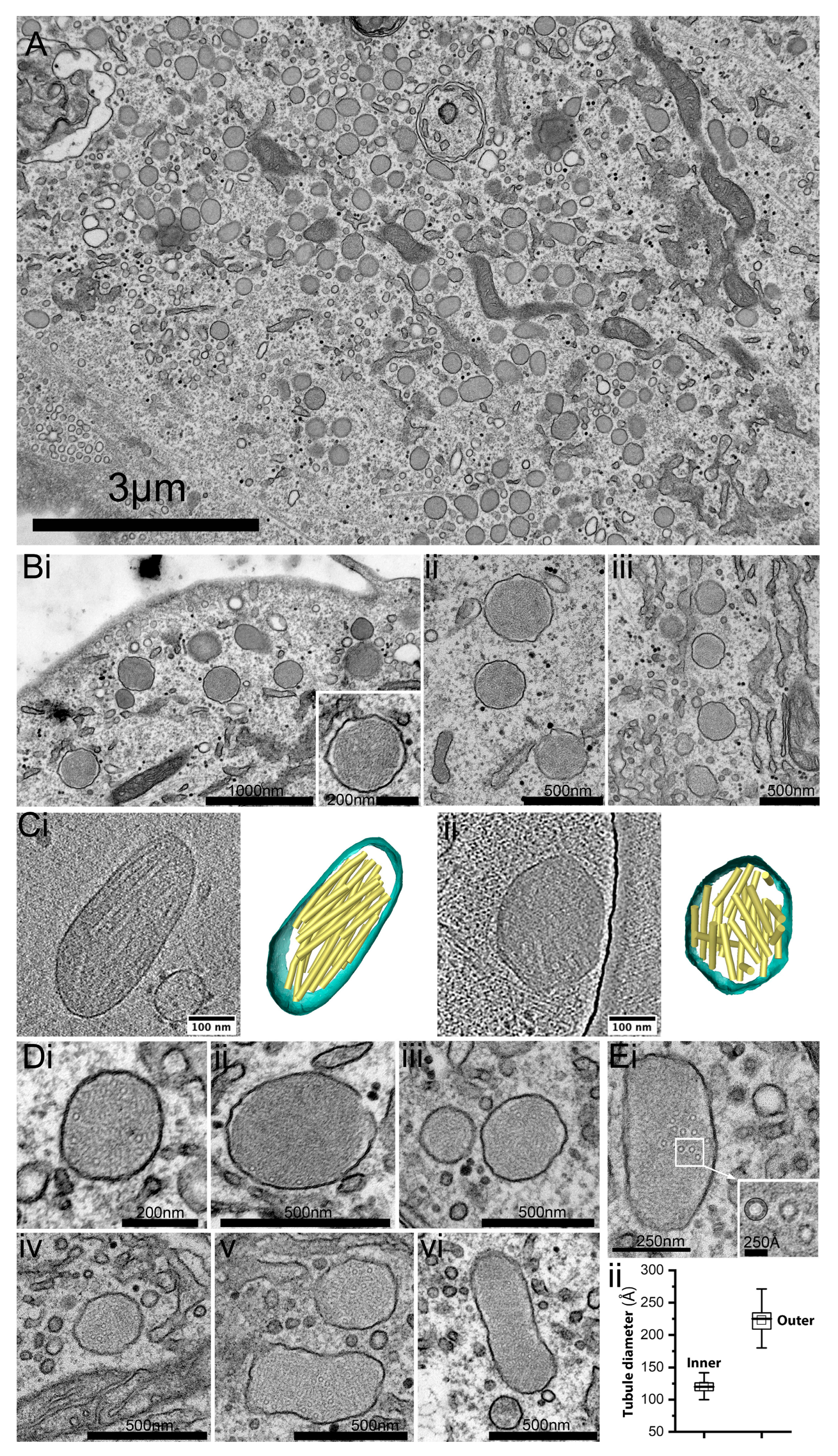
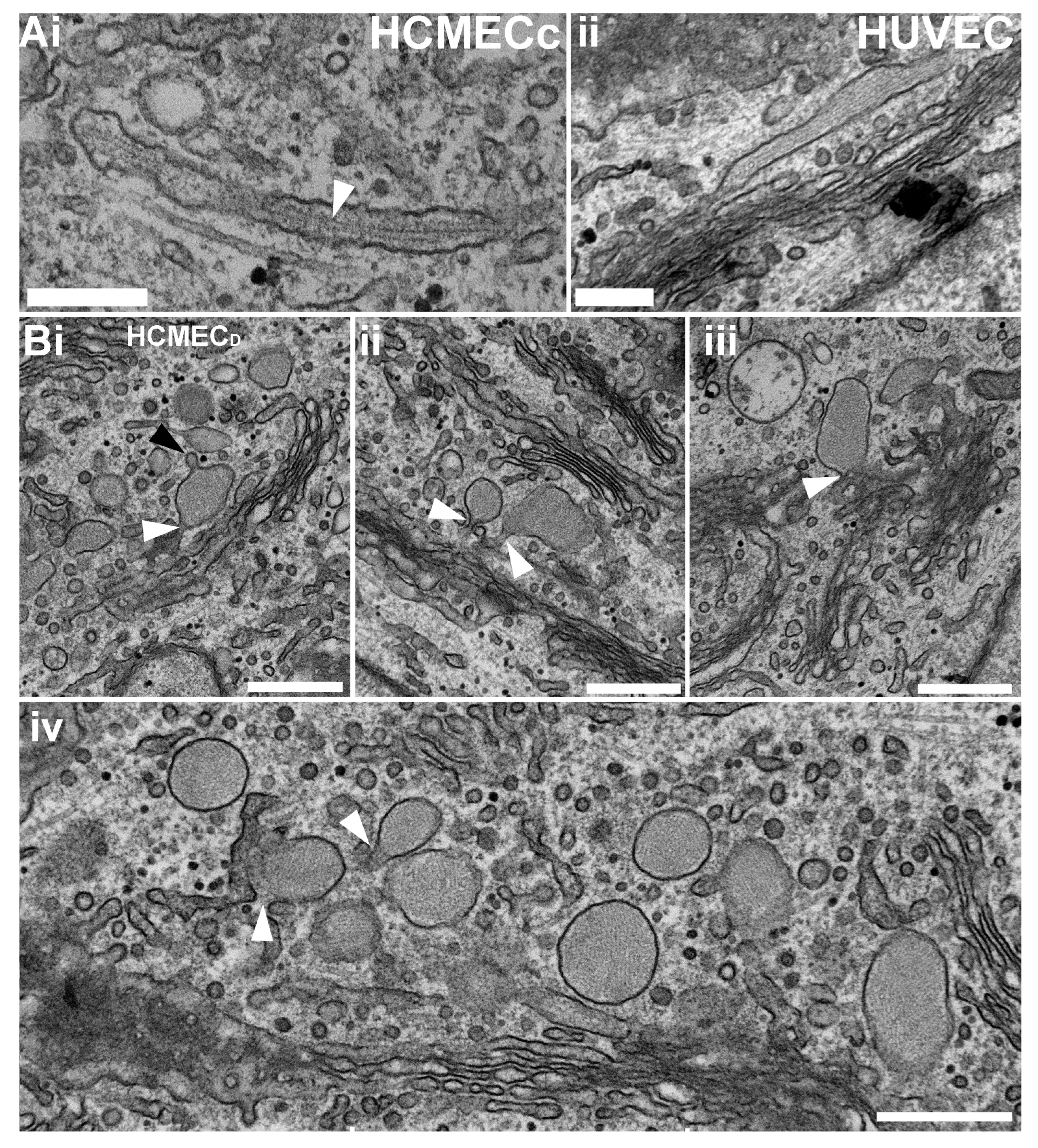
2.2. Ultrastructural Analysis of HCMECD WPBs Reveals Disordered VWF Tubules
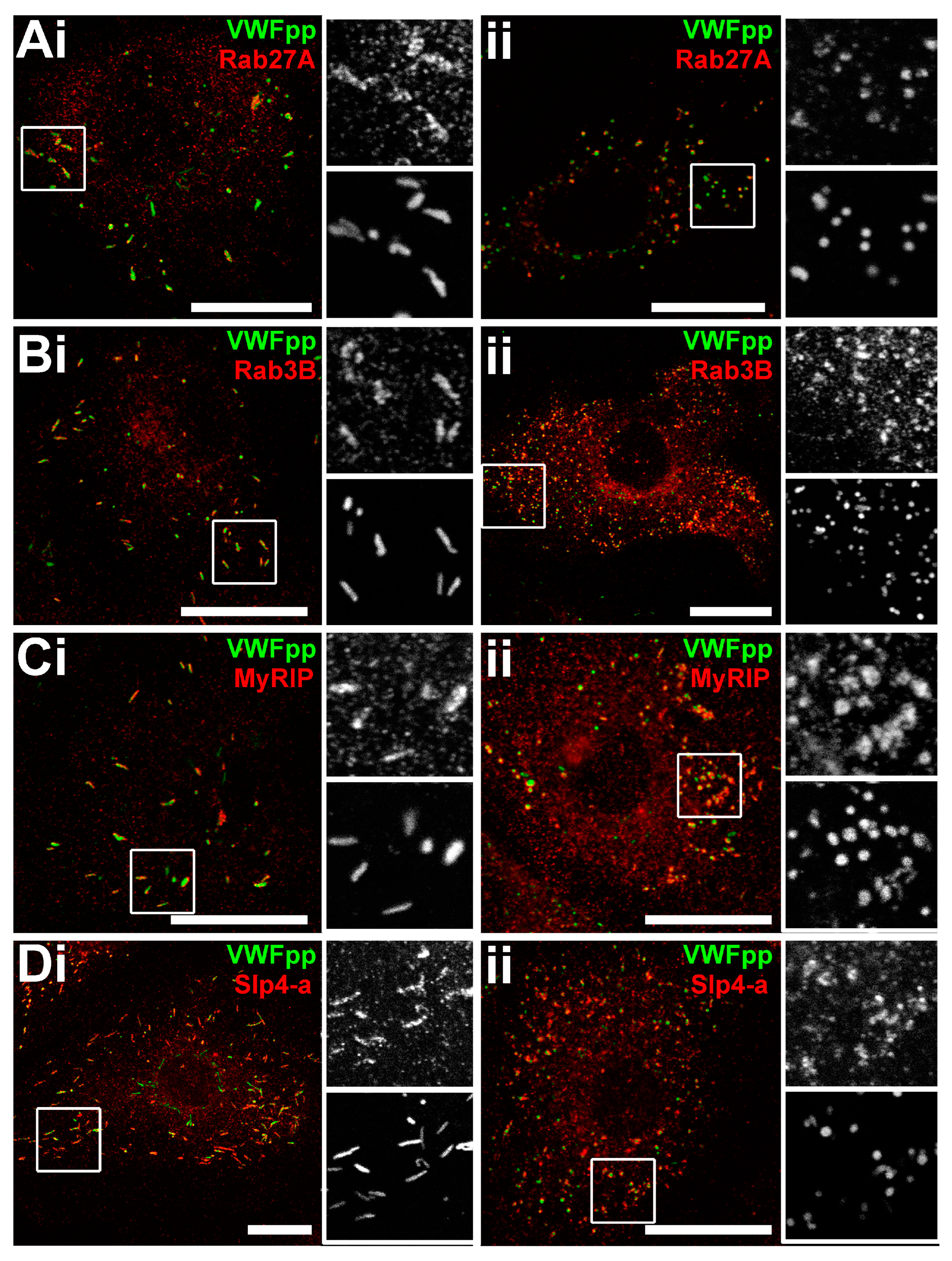
2.3. Rounded WPBs of HCMECD form at the Trans-Golgi Network with Disordered VWF Tubules
2.4. Rounded WPBs of HCMECD Are Smaller, by Volume, Than Rod-Shaped WPBs of HCMECC
2.5. WPBs in HCMECD Have a Slightly Elevated Luminal pH
2.6. The Kinetics of Histamine-Evoked WPB Exocytosis in HCMECD Are Similar to That in HCMECC
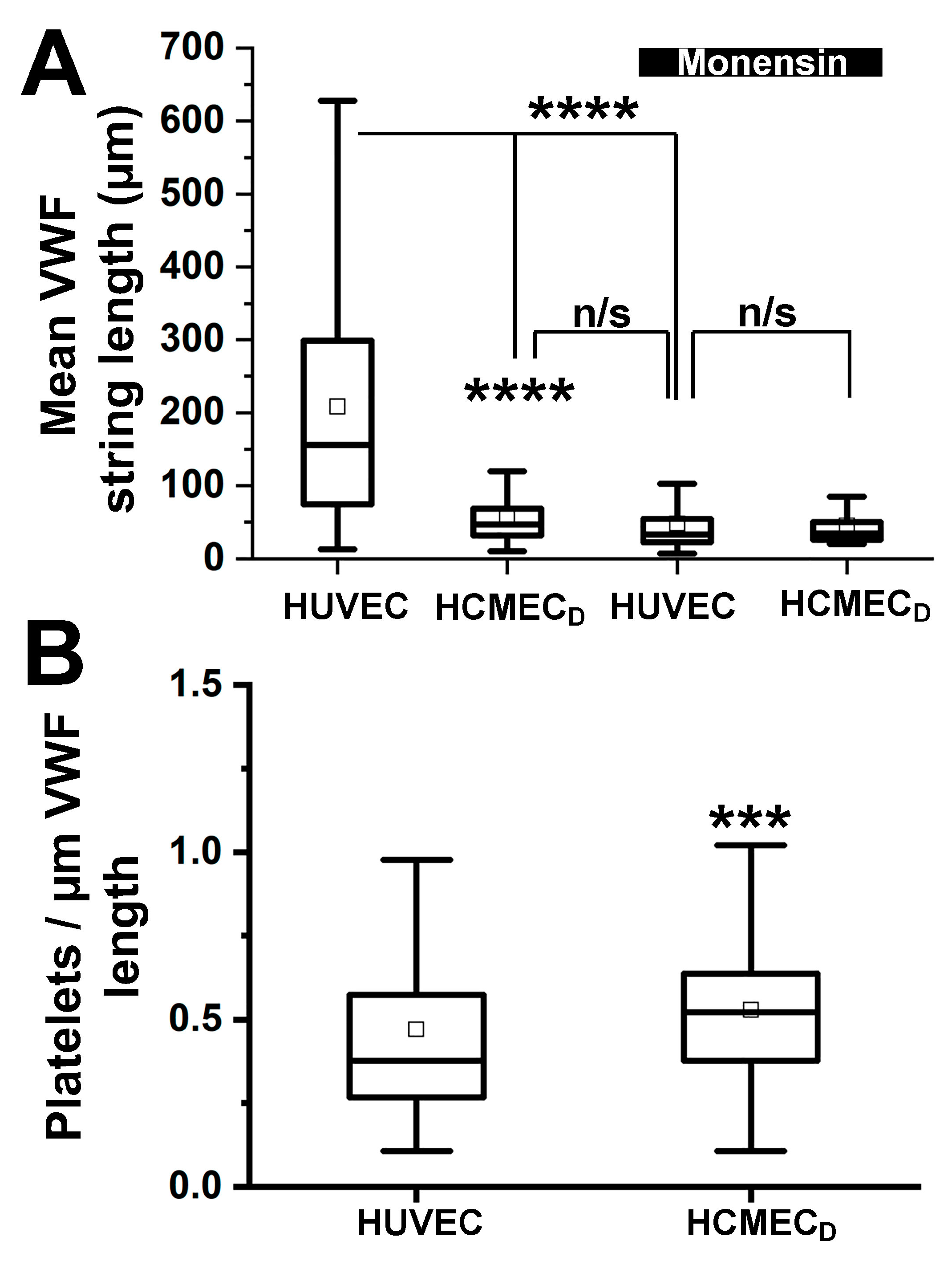
2.7. VWF Strings Secreted from HCMECD Are Short Compared to Those Secreted from HUVEC although Platelet Binding per Unit Length Is No Different
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Tissue Culture, Transfection and Immunocytochemistry
4.2. Transmission Electron Microscopy (TEM) and Electron Cryomicroscopy
4.3. Estimation of the Volume of Rounded and Rod-Shaped WPBs in HCMECD and HCMECC
4.4. Live Cell Imaging of WPB Exocytosis and Intra-WPB pH
4.5. VWF String Length and Platelet Binding Density
4.6. Ethical Considerations
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Premer, C.; Kanelidis, A.J.; Hare, J.M.; Schulman, I.H. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. In Mayo Clinic Proceedings: Innovations, Quality & Outcomes; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 3, pp. 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sena, C.M.; Leandro, A.; Azul, L.; Seica, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisslthaler, B.; Fleming, I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ. Res. 2009, 105, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Lopes-da-Silva, M.; McCormack, J.J.; Burden, J.J.; Harrison-Lavoie, K.J.; Ferraro, F.; Cutler, D.F. A GBF1-Dependent Mechanism for Environmentally Responsive Regulation of ER-Golgi Transport. Dev. Cell 2019, 49, 786–801 e786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, Z.M. Von Willebrand factor, platelets and endothelial cell interactions. J. Thromb. Haemost. 2003, 1, 1335–1342. [Google Scholar] [CrossRef]
- Karampini, E.; Bierings, R.; Voorberg, J. Orchestration of Primary Hemostasis by Platelet and Endothelial Lysosome-Related Organelles. Arterioscl. Throm. Vas. 2020, 40, 1441–1453. [Google Scholar] [CrossRef]
- Sanders, Y.V.; Eikenboom, J.; de Wee, E.M.; van der Bom, J.G.; Cnossen, M.H.; Degenaar-Dujardin, M.E.; Fijnvandraat, K.; Kamphuisen, P.W.; Laros-van Gorkom, B.A.; Meijer, K.; et al. Reduced prevalence of arterial thrombosis in von Willebrand disease. J. Thromb. Haemost. 2013, 11, 845–854. [Google Scholar] [CrossRef]
- van Schie, M.C.; de Maat, M.P.; Isaacs, A.; van Duijn, C.M.; Deckers, J.W.; Dippel, D.W.; Leebeek, F.W. Variation in the von Willebrand factor gene is associated with von Willebrand factor levels and with the risk for cardiovascular disease. Blood 2011, 117, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whincup, P.H.; Danesh, J.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Rumley, A.; Lowe, G.D. von Willebrand factor and coronary heart disease: Prospective study and meta-analysis. Eur. Heart J. 2002, 23, 1764–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieberdink, R.G.; van Schie, M.C.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; de Maat, M.P.; Leebeek, F.W.; Breteler, M.M. High von Willebrand factor levels increase the risk of stroke: The Rotterdam study. Stroke 2010, 41, 2151–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.G.; Kienast, J.; Pyke, S.D.; Haverkate, F.; van de Loo, J.C. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N. Engl. J. Med. 1995, 332, 635–641. [Google Scholar] [CrossRef]
- Huang, R.H.; Wang, Y.; Roth, R.; Yu, X.; Purvis, A.R.; Heuser, J.E.; Egelman, E.H.; Sadler, J.E. Assembly of Weibel-Palade body-like tubules from N-terminal domains of von Willebrand factor. Proc. Natl. Acad. Sci. USA 2008, 105, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Berriman, J.A.; Li, S.; Hewlett, L.J.; Wasilewski, S.; Kiskin, F.N.; Carter, T.; Hannah, M.J.; Rosenthal, P.B. Structural organization of Weibel-Palade bodies revealed by cryo-EM of vitrified endothelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 17407–17412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014, 124, 1412–1425. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.F.; Eng, E.T.; Nishida, N.; Lu, C.; Walz, T.; Springer, T.A. A pH-regulated dimeric bouquet in the structure of von Willebrand factor. EMBO J. 2011, 30, 4098–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.R.; Li, J.; Springer, T.A.; Brown, A. Structures of VWF tubules before and after concatemerization reveal a mechanism of disulfide bond exchange. Blood 2022, 140, 1419–1430. [Google Scholar] [CrossRef]
- Javitt, G.; Yeshaya, N.; Khmelnitsky, L.; Fass, D. Assembly of von Willebrand factor tubules with in vivo helical parameters requires A1 domain insertion. Blood 2022, 140, 2835–2843. [Google Scholar] [CrossRef]
- Dong, J.F.; Moake, J.L.; Nolasco, L.; Bernardo, A.; Arceneaux, W.; Shrimpton, C.N.; Schade, A.J.; McIntire, L.V.; Fujikawa, K.; Lopez, J.A. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002, 100, 4033–4039. [Google Scholar] [CrossRef] [Green Version]
- Michaux, G.; Abbitt, K.B.; Collinson, L.M.; Haberichter, S.L.; Norman, K.E.; Cutler, D.F. The physiological function of von Willebrand’s factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev. Cell 2006, 10, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, F.; Patella, F.; Costa, J.R.; Ketteler, R.; Kriston-Vizi, J.; Cutler, D.F. Modulation of endothelial organelle size as an antithrombotic strategy. J. Thromb. Haemost. 2020, 18, 3296–3308. [Google Scholar] [CrossRef]
- Patella, F.; Vendramin, C.; Charles, O.; Scully, M.A.; Cutler, D.F. Shrinking Weibel-Palade bodies prevents high platelet recruitment in assays using thrombotic thrombocytopenic purpura plasma. Res. Pract. Thromb. Haemost. 2021, 5, e12626. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.; da Mafalda Lopes, S.; Grimes, W.; Lee, H.K.; Ketteler, R.; Kriston-Vizi, J.; Cutler, D.F. Weibel-Palade body size modulates the adhesive activity of its von Willebrand Factor cargo in cultured endothelial cells. Sci. Rep. 2016, 6, 32473. [Google Scholar] [CrossRef] [PubMed]
- Kat, M.; Karampini, E.; Hoogendijk, A.J.; Bürgisser, P.E.; Mulder, A.A.; Van Alphen, F.P.J.; Olins, J.; Geerts, D.; Van den Biggelaar, M.; Margadant, C.; et al. Syntaxin 5 determines Weibel-Palade body size and von Willebrand factor secretion by controlling Golgi architecture. Haematologica 2022, 107, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Kat, M.; Margadant, C.; Voorberg, J.; Bierings, R. Dispatch and delivery at the ER-Golgi interface: How endothelial cells tune their hemostatic response. FEBS J. 2022, 289, 6863–6870. [Google Scholar] [CrossRef]
- Tsutsui, H. Mitochondrial oxidative stress and heart failure. Intern. Med. 2006, 45, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewlett, L.; Zupančič, G.; Mashanov, G.; Knipe, L.; Ogden, D.; Hannah, M.J.; Carter, T. Temperature-Dependence of Weibel-Palade Body Exocytosis and Cell Surface Dispersal of von Willebrand Factor and Its Propolypeptide. PLoS ONE 2011, 6, e27314. [Google Scholar] [CrossRef] [Green Version]
- Bierings, R.; Hellen, N.; Kiskin, N.; Knipe, L.; Fonseca, A.V.; Patel, B.; Meli, A.; Rose, M.; Hannah, M.J.; Carter, T. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood 2012, 120, 2757–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenner, H.L.; Collinson, L.M.; Michaux, G.; Cutler, D.F. High-pressure freezing provides insights into Weibel-Palade body biogenesis. J. Cell Sci. 2007, 120, 2117–2125. [Google Scholar] [CrossRef] [Green Version]
- Zupancic, G.; Ogden, D.; Magnus, C.J.; Wheeler-Jones, C.; Carter, T.D. Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. J. Physiol. 2002, 544, 741–755. [Google Scholar] [CrossRef]
- Zeng, J.; Shu, Z.; Liang, Q.; Zhang, J.; Wu, W.; Wang, X.; Zhou, A. Structural basis of von Willebrand factor multimerization and tubular storage. Blood 2022, 139, 3314–3324. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Eura, Y.; Kokame, K. V-ATPase V0a1 promotes Weibel–Palade body biogenesis through the regulation of membrane fission. eLife 2021, 10, e71526. [Google Scholar] [CrossRef] [PubMed]
- Terglane, J.; Menche, D.; Gerke, V. Acidification of endothelial Weibel-Palade bodies is mediated by the vacuolar-type H+-ATPase. PLoS ONE 2022, 17, e0270299. [Google Scholar] [CrossRef] [PubMed]
- Erent, M.; Meli, A.; Moisoi, N.; Babich, V.; Hannah, M.J.; Skehel, P.; Knipe, L.; Zupancic, G.; Ogden, D.; Carter, T. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J. Physiol. 2007, 583, 195–212. [Google Scholar] [CrossRef]
- Kiskin, N.I.; Hellen, N.; Babich, V.; Hewlett, L.; Knipe, L.; Hannah, M.J.; Carter, T. Protein mobilities and P-selectin storage in Weibel-Palade bodies. J. Cell Sci. 2010, 123, 2964–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannah, M.J.; Williams, R.; Kaur, J.; Hewlett, L.J.; Cutler, D.F. Biogenesis of Weibel-Palade bodies. Semin. Cell Dev. Biol. 2002, 13, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Rondaij, M.G.; Bierings, R.; Kragt, A.; van Mourik, J.A.; Voorberg, J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.S.C.; Matouk, C.C.; Marsden, P.A. Epigenetics of the vascular endothelium. J. Appl. Physiol. 2010, 109, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol.-Heart C 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Shen, Y.; Maurer, F.; Medcalf, R.L. Transcriptional regulation of the tissue-type plasminogen-activator gene in human endothelial cells: Identification of nuclear factors that recognise functional elements in the tissue-type plasminogen-activator gene promoter. Eur. J. Biochem. 1998, 258, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Dunoyer-Geindre, S.; Fish, R.J.; Kruithof, E.K.O. Regulation of the endothelial plasminogen activator system by fluvastatin Role of Rho family proteins, actin polymerisation and p38 MAP kinase. Thromb. Haemost. 2011, 105, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Keber, I.; Keber, D.; Stegnar, M.; Vene, N. Tissue plasminogen activator release in chronic venous hypertension due to heart failure. Thromb. Haemost. 1992, 68, 321–324. [Google Scholar] [CrossRef]
- van Agtmaal, E.L.; Bierings, R.; Dragt, B.S.; Leyen, T.A.; Fernandez-Borja, M.; Horrevoets, A.J.; Voorberg, J. The shear stress-induced transcription factor KLF2 affects dynamics and angiopoietin-2 content of Weibel-Palade bodies. PLoS ONE 2012, 7, e38399. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Yang, L.; Kriston-Vizi, J.; Cutler, D.F.; Li, W. BLOC-2 subunit HPS6 deficiency affects the tubulation and secretion of von Willebrand factor from mouse endothelial cells. J. Genet. Genom. 2016, 43, 686–693. [Google Scholar] [CrossRef]
- Karampini, E.; Burgisser, P.E.; Olins, J.; Mulder, A.A.; Jost, C.R.; Geerts, D.; Voorberg, J.; Bierings, R. Sec22b determines Weibel-Palade body length by controlling anterograde ER-Golgi transport. Haematologica 2021, 106, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.B.; Foster, P.A.; Kaufman, R.J.; Vokac, E.A.; Moussalli, M.; Kroner, P.A.; Montgomery, R.R. Intracellular trafficking of factor VIII to von Willebrand factor storage granules. J. Clin. Investig. 1998, 101, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Bouwens, E.A.M.; Mourik, M.J.; van den Biggelaar, M.; Eikenboom, J.C.J.; Voorberg, J.; Valentijn, K.M.; Mertens, K. Factor VIII alters tubular organization and functional properties of von Willebrand factor stored in Weibel-Palade bodies. Blood 2011, 118, 5947–5956. [Google Scholar] [CrossRef] [Green Version]
- van den Biggelaar, M.; Meijer, A.B.; Voorberg, J.; Mertens, K. Intracellular cotrafficking of factor VIII and von Willebrand factor type 2N variants to storage organelles. Blood 2009, 113, 3102–3109. [Google Scholar] [CrossRef] [Green Version]
- Holder, A.L.; Wolf, S.; Walshe, C.; Pandya, P.; Stanford, R.E.; Smith, J.D.; Rose, M.L.; Lawson, C. Expression of endothelial intercellular adhesion molecule-1 is determined by genotype: Effects on efficiency of leukocyte adhesion to human endothelial cells. Hum. Immunol. 2008, 69, 71–78. [Google Scholar] [CrossRef]
- McDouall, R.M.; Batten, P.; McCormack, A.; Yacoub, M.H.; Rose, M.L. MHC class II expression on human heart microvascular endothelial cells: Exquisite sensitivity to interferon-gamma and natural killer cells. Transplantation 1997, 64, 1175–1180. [Google Scholar] [CrossRef]
- Hannah, M.J.; Skehel, P.; Erent, M.; Knipe, L.; Ogden, D.; Carter, T. Differential kinetics of cell surface loss of von Willebrand factor and its propolypeptide after secretion from Weibel-Palade bodies in living human endothelial cells. J. Biol. Chem. 2005, 280, 22827–22830. [Google Scholar] [CrossRef] [Green Version]
- Mastronarde, D.N. Dual-axis tomography: An approach with alignment methods that preserve resolution. J. Struct. Biol. 1997, 120, 343–352. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, H.; Wang, H.; Tao, C.L.; Bi, G.Q.; Zhou, Z.H. Isotropic reconstruction for electron tomography with deep learning. Nat. Commun. 2022, 13, 6482. [Google Scholar] [CrossRef]
- Weibel, E.R. Practical methods for biological morphometry. In Stereologkal Methods; Academic Press Inc.: London, UK, 1979; Volume 1. [Google Scholar]
- Parsons, T.D.; Coorssen, J.R.; Horstmann, H.; Almers, W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron 1995, 15, 1085–1096. [Google Scholar] [CrossRef] [Green Version]
- Knipe, L.; Meli, A.; Hewlett, L.; Bierings, R.; Dempster, J.; Skehel, P.; Hannah, M.J.; Carter, T. A revised model for the secretion of tPA and cytokines from cultured endothelial cells. Blood 2010, 116, 2183–2191. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meli, A.; McCormack, A.; Conte, I.; Chen, Q.; Streetley, J.; Rose, M.L.; Bierings, R.; Hannah, M.J.; Molloy, J.E.; Rosenthal, P.B.; et al. Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts. Int. J. Mol. Sci. 2023, 24, 4553. https://doi.org/10.3390/ijms24054553
Meli A, McCormack A, Conte I, Chen Q, Streetley J, Rose ML, Bierings R, Hannah MJ, Molloy JE, Rosenthal PB, et al. Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts. International Journal of Molecular Sciences. 2023; 24(5):4553. https://doi.org/10.3390/ijms24054553
Chicago/Turabian StyleMeli, Athinoula, Ann McCormack, Ianina Conte, Qu Chen, James Streetley, Marlene L. Rose, Ruben Bierings, Matthew J. Hannah, Justin E. Molloy, Peter B. Rosenthal, and et al. 2023. "Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts" International Journal of Molecular Sciences 24, no. 5: 4553. https://doi.org/10.3390/ijms24054553
APA StyleMeli, A., McCormack, A., Conte, I., Chen, Q., Streetley, J., Rose, M. L., Bierings, R., Hannah, M. J., Molloy, J. E., Rosenthal, P. B., & Carter, T. (2023). Altered Storage and Function of von Willebrand Factor in Human Cardiac Microvascular Endothelial Cells Isolated from Recipient Transplant Hearts. International Journal of Molecular Sciences, 24(5), 4553. https://doi.org/10.3390/ijms24054553





