Expression of Non-T Cell Activation Linker (NTAL) in Jurkat Cells Negatively Regulates TCR Signaling: Potential Role in Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
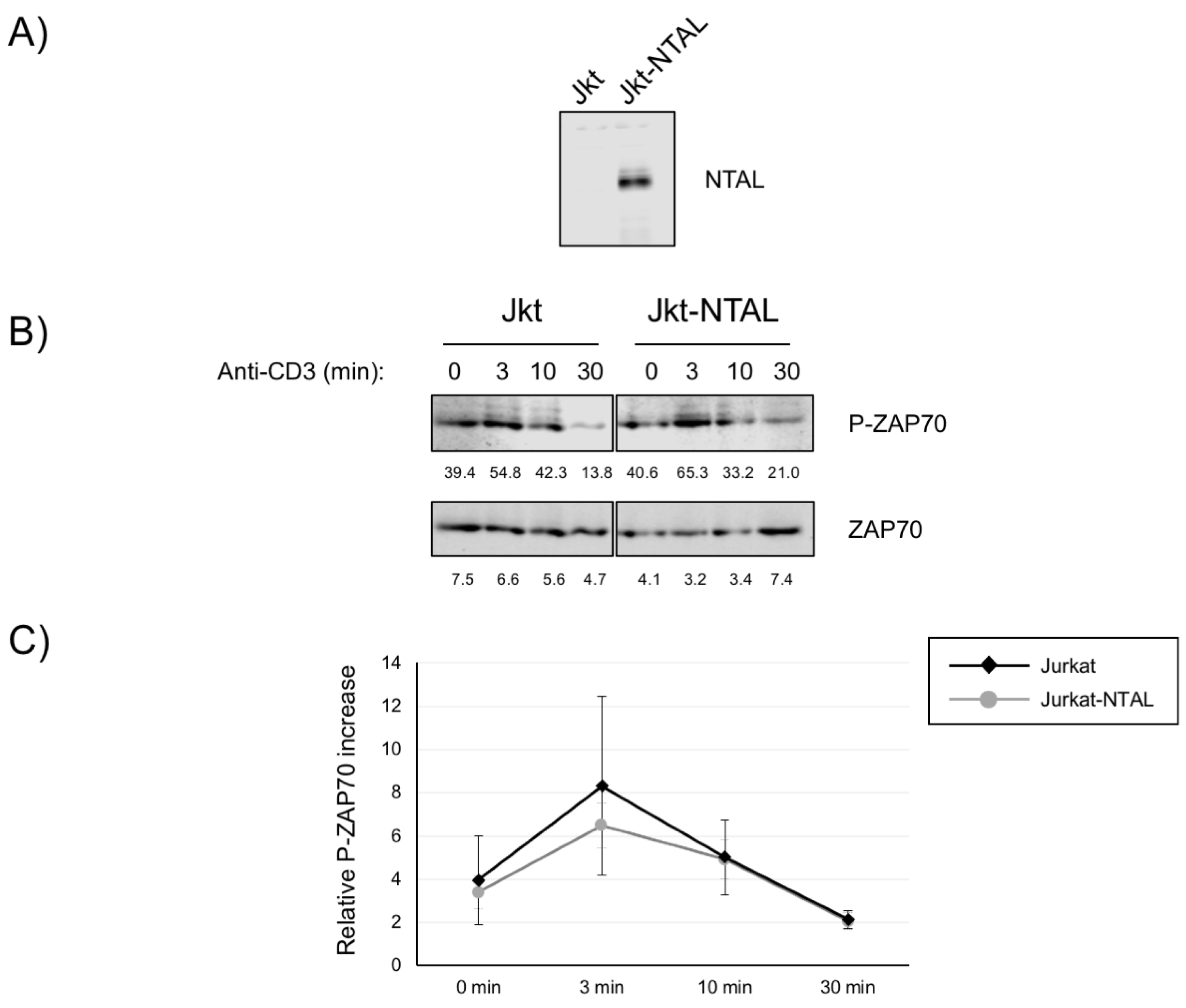
2.1. Impact of NTAL Expression in Jurkat Cells for ZAP70 Activation
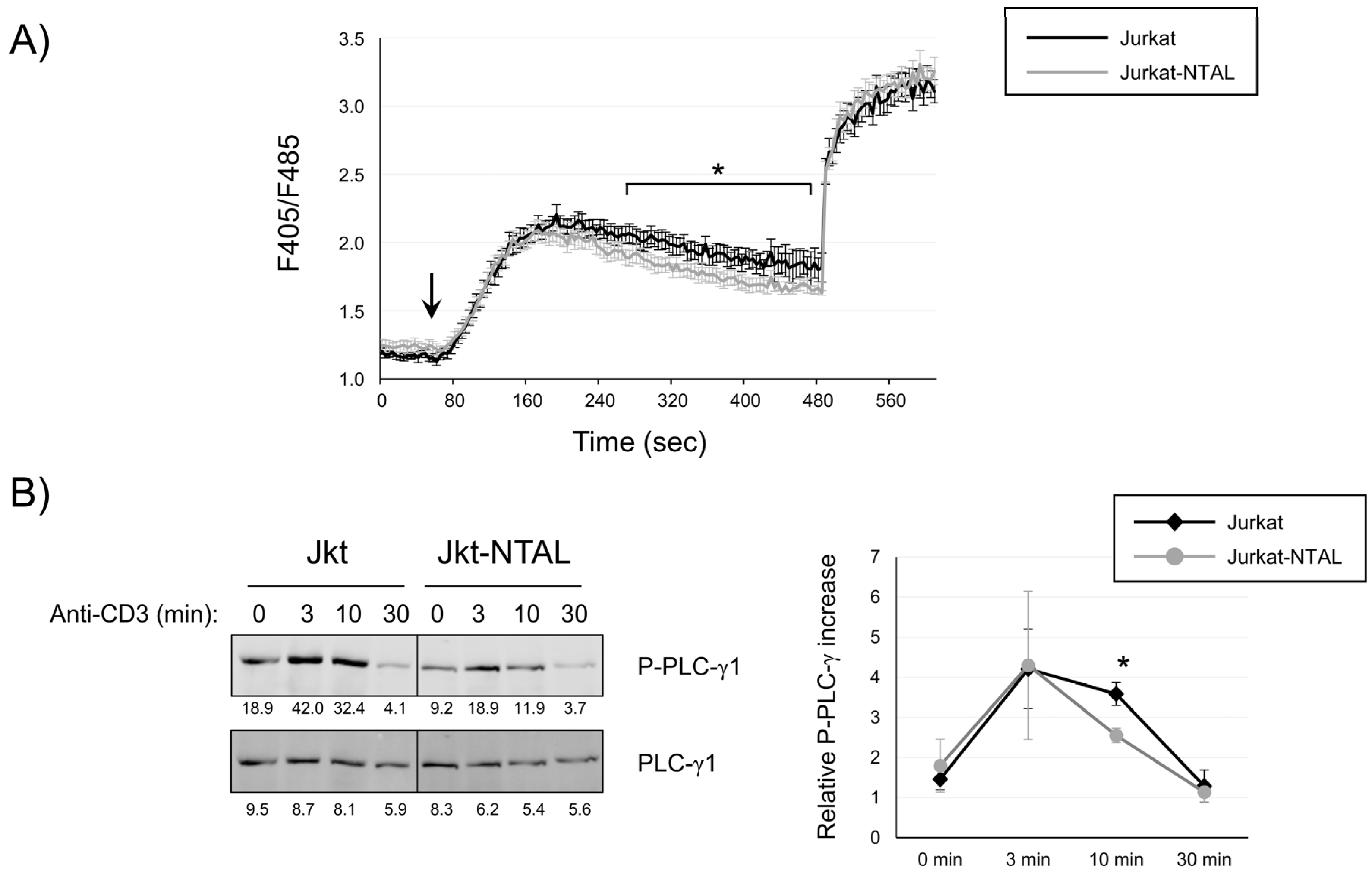
2.2. NTAL Expression Modulates Calcium Signaling in Jurkat Cells
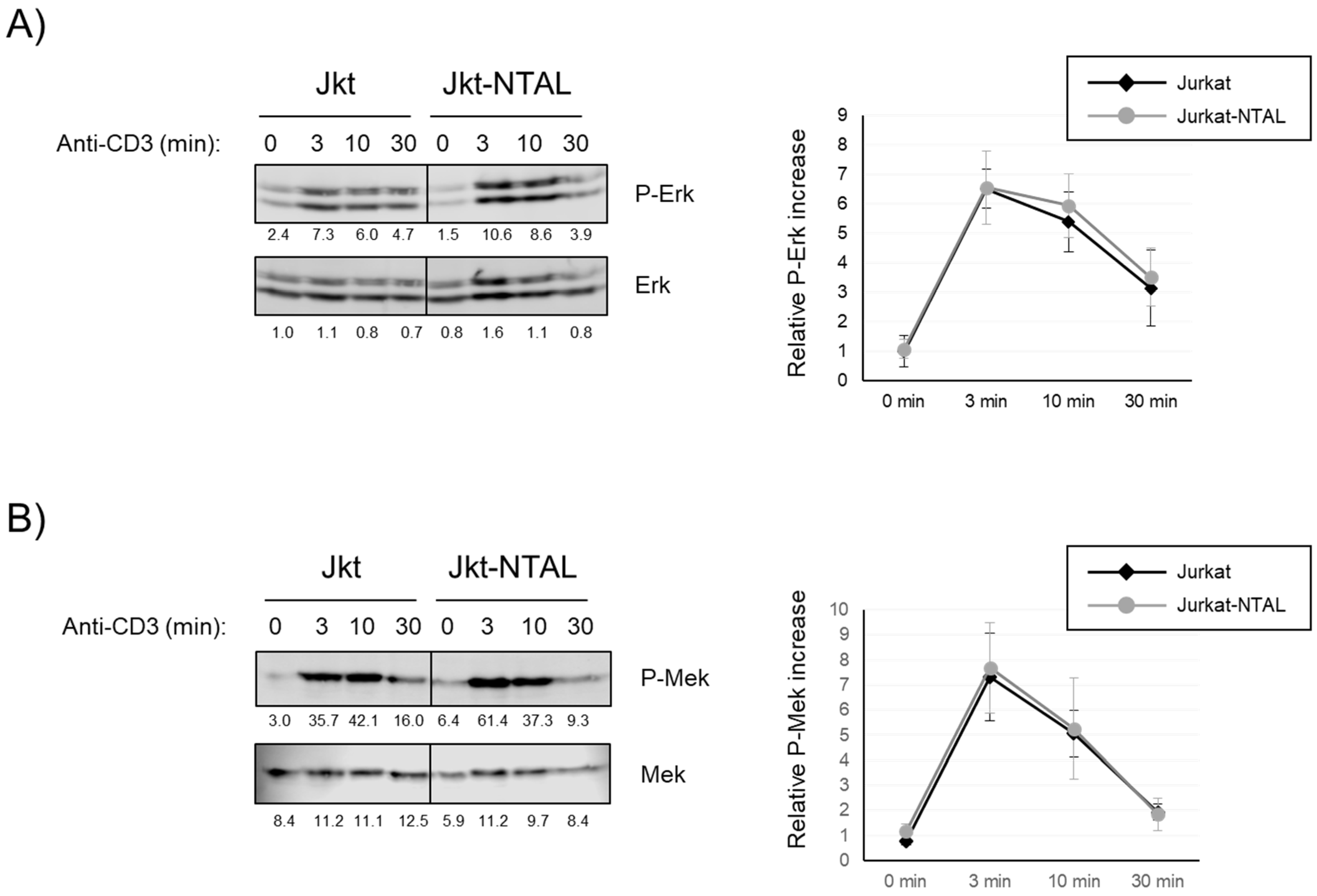
2.3. Effect of NTAL Expression in Jurkat Cells on the MAP Kinase Signaling Pathway
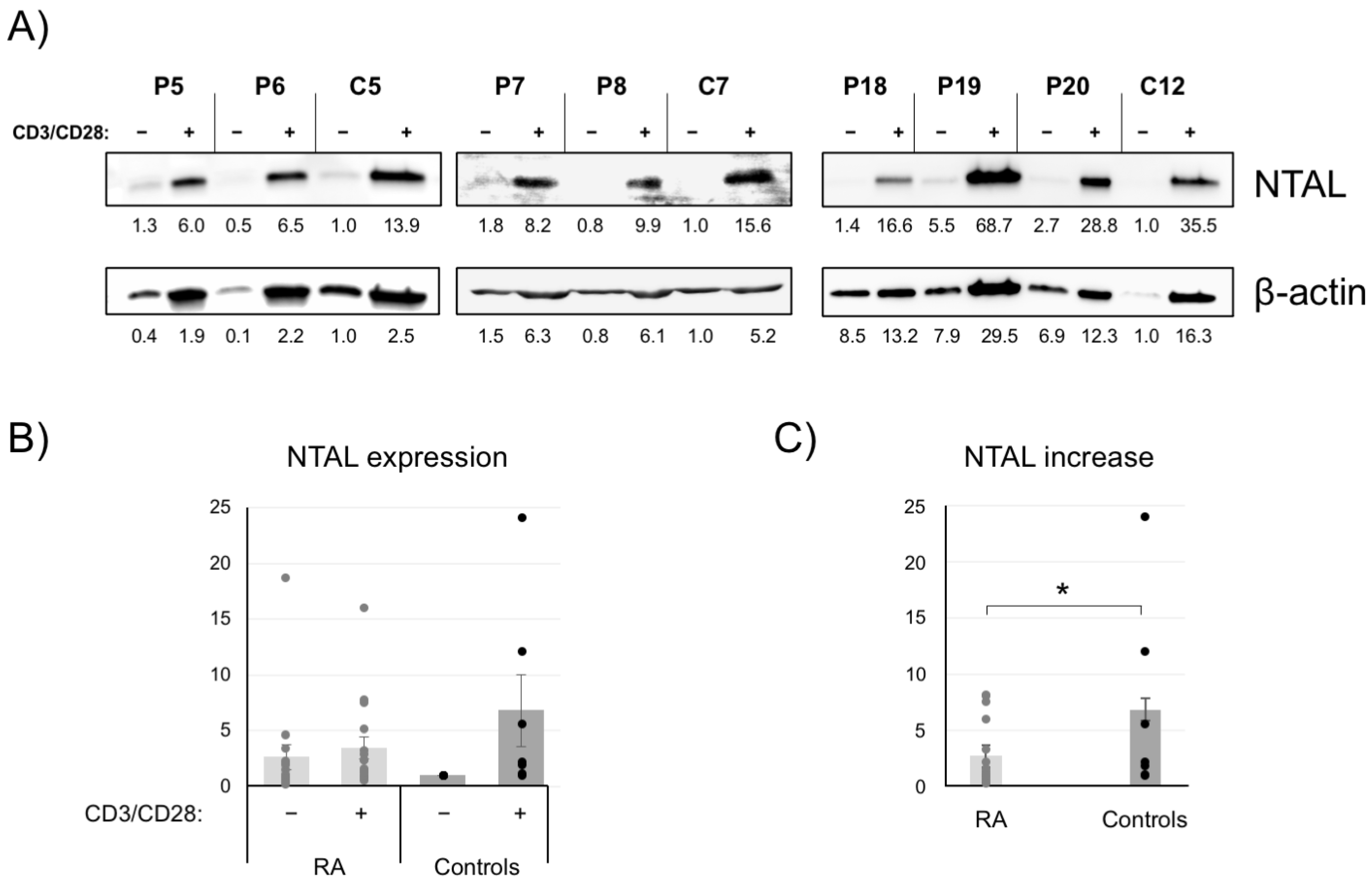
2.4. NTAL Expression in Human Peripheral T Cells
2.5. Increased Erk Activation in CD4+ T Cells from RA Patients
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Cloning and Lentiviral Transfection
4.2. Antibodies and Reagents
4.3. Purification and Culture of CD4+ T-Cells
4.4. Preparation of Cell Lysates and Western Blotting
4.5. Ca2+ Mobilization Assays
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguado, E.; Martinez-Florensa, M.; Aparicio, P. Activation of T lymphocytes and the role of the adapter LAT. Transpl. Immunol. 2006, 17, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Bongrand, P. Early T cell activation: Integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol. 2015, 33, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.R.; Orstavik, S.; Torgersen, K.M.; Danbolt, N.C.; Berg, S.F.; Ryan, J.C.; Tasken, K.; Imboden, J.B.; Vaage, J.T. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 1998, 187, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sloan-Lancaster, J.; Kitchen, J.; Trible, R.P.; Samelson, L.E. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 1998, 92, 83–92. [Google Scholar] [CrossRef]
- Aguado, E.; Richelme, S.; Nunez-Cruz, S.; Miazek, A.; Mura, A.M.; Richelme, M.; Guo, X.J.; Sainty, D.; He, H.T.; Malissen, B.; et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 2002, 296, 2036–2040. [Google Scholar] [CrossRef]
- Sommers, C.L.; Park, C.S.; Lee, J.; Feng, C.; Fuller, C.L.; Grinberg, A.; Hildebrand, J.A.; Lacana, E.; Menon, R.K.; Shores, E.W.; et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 2002, 296, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Cruz, S.; Aguado, E.; Richelme, S.; Chetaille, B.; Mura, A.M.; Richelme, M.; Pouyet, L.; Jouvin-Marche, E.; Xerri, L.; Malissen, B.; et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nat. Immunol. 2003, 4, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Mingueneau, M.; Roncagalli, R.; Gregoire, C.; Kissenpfennig, A.; Miazek, A.; Archambaud, C.; Wang, Y.; Perrin, P.; Bertosio, E.; Sansoni, A.; et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 2009, 31, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, J.A.; Simeoni, L.; Schraven, B. Transmembrane adapters: Attractants for cytoplasmic effectors. Immunol. Rev. 2003, 191, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Brdicka, T.; Imrich, M.; Angelisova, P.; Brdickova, N.; Horvath, O.; Spicka, J.; Hilgert, I.; Luskova, P.; Draber, P.; Novak, P.; et al. Non-T cell activation linker (NTAL): A transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 2002, 196, 1617–1626. [Google Scholar] [CrossRef]
- Janssen, E.; Zhu, M.; Zhang, W.; Koonpaew, S.; Zhang, W. LAB: A new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 2003, 4, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Koonpaew, S.; Janssen, E.; Zhu, M.; Zhang, W. The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J. Biol. Chem. 2004, 279, 11229–11235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sommers, C.L.; Burshtyn, D.N.; Stebbins, C.C.; DeJarnette, J.B.; Trible, R.P.; Grinberg, A.; Tsay, H.C.; Jacobs, H.M.; Kessler, C.M.; et al. Essential role of LAT in T cell development. Immunity 1999, 10, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Horvath, O.; Hamm-Baarke, A.; Richelme, M.; Gregoire, C.; Guinamard, R.; Horejsi, V.; Angelisova, P.; Spicka, J.; Schraven, B.; et al. Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol. Cell. Biol. 2005, 25, 4455–4465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Koonpaew, S.; Liu, Y.; Shen, S.; Denning, T.; Dzhagalov, I.; Rhee, I.; Zhang, W. Negative regulation of T cell activation and autoimmunity by the transmembrane adaptor protein LAB. Immunity 2006, 25, 757–768. [Google Scholar] [CrossRef]
- Janssen, E.; Zhu, M.; Craven, B.; Zhang, W. Linker for activation of B cells: A functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J. Immunol. 2004, 172, 6810–6819. [Google Scholar] [CrossRef]
- Alamanos, Y.; Drosos, A.A. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005, 4, 130–136. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Snieder, H.; Rigby, A.S.; Koskenvuo, M.; Kaprio, J.; Aho, K.; Silman, A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000, 43, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Amariuta, T.; Luo, Y.; Knevel, R.; Okada, Y.; Raychaudhuri, S. Advances in genetics toward identifying pathogenic cell states of rheumatoid arthritis. Immunol. Rev. 2020, 294, 188–204. [Google Scholar] [CrossRef]
- Hu, X.; Kim, H.; Raj, T.; Brennan, P.J.; Trynka, G.; Teslovich, N.; Slowikowski, K.; Chen, W.M.; Onengut, S.; Baecher-Allan, C.; et al. Regulation of gene expression in autoimmune disease loci and the genetic basis of proliferation in CD4+ effector memory T cells. PLoS Genet. 2014, 10, e1004404. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K. Personalized medicine: Theranostics (therapeutics diagnostics) essential for rational use of tumor necrosis factor-alpha antagonists. Discov. Med. 2013, 15, 201–211. [Google Scholar] [PubMed]
- Bader, L.; Gullaksen, S.E.; Blaser, N.; Brun, M.; Bringeland, G.H.; Sulen, A.; Gjesdal, C.G.; Vedeler, C.; Gavasso, S. Candidate Markers for Stratification and Classification in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Arbulo-Echevarria, M.M.; Munoz-Miranda, J.P.; Caballero-Garcia, A.; Poveda-Diaz, J.L.; Fernandez-Ponce, C.; Duran-Ruiz, M.C.; Miazek, A.; Garcia-Cozar, F.; Aguado, E. Non-T cell activation linker (NTAL) proteolytic cleavage as a terminator of activatory intracellular signals. J. Leukoc. Biol. 2016, 100, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, V.; Mege, D.; Germain, V.; Pelosi, M.; Dufour, E.; Michel, F.; Magistrelli, G.; Isacchi, A.; Acuto, O. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 1999, 274, 6285–6294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gluck, S.; Zhang, L.; Moran, M.F. Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis. Mol. Cell. Biol. 1998, 18, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, N.; Poltorak, M.; Kowtharapu, B.S.; Arndt, B.; Stone, J.C.; Schraven, B.; Simeoni, L. TCR-mediated Erk activation does not depend on Sos and Grb2 in peripheral human T cells. EMBO Rep. 2012, 13, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.M.; Alessandrini, A.; Erikson, R.L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 1992, 258, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Koonpaew, S.; Granillo, O.; Zhang, W. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 2004, 200, 991–1000. [Google Scholar] [CrossRef]
- Volna, P.; Lebduska, P.; Draberova, L.; Simova, S.; Heneberg, P.; Boubelik, M.; Bugajev, V.; Malissen, B.; Wilson, B.S.; Horejsi, V.; et al. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 2004, 200, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Aguado, E.; Malissen, M. Role of the LAT adaptor in T-cell development and Th2 differentiation. Adv. Immunol. 2005, 87, 1–25. [Google Scholar] [PubMed]
- Vico-Barranco, I.; Arbulo-Echevarria, M.M.; Serrano-Garcia, I.; Perez-Linaza, A.; Miranda-Sayago, J.M.; Miazek, A.; Narbona-Sanchez, I.; Aguado, E. A Novel, LAT/Lck Double Deficient T Cell Subline J.CaM1.7 for Combined Analysis of Early TCR Signaling. Cells 2021, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Rouquette-Jazdanian, A.K.; Sommers, C.L.; Kortum, R.L.; Morrison, D.K.; Samelson, L.E. LAT-independent Erk activation via Bam32-PLC-gamma1-Pak1 complexes: GTPase-independent Pak1 activation. Mol. Cell 2012, 48, 298–312. [Google Scholar] [CrossRef]
- Duque-Afonso, J.; Solari, L.; Essig, A.; Berg, T.; Pahl, H.L.; Lubbert, M. Regulation of the adaptor molecule LAT2, an in vivo target gene of AML1/ETO (RUNX1/RUNX1T1), during myeloid differentiation. Br. J. Haematol. 2011, 153, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Duque-Afonso, J.; Yalcin, A.; Berg, T.; Abdelkarim, M.; Heidenreich, O.; Lubbert, M. The HDAC class I-specific inhibitor entinostat (MS-275) effectively relieves epigenetic silencing of the LAT2 gene mediated by AML1/ETO. Oncogene 2011, 30, 3062–3072. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Deshpande, P.; Li, G.; Yu, M.; Pryshchep, S.; Cavanagh, M.; Weyand, C.M.; Goronzy, J.J. K-RAS GTPase- and B-RAF kinase-mediated T-cell tolerance defects in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2012, 109, E1629–E1637. [Google Scholar] [CrossRef]
- Zayoud, M.; Marcu-Malina, V.; Vax, E.; Jacob-Hirsch, J.; Elad-Sfadia, G.; Barshack, I.; Kloog, Y.; Goldstein, I. Ras Signaling Inhibitors Attenuate Disease in Adjuvant-Induced Arthritis via Targeting Pathogenic Antigen-Specific Th17-Type Cells. Front. Immunol. 2017, 8, 799. [Google Scholar] [CrossRef]
- Begovich, A.B.; Carlton, V.E.; Honigberg, L.A.; Schrodi, S.J.; Chokkalingam, A.P.; Alexander, H.C.; Ardlie, K.G.; Huang, Q.; Smith, A.M.; Spoerke, J.M.; et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004, 75, 330–337. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narbona-Sánchez, I.; Pérez-Linaza, A.; Serrano-García, I.; Vico-Barranco, I.; Fernández-Aguilar, L.M.; Poveda-Díaz, J.L.; Sánchez del Pino, M.J.; Medina-Varo, F.; Arbulo-Echevarria, M.M.; Aguado, E. Expression of Non-T Cell Activation Linker (NTAL) in Jurkat Cells Negatively Regulates TCR Signaling: Potential Role in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 4574. https://doi.org/10.3390/ijms24054574
Narbona-Sánchez I, Pérez-Linaza A, Serrano-García I, Vico-Barranco I, Fernández-Aguilar LM, Poveda-Díaz JL, Sánchez del Pino MJ, Medina-Varo F, Arbulo-Echevarria MM, Aguado E. Expression of Non-T Cell Activation Linker (NTAL) in Jurkat Cells Negatively Regulates TCR Signaling: Potential Role in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2023; 24(5):4574. https://doi.org/10.3390/ijms24054574
Chicago/Turabian StyleNarbona-Sánchez, Isaac, Alba Pérez-Linaza, Isabel Serrano-García, Inmaculada Vico-Barranco, Luis M. Fernández-Aguilar, José L. Poveda-Díaz, María J. Sánchez del Pino, Fermín Medina-Varo, Mikel M. Arbulo-Echevarria, and Enrique Aguado. 2023. "Expression of Non-T Cell Activation Linker (NTAL) in Jurkat Cells Negatively Regulates TCR Signaling: Potential Role in Rheumatoid Arthritis" International Journal of Molecular Sciences 24, no. 5: 4574. https://doi.org/10.3390/ijms24054574
APA StyleNarbona-Sánchez, I., Pérez-Linaza, A., Serrano-García, I., Vico-Barranco, I., Fernández-Aguilar, L. M., Poveda-Díaz, J. L., Sánchez del Pino, M. J., Medina-Varo, F., Arbulo-Echevarria, M. M., & Aguado, E. (2023). Expression of Non-T Cell Activation Linker (NTAL) in Jurkat Cells Negatively Regulates TCR Signaling: Potential Role in Rheumatoid Arthritis. International Journal of Molecular Sciences, 24(5), 4574. https://doi.org/10.3390/ijms24054574






