Molecular and Physiological Functions of PACAP in Sweat Secretion
Abstract
1. Introduction
2. Results
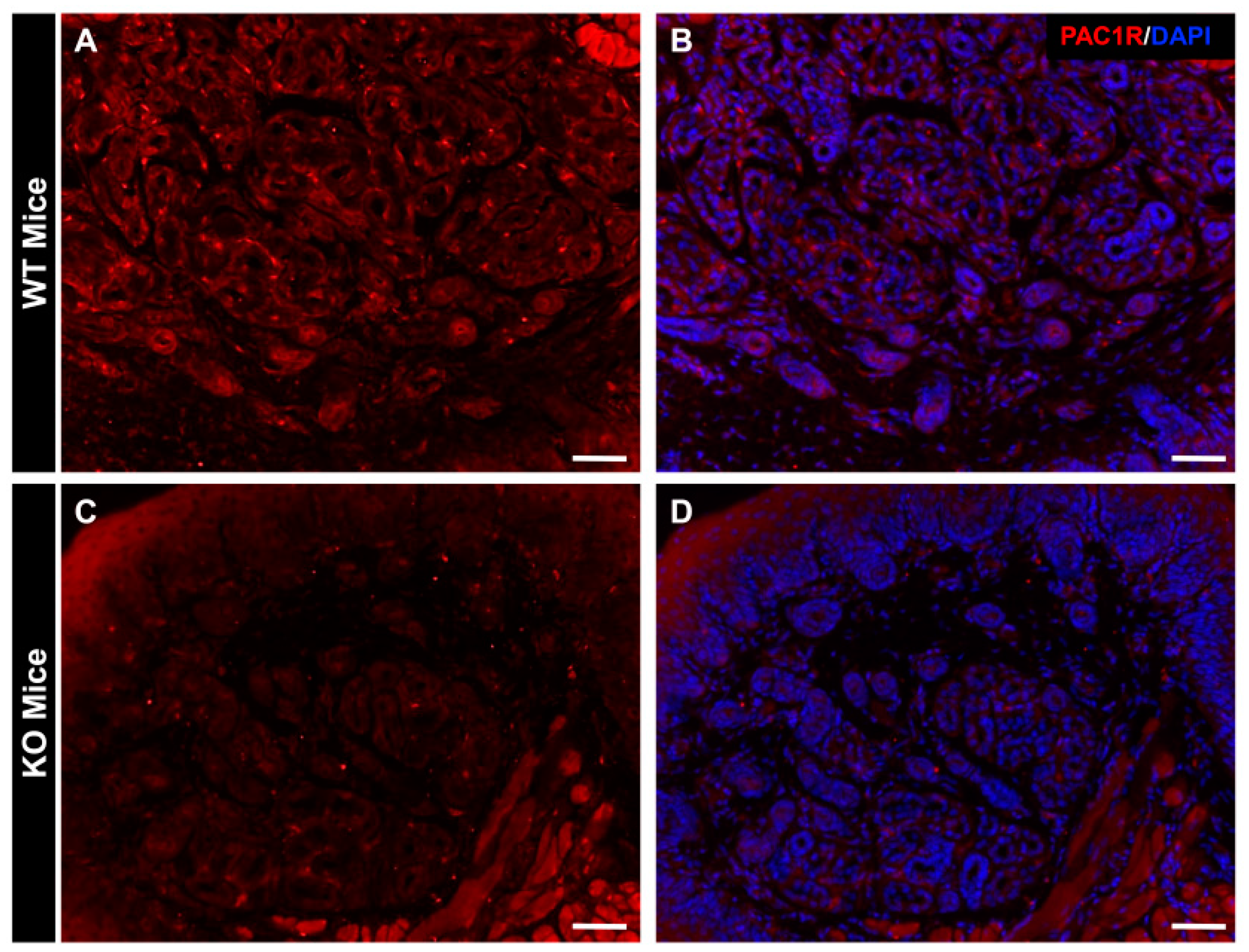
2.1. Distribution of PAC1R in Eccrine Gland in WT Mice and PAC1R KO Mice
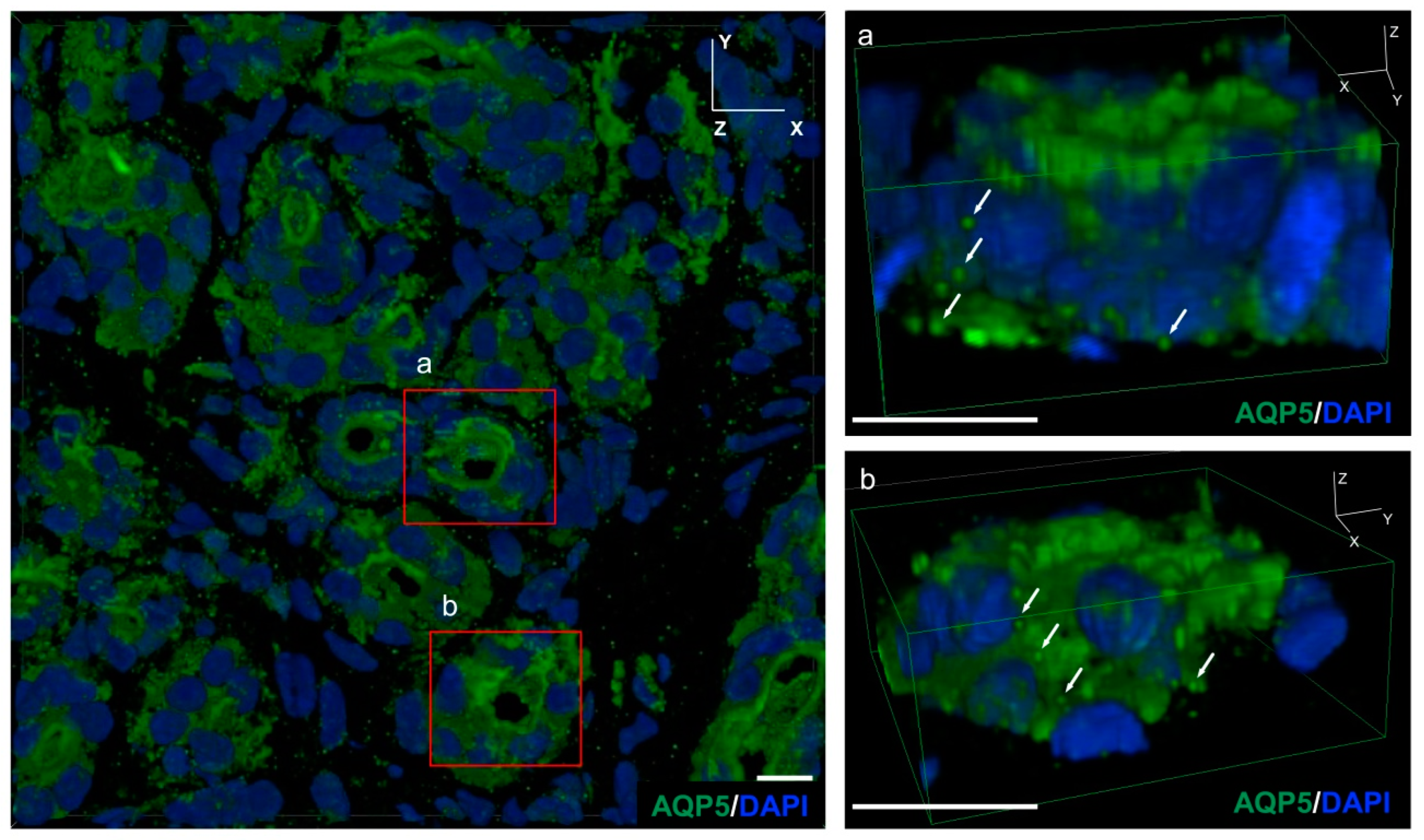
2.2. AQP5-Positive Vesicles Present in the Secretory Cells of the Mouse Eccrine Gland
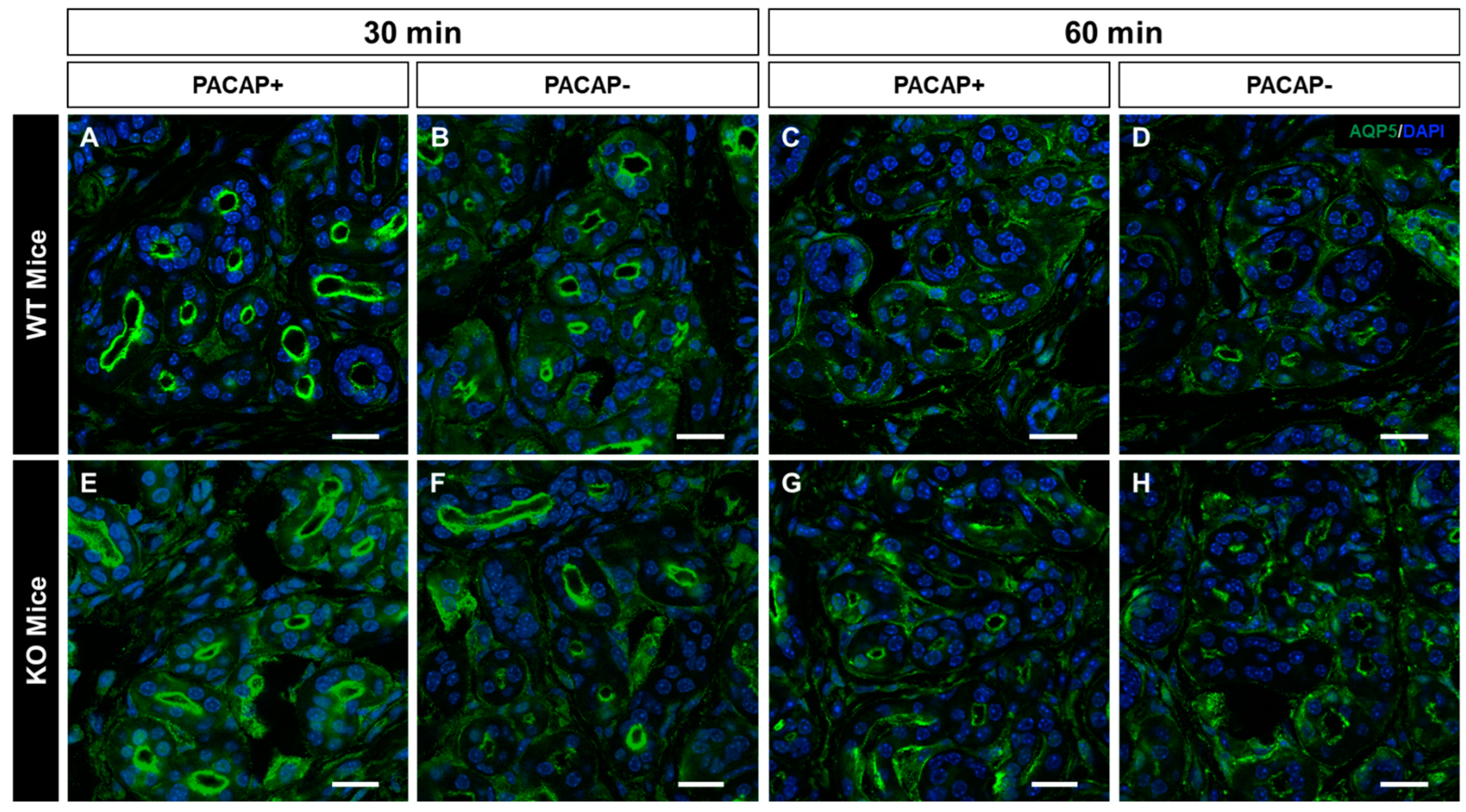
2.3. Changes in the Intracellular Localization of AQP5 Following Intradermal Injection of PACAP
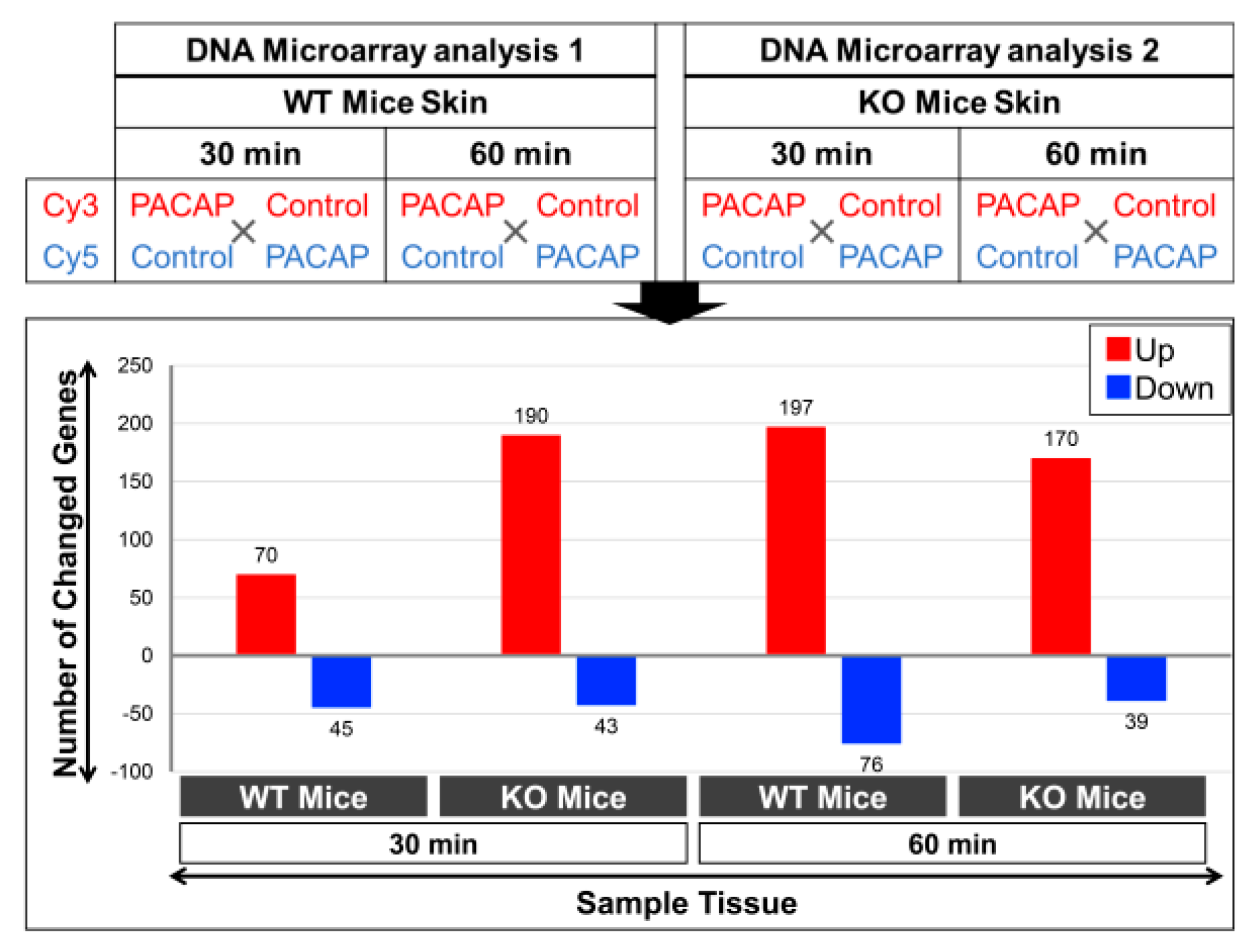
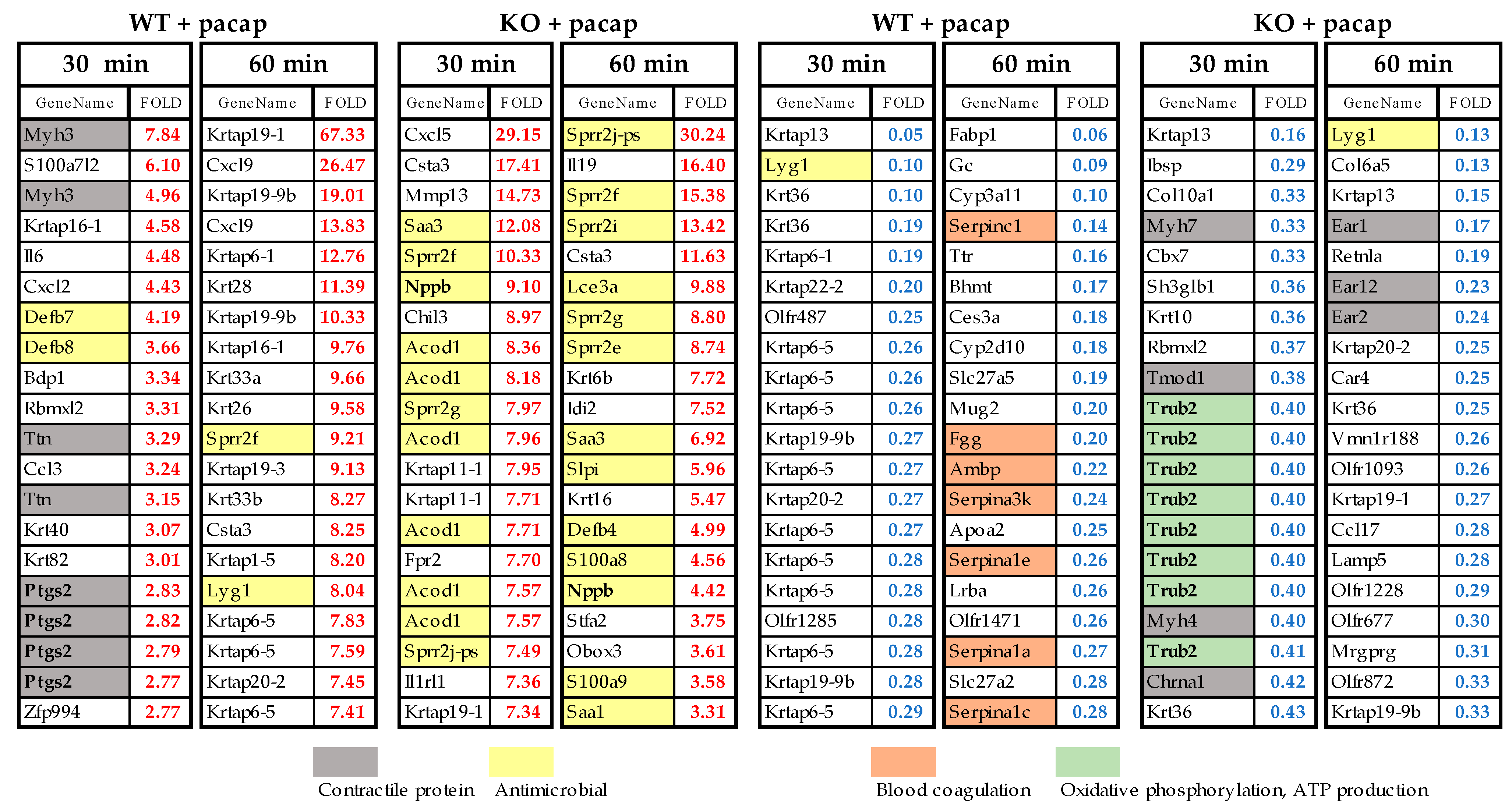
2.4. Changes in Gene Expression Levels in WT Mice and PAC1R KO Mice after Intradermal Administration of PACAP at 30 and 60 Minutes
2.5. Top 20 Genes Whose Expression Was Altered by PACAP in WT and PAC1R KO Mice
3. Discussion
3.1. PACAP Promotes Plasma Membrane Translocation of AQP5 via PAC1R
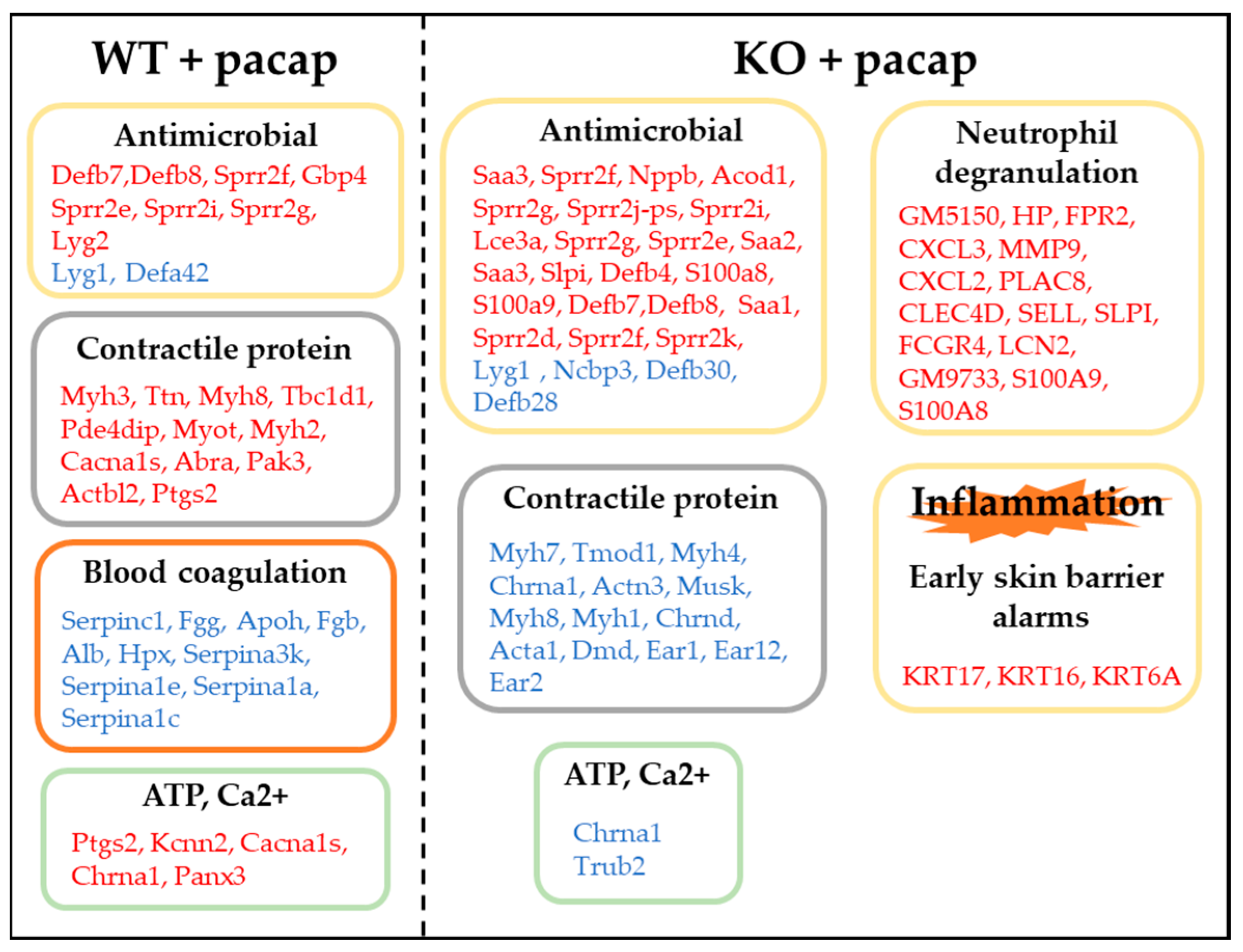
3.2. Results from the Whole Genome DNA Microarray Analysis
3.2.1. Expression of Genes Involved in Sweat Secretion
3.2.2. Expression of Genes Involved in Inflammation
3.2.3. Expression of Genes Involved in Contractile Proteins
3.2.4. Expression of Genes Involved in Ca2+·ATP
3.2.5. Expression of Genes Involved in Blood Coagulation
3.2.6. The TBC1D1 Gene
4. Material and Methods
4.1. Animals
4.2. Administration of PACAP to the Plantar Surface of Mice’s Forepaws
4.3. Histology
4.4. Determination of the Location of PAC1R in Mouse Skin
4.5. Confirming the Intracellular Location of AQP5 in Sweat Gland
4.6. Whole Genome DNA Microarray Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, S.M.; Warshaw, E.M. Dyshidrosis: Epidemiology, clinical characteristics, and therapy. Dermatitis 2006, 17, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Suma, A.; Murota, H.; Kitaba, S.; Yamaoka, T.; Kato, K.; Matsui, S.; Takahashi, A.; Yokomi, A.; Katayama, I. Idiopathic pure sudomotor failure responding to oral antihistamine with sweating activities. Acta Derm. Venereol. 2014, 94, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Obed, D.; Salim, M.; Bingoel, A.S.; Hofmann, T.R.; Vogt, P.M.; Krezdorn, N. Botulinum Toxin Versus Placebo: A meta-analysis of treatment and quality-of-life outcomes for hyperhidrosis. Aesthetic Plast. Surg. 2021, 45, 1783–1791. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, J.Y.; Suh, J.H.; Park, C.B.; Kwoun, H.; Park, S.S. Thoracoscopic sympathetic block to predict compensatory hyperhidrosis in primary hyperhidrosis. J. Thorac. Dis. 2021, 13, 3509–3517. [Google Scholar] [CrossRef]
- Wohlrab, J.; Bechara, F.G.; Schick, C.; Naumann, M. Hyperhidrosis: A central nervous dysfunction of sweat secretion. Dermatol. Ther. 2023, 13, 453–463. [Google Scholar] [CrossRef]
- Cruddas, L.; Baker, D.M. Treatment of primary hyperhidrosis with oral anticholinergic medications: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 952–963. [Google Scholar] [CrossRef]
- Shimizu, H.; Tamada, Y.; Shimizu, J.; Ohshima, Y.; Matsumoto, Y.; Sugenoya, J. Effectiveness of iontophoresis with alternating current (AC) in the treatment of patients with palmoplantar hyperhidrosis. J. Dermatol. 2003, 30, 444–449. [Google Scholar] [CrossRef]
- Hu, Y.; Converse, C.; Lyons, M.C.; Hsu, W.H. Neural control of sweat secretion: A review. Br. J. Dermatol. 2018, 178, 1246–1256. [Google Scholar] [CrossRef]
- Inoue, R. New findings on the mechanism of perspiration including aquaporin-5 water channel. Curr. Probl. Dermatol. 2016, 51, 11–21. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Tamas, A.; Reglodi, D. The neuroprotective and biomarker potential of PACAP in human traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 827. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Farkas, J.; Watanabe, J.; Ohtaki, H.; Dohi, K.; Arata, S.; Shioda, S. Role of PACAP in neural stem/progenitor cell and astrocyte--from neural development to neural repair. Curr. Pharm. Des. 2011, 17, 973–984. [Google Scholar] [CrossRef]
- Tsuchikawa, D.; Nakamachi, T.; Tsuchida, M.; Wada, Y.; Hori, M.; Farkas, J.; Yoshikawa, A.; Kagami, N.; Imai, N.; Shintani, N.; et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J. Mol. Neurosci. 2012, 48, 508–517. [Google Scholar] [CrossRef]
- Matoba, Y.; Nonaka, N.; Takagi, Y.; Imamura, E.; Narukawa, M.; Nakamachi, T.; Shioda, S.; Banks, W.A.; Nakamura, M. Pituitary adenylate cyclase-activating polypeptide enhances saliva secretion via direct binding to PACAP receptors of major salivary glands in mice. Anat. Rec. 2016, 299, 1293–1299. [Google Scholar] [CrossRef]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef]
- Sasaki, S.; Watanabe, J.; Ohtaki, H.; Matsumoto, M.; Murai, N.; Nakamachi, T.; Hannibal, J.; Fahrenkrug, J.; Hashimoto, H.; Watanabe, H.; et al. Pituitary adenylate cyclase-activating polypeptide promotes eccrine gland sweat secretion. Br. J. Dermatol. 2017, 176, 413–422. [Google Scholar] [CrossRef]
- Yamashita, M.; Takenoya, F.; Hirabayashi, T.; Shibato, J.; Rakwal, R.; Takasaki, I.; Harvey, B.J.; Chiba, Y.; Shioda, S. Effect of PACAP on sweat secretion by immortalized human sweat gland cells. Peptides 2021, 146, 170647. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, F. Effect of VIP on sweat secretion and cAMP accumulation in isolated simian eccrine glands. Am. J. Physiol. 1987, 253, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Nakahigashi, K.; Nomura, T.; Miyachi, Y.; Kabashima, K. Normal immunostaining pattern for aquaporin-5 in the lesions of palmoplantar hyperhidrosis. Case Rep. Dermatol. 2013, 5, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Oberg, F.; Sjohamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Tornroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; McGinn, R.; Halili, L.; Singh, M.S.; Kondo, N.; Kenny, G.P. Cutaneous vascular and sweating responses to intradermal administration of ATP: A role for nitric oxide synthase and cyclooxygenase? J. Physiol. 2015, 593, 2515–2525. [Google Scholar] [CrossRef]
- Cui, C.Y.; Sima, J.; Yin, M.; Michel, M.; Kunisada, M.; Schlessinger, D. Identification of potassium and chloride channels in eccrine sweat glands. J. Dermatol. Sci. 2016, 81, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Fujii, N.; Kenny, G.P.; Inoue, Y.; Kondo, N. Do nitric oxide synthase and cyclooxygenase contribute to sweating response during passive heating in endurance-trained athletes? Physiol. Rep. 2017, 5, e13403. [Google Scholar] [CrossRef]
- Fujii, N.; Kenny, G.P.; Amano, T.; Honda, Y.; Kondo, N.; Nishiyasu, T. Evidence for TRPV4 channel induced skin vasodilatation through NOS, COX, and KCa channel mechanisms with no effect on sweat rate in humans. Eur. J. Pharmacol. 2019, 858, 172462. [Google Scholar] [CrossRef] [PubMed]
- Mochel, F.; Rastetter, A.; Ceulemans, B.; Platzer, K.; Yang, S.; Shinde, D.N.; Helbig, K.L.; Lopergolo, D.; Mari, F.; Renieri, A.; et al. Variants in the SK2 channel gene (KCNN2) lead to dominant neurodevelopmental movement disorders. Brain 2020, 143, 3564–3573. [Google Scholar] [CrossRef]
- Lin, J.B.; Kang, M.Q.; Huang, L.P.; Zhuo, Y.; Li, X.; Lai, F.C. CHRNA1 promotes the pathogenesis of primary focal hyperhidrosis. Mol. Cell. Neurosci. 2021, 111, 103598. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, A.R.; Vaeth, M.; Wagner, L.E., 2nd; Eckstein, M.; Hecht, L.; Yang, J.; Crottes, D.; Seidl, M.; Shin, H.P.; Weidinger, C.; et al. Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J. Clin. Investig. 2016, 126, 4303–4318. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shah, S.; Du, X.; Zhang, H.; Gamper, N. Activation of Ca(2+) -activated Cl(-) channel ANO1 by localized Ca(2+) signals. J. Physiol. 2016, 594, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Sohara, E.; Rai, T.; Satoh, T.; Yokozeki, H.; Sasaki, S.; Uchida, S. Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. J. Dermatol. Sci. 2013, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Cagini, L.; Antognelli, C.; Puma, F.; Pacifico, E.; Talesa, V.N. TNF-α regulates natriuretic peptides and aquaporins in human bronchial epithelial cells BEAS-2B. Mediat. Inflamm. 2013, 2013, 159349. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, B. Non-transport functions of Aquaporins. Adv. Exp. Med. Biol. 2023, 1398, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Mentino, D.; De Marco, A.; Del Vecchio, C.; Garra, S.; Cazzato, G.; Foti, C.; Crovella, S.; Calamita, G. Aquaporins are one of the critical factors in the disruption of the skin barrier in inflammatory skin diseases. Int. J. Mol. Sci. 2022, 23, 4020. [Google Scholar] [CrossRef]
- Steinhoff, M.; McGregor, G.P.; Radleff-Schlimme, A.; Steinhoff, A.; Jarry, H.; Schmidt, W.E. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: Expression of PACAP-38 is increased in patients with psoriasis. Regul. Pept. 1999, 80, 49–55. [Google Scholar] [CrossRef]
- Seeliger, S.; Buddenkotte, J.; Schmidt-Choudhury, A.; Rosignoli, C.; Shpacovitch, V.; von Arnim, U.; Metze, D.; Rukwied, R.; Schmelz, M.; Paus, R.; et al. Pituitary adenylate cyclase activating polypeptide. Am. J. Pathol. 2010, 177, 2563–2575. [Google Scholar] [CrossRef]
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate immunity and microbial dysbiosis in hidradenitis suppurativa—Vicious cycle of chronic inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Pajouhi, N.; Owji, M.; Naghibalhossaini, F.; Omrani, G.H.; Varedi, M. Modulation by thyroid hormone of myosin light chain phosphorylation and aquaporin 5 protein expression in the intact lung. J. Physiol. Biochem. 2015, 71, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, D.; Zhong, L.; Zhang, W.; Kang, J.; Zhou, J.; Ka, W.; Sun, D.; Xia, X.; Xie, L.; et al. Quantitative proteomics reveals TMOD1-related proteins associated with water balance regulation. PLoS ONE 2019, 14, e0219932. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins--new players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef]
- O’Donnell, B.L.; Penuela, S. Pannexin 3 channels in health and disease. Purinergic Signal. 2021, 17, 577–589. [Google Scholar] [CrossRef]
- Zhang, P.; Ishikawa, M.; Rhodes, C.; Doyle, A.; Ikeuchi, T.; Nakamura, K.; Chiba, Y.; He, B.; Yamada, Y. Pannexin-3 deficiency delays skin wound healing in mice due to defects in channel functionality. J. Investig. Dermatol. 2019, 139, 909–918. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Jourdain, A.A.; Calvo, S.E.; Ballarano, C.A.; Doench, J.G.; Root, D.E.; Mootha, V.K. A genome-wide CRISPR death screen identifies genes essential for oxidative phosphorylation. Cell Metab. 2016, 24, 875–885. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, G.; Peng, P.; Zhang, Y.; Xin, X. Down-regulated expression of AQP5 on lung in rat DIC model induced by LPS and its effect on the development of pulmonary edema. Pulm. Pharmacol. Ther. 2013, 26, 661–665. [Google Scholar] [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. Molecular mechanisms linking inflammation to autoimmunity in Sjögren’s syndrome: Identification of new targets. Int. J. Mol. Sci. 2022, 23, 13229. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 vesicle traffic: A cornerstone of insulin action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 2015, 58, 19–30. [Google Scholar] [CrossRef]
- Tengvall, K.; Sundstrom, E.; Wang, C.; Bergvall, K.; Wallerman, O.; Pederson, E.; Karlsson, A.; Harvey, N.D.; Blott, S.C.; Olby, N.; et al. Bayesian model and selection signature analyses reveal risk factors for canineatopic dermatitis. Commun. Biol. 2022, 5, 1348. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Arata, S.; Nakajo, S.; Ikenaka, K.; Kikuyama, S.; Shioda, S. Expression of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC1-R) in reactive astrocytes. Brain Res. Mol. Brain Res. 2003, 115, 10–20. [Google Scholar] [CrossRef]
- Ogawa, T.; Rakwal, R.; Shibato, J.; Sawa, C.; Saito, T.; Murayama, A.; Kuwagata, M.; Kageyama, H.; Yagi, M.; Satoh, K.; et al. Seeking gene candidates responsible for developmental origins of health and disease. Congenit. Anom. 2011, 51, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Nakamura, K.; Wada, Y.; Tsuchikawa, D.; Yoshikawa, A.; Tamaki, K.; Shioda, S. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Model. Mech. 2012, 5, 270–283. [Google Scholar] [CrossRef]
| Gene Name | Fold | |||
|---|---|---|---|---|
| 30 min | 60 min | |||
| WT | KO | WT | KO | |
| Ptgs2 | 2.83 | 1.37 | 1.25 | 1.84 |
| Kcnn2 | 2.60 | 0.68 | 1.18 | 0.96 |
| Cacna1s | 2.22 | 0.58 | 0.95 | 1.20 |
| Chrna1 | 1.93 | 0.42 | 1.28 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, M.; Shibato, J.; Rakwal, R.; Nonaka, N.; Hirabayashi, T.; Harvey, B.J.; Shioda, S.; Takenoya, F. Molecular and Physiological Functions of PACAP in Sweat Secretion. Int. J. Mol. Sci. 2023, 24, 4572. https://doi.org/10.3390/ijms24054572
Yamashita M, Shibato J, Rakwal R, Nonaka N, Hirabayashi T, Harvey BJ, Shioda S, Takenoya F. Molecular and Physiological Functions of PACAP in Sweat Secretion. International Journal of Molecular Sciences. 2023; 24(5):4572. https://doi.org/10.3390/ijms24054572
Chicago/Turabian StyleYamashita, Michio, Junko Shibato, Randeep Rakwal, Naoko Nonaka, Takahiro Hirabayashi, Brian J. Harvey, Seiji Shioda, and Fumiko Takenoya. 2023. "Molecular and Physiological Functions of PACAP in Sweat Secretion" International Journal of Molecular Sciences 24, no. 5: 4572. https://doi.org/10.3390/ijms24054572
APA StyleYamashita, M., Shibato, J., Rakwal, R., Nonaka, N., Hirabayashi, T., Harvey, B. J., Shioda, S., & Takenoya, F. (2023). Molecular and Physiological Functions of PACAP in Sweat Secretion. International Journal of Molecular Sciences, 24(5), 4572. https://doi.org/10.3390/ijms24054572






