Emerging RNA-Based Therapeutic and Diagnostic Options: Recent Advances and Future Challenges in Genitourinary Cancers
Abstract
:1. Introduction
2. Renal Cell Carcinoma
3. Bladder Cancer
4. Prostate Cancer
5. Other Genitourinary Cancers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarrabi, K.; Paroya, A.; Wu, S. Emerging therapeutic agents for genitourinary cancers. J. Hematol. Oncol. 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukleja, J.; Kusaka, E.; Miyamoto, D.T. Immunotherapy Combined With Radiation Therapy for Genitourinary Malignancies. Front. Oncol. 2021, 11, 663852. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Park, E.G.; Pyo, S.J.; Cui, Y.; Yoon, S.H.; Nam, J.W. Tumor immune microenvironment lncRNAs. Brief Bioinform. 2022, 23, bbab504. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Perazella, M.A.; Dreicer, R.; Rosner, M.H. Renal Cell Carcinoma for the Nephrologist. Kidney Int. 2018, 94, 471–483. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Gu, L.; Li, X.; Gao, Y.; Lyu, X.; Chen, L.; Luo, G.; Wang, L.; Xie, Y.; et al. LncRNAs Act as Prognostic and Diagnostic Biomarkers in Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 74325–74336. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, A.M.; Zhang, J.Y.; He, X.M.; Pan, Y.S.; Cheng, G.; Qin, C.; Hua, L.X.; Wang, Z.J. Prognostic Significance of Long Non-Coding RNA MALAT-1 in Various Human Carcinomas: A Meta-Analysis. Genet. Mol. Res. 2016, 15, gmr.15017433. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.; Chen, S.-J.; Che, J.; Zheng, J. Upregulation of Long Non-Coding RNA MALAT1 Correlates with Tumor Progression and Poor Prognosis in Clear Cell Renal Cell Carcinoma. Tumour Biol. 2015, 36, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Nakajima, K.; Tabatabai, Z.L.; Ishii, N.; Dahiya, R. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with MiR-205. Cancer Res. 2015, 75, 1322–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Lee, D.Y.; Ben-David, Y. The Roles of MicroRNAs in Tumorigenesis and Angiogenesis. Int. J. Physiol. Pathophysiol. Pharm. 2011, 3, 140–155. [Google Scholar]
- Xiao, H.; Tang, K.; Liu, P.; Chen, K.; Hu, J.; Zeng, J.; Xiao, W.; Yu, G.; Yao, W.; Zhou, H.; et al. LncRNA MALAT1 Functions as a Competing Endogenous RNA to Regulate ZEB2 Expression by Sponging MiR-200s in Clear Cell Kidney Carcinoma. Oncotarget 2015, 6, 38005–38015. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Mu, Z.; Wei, N.; Sun, M.; Wang, W.; Xin, N.; Shao, Y.; Zhao, C. Long Non-Coding RNA ZFAS1 Promotes Proliferation and Metastasis of Clear Cell Renal Cell Carcinoma via Targeting MiR-10a/SKA1 Pathway. Biomed. Pharm. 2019, 111, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, X.; Siqin, B.; Ren, C.; Yi, F. Long Non-Coding RNA CYTOR Modulates Cancer Progression through MiR-136-5p/MAT2B Axis in Renal Cell Carcinoma. Toxicol. Appl. Pharm. 2022, 447, 116067. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, D.-L.; Wang, J.-M.; Jiang, J.-Y.; Du, X.; Zeng, X.-Y.; Du, Z.-X. TRIM29 Inhibits MiR-873-5P Biogenesis via CYTOR to Upregulate Fibronectin 1 and Promotes Invasion of Papillary Thyroid Cancer Cells. Cell Death Dis. 2020, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, J.; Liu, X.; Xu, Y.; Zhai, R.; Zhang, J.; Wang, M.; Wang, M.; Liu, L. LncRNA CYTOR Promotes Aberrant Glycolysis and Mitochondrial Respiration via HNRNPC-Mediated ZEB1 Stabilization in Oral Squamous Cell Carcinoma. Cell Death Dis. 2022, 13, 703. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, X. Long Non-Coding RNA (LncRNA) CYTOR Promotes Hepatocellular Carcinoma Proliferation by Targeting the MicroRNA-125a-5p/LASP1 Axis. Bioengineered 2022, 13, 3666–3679. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z.; Li, F. Overexpressed MicroRNA-136 Works as a Cancer Suppressor in Gallbladder Cancer through Suppression of JNK Signaling Pathway via Inhibition of MAP2K4. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G670–G681. [Google Scholar] [CrossRef]
- Gao, R.-Z.; Que, Q.; Lin, P.; Pang, Y.-Y.; Wu, H.-Y.; Li, X.-J.; Chen, G.; He, Y.; Yang, H. Clinical Roles of MiR-136-5p and Its Target Metadherin in Thyroid Carcinoma. Am. J. Transl. Res. 2019, 11, 6754–6774. [Google Scholar] [PubMed]
- Han, C.; Fu, Y.; Zeng, N.; Yin, J.; Li, Q. LncRNA FAM83H-AS1 Promotes Triple-Negative Breast Cancer Progression by Regulating the MiR-136-5p/Metadherin Axis. Aging 2020, 12, 3594–3616. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Q.; Xue, F.; Wu, Y. LncRNA-CYTOR Works as an Oncogene Through the CYTOR/MiR-3679-5p/MACC1 Axis in Colorectal Cancer. DNA Cell Biol. 2019, 38, 572–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Zhang, D.; Zhong, Z.; Zhang, W. LncRNA ROR Promotes the Progression of Renal Cell Carcinoma through the MiR-206/VEGF Axis. Mol. Med. Rep. 2019, 20, 3782–3792. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Hu, W.; Wang, Y.; An, Y.; Song, L.; Shang, P.; Yue, Z. Long Non-Coding RNA UCA1 Promotes Malignant Phenotypes of Renal Cancer Cells by Modulating the MiR-182-5p/DLL4 Axis as a CeRNA. Mol. Cancer 2020, 19, 18. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Gu, W.; Yan, Y.; Yao, X.; Zheng, J. MALAT1 Accelerates the Development and Progression of Renal Cell Carcinoma by Decreasing the Expression of MiR-203 and Promoting the Expression of BIRC5. Cell Prolif. 2019, 52, e12640. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Hu, Q.; Nie, E.; Yu, T.; Wu, Y.; Zhi, T.; Jiang, K.; Shen, F.; Wang, Y.; Zhang, J.; et al. Hypoxia Induces H19 Expression through Direct and Indirect Hif-1α Activity, Promoting Oncogenic Effects in Glioblastoma. Sci. Rep. 2017, 7, 45029. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cai, Y.; Zhao, X.; Jia, X.; Zhang, J.; Liu, J.; Zhen, H.; Wang, T.; Tang, X.; Liu, Y.; et al. Down-Regulated Long Non-Coding RNA H19 Inhibits Carcinogenesis of Renal Cell Carcinoma. Neoplasma 2015, 62, 412–418. [Google Scholar] [CrossRef]
- He, H.; Wang, N.; Yi, X.; Tang, C.; Wang, D. Long Non-Coding RNA H19 Regulates E2F1 Expression by Competitively Sponging Endogenous MiR-29a-3p in Clear Cell Renal Cell Carcinoma. Cell Biosci. 2017, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Raveh, E.; Matouk, I.J.; Gilon, M.; Hochberg, A. The H19 Long Non-Coding RNA in Cancer Initiation, Progression and Metastasis—A Proposed Unifying Theory. Mol. Cancer 2015, 14, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liu, J.; Zheng, Y.; You, L.; Kuang, D.; Liu, T. Suppressed Expression of Long Non-Coding RNA HOTAIR Inhibits Proliferation and Tumourigenicity of Renal Carcinoma Cells. Tumour Biol. 2014, 35, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, H.; Tamai, K.; Shibuya, R.; Nakamura, M.; Mochizuki, M.; Yamaguchi, K.; Kawamura, S.; Tochigi, T.; Sato, I.; Okanishi, T.; et al. Long Non-Coding RNA HOTAIR Promotes Cell Migration by Upregulating Insulin Growth Factor-Binding Protein 2 in Renal Cell Carcinoma. Sci. Rep. 2017, 7, 12016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Q.; Li, O.; Zheng, W.; Xiao, W.-Z.; Zhang, L.; Wu, D.; Cai, G.-Y.; He, J.C.; Chen, X.-M. LncRNA HOTAIR Regulates HIF-1α/AXL Signaling through Inhibition of MiR-217 in Renal Cell Carcinoma. Cell Death Dis. 2017, 8, e2772. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Yeh, C.-R.; Sun, Y.; Lin, C.; Chou, J.; Ou, Z.; Chang, C.; Qi, J.; Yeh, S. Estrogen Receptor β Promotes Renal Cell Carcinoma Progression via Regulating LncRNA HOTAIR-MiR-138/200c/204/217 Associated CeRNA Network. Oncogene 2018, 37, 5037–5053. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Wang, Y.; Liu, S.; Yuan, X.; Pan, F.; Geng, P. Decreased Expression of a Novel LncRNA CADM1-AS1 Is Associated with Poor Prognosis in Patients with Clear Cell Renal Cell Carcinomas. Int. J. Clin. Exp. Pathol. 2014, 7, 2758–2767. [Google Scholar]
- Zhang, H.-M.; Yang, F.-Q.; Yan, Y.; Che, J.-P.; Zheng, J.-H. High Expression of Long Non-Coding RNA SPRY4-IT1 Predicts Poor Prognosis of Clear Cell Renal Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5801–5809. [Google Scholar]
- Song, S.; Wu, Z.; Wang, C.; Liu, B.; Ye, X.; Chen, J.; Yang, Q.; Ye, H.; Xu, B.; Wang, L. RCCRT1 Is Correlated with Prognosis and Promotes Cell Migration and Invasion in Renal Cell Carcinoma. Urology 2014, 84, 730.e1–730.e7. [Google Scholar] [CrossRef]
- Xue, S.; Li, Q.-W.; Che, J.-P.; Guo, Y.; Yang, F.-Q.; Zheng, J.-H. Decreased Expression of Long Non-Coding RNA NBAT-1 Is Associated with Poor Prognosis in Patients with Clear Cell Renal Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3765–3774. [Google Scholar]
- Su, H.; Sun, T.; Wang, H.; Shi, G.; Zhang, H.; Sun, F.; Ye, D. Decreased TCL6 Expression Is Associated with Poor Prognosis in Patients with Clear Cell Renal Cell Carcinoma. Oncotarget 2017, 8, 5789–5799. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Huang, X.; Cai, M.; Huang, P. Potential Biomarkers for Predicting the Overall Survival Outcome of Kidney Renal Papillary Cell Carcinoma: An Analysis of Ferroptosis-Related LNCRNAs. BMC Urol. 2022, 22, 152. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Chen, Y. Targeting Long Non-Coding RNAs in Cancers: Progress and Prospects. Int. J. Biochem. Cell Biol. 2013, 45, 1895–1910. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and Other Non-Coding RNAs as Targets for Anticancer Drug Development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [Green Version]
- Lennox, K.A.; Behlke, M.A. Cellular Localization of Long Non-Coding RNAs Affects Silencing by RNAi More than by Antisense Oligonucleotides. Nucleic Acids Res. 2016, 44, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.Y.-W.; Zhang, H.; Fu, Y.; Chen, Y.-T.; Tang, Q.-Y.; Liu, Y.-X.; Zhang, Y.-X.; Wang, S.-Z.; Wesselius, A.; Li, W.-C.; et al. Integrative Multi-Omics Analysis for the Determination of Non-Muscle Invasive vs. Muscle Invasive Bladder Cancer: A Pilot Study. Curr. Oncol. 2022, 29, 5442–5456. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Noon, A.P.; on behalf of the EAU Young Academic Urologists—Urothelial Cancer Working Party. Epidemiology, Aetiology and Screening of Bladder Cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- DeGeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician 2017, 96, 507–514. [Google Scholar] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of MicroRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. [Google Scholar] [CrossRef]
- Parizi, P.K.; Yarahmadi, F.; Tabar, H.M.; Hosseini, Z.; Sarli, A.; Kia, N.; Tafazoli, A.; Esmaeili, S.-A. MicroRNAs and Target Molecules in Bladder Cancer. Med. Oncol. 2020, 37, 118. [Google Scholar] [CrossRef]
- Böhmer, D.; Grün, A. Lacking Evidence to Recommend Neoadjuvant Chemotherapy and Definitive Radiotherapy in Muscle-Invasive Bladder Cancer. Curr. Oncol. Rep. 2021, 23, 18. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT Promoter Mutations in Bladder Cancer Affect Patient Survival and Disease Recurrence through Modification by a Common Polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [Green Version]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flández, M.; Marqués, M.; Márquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase Reverse Transcriptase Promoter Mutations in Bladder Cancer: High Frequency across Stages, Detection in Urine, and Lack of Association with Outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Kurtis, B.; Zhuge, J.; Ojaimi, C.; Ye, F.; Cai, D.; Zhang, D.; Fallon, J.T.; Zhong, M. Recurrent TERT Promoter Mutations in Urothelial Carcinoma and Potential Clinical Applications. Ann. Diagn. Pathol. 2016, 21, 7–11. [Google Scholar] [CrossRef]
- Leão, R.; Lee, D.; Figueiredo, A.; Hermanns, T.; Wild, P.; Komosa, M.; Lau, I.; Mistry, M.; Nunes, N.M.; Price, A.J.; et al. Combined Genetic and Epigenetic Alterations of the TERT Promoter Affect Clinical and Biological Behavior of Bladder Cancer. Int. J. Cancer 2019, 144, 1676–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qiao, L.; Zang, Y.; Ni, W.; Xu, Z. Circular RNA FOXO3 Suppresses Bladder Cancer Progression and Metastasis by Regulating MiR-9-5p/TGFBR2. Cancer Manag. Res. 2020, 12, 5049–5056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Q.; Zhong, P. Circ_0067934 Increases Bladder Cancer Cell Proliferation, Migration and Invasion through Suppressing MiR-1304 Expression and Increasing Myc Expression Levels. Exp. Ther. Med. 2020, 19, 3751–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Deng, H.; He, H.; Xu, R.; Wang, Y.; Zhu, X.; Zhang, J.; Zeng, Q.; Zhao, X. The Circ_0004463/MiR-380-3p/FOXO1 Axis Modulates Mitochondrial Respiration and Bladder Cancer Cell Apoptosis. Cell Cycle 2020, 19, 3563–3580. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, L.; Sun, F. Puerarin Inhibits the Progression of Bladder Cancer by Regulating Circ_0020394/MiR-328-3p/NRBP1 Axis. Cancer Biother. Radiopharm. 2022, 37, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Mojarrad, M.; Moghbeli, M. Genetic and Molecular Biology of Bladder Cancer among Iranian Patients. Mol. Genet. Genom. Med. 2020, 8, e1233. [Google Scholar] [CrossRef]
- Huang, W.; Lu, Y.; Wang, F.; Huang, X.; Yu, Z. Circular RNA CircRNA_103809 Accelerates Bladder Cancer Progression and Enhances Chemo-Resistance by Activation of MiR-516a-5p/FBXL18 Axis. Cancer Manag. Res. 2020, 12, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizaveh, S.; Ashrafizadeh, M.; Zarrabi, A.; Husmandi, K.; Zabolian, A.; Shahinozzaman, M.; Aref, A.R.; Hamblin, M.R.; Nabavi, N.; Crea, F.; et al. Long Non-Coding RNAs in the Doxorubicin Resistance of Cancer Cells. Cancer Lett. 2021, 508, 104–114. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-ΚB) Signaling Pathway by Non-Coding RNAs in Cancer: Inhibiting or Promoting Carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Zhang, X.; Liu, J.; Gui, J.; Cui, M.; Li, Y. MiR-211-5p Is down-Regulated and a Prognostic Marker in Bladder Cancer. J. Gene Med. 2020, 22, e3270. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Zhao, J.; Xiong, M.; Almaraihah, R.; Chen, Z.; Hou, T. Circ_0008532 Promotes Bladder Cancer Progression by Regulation of the MiR-155-5p/MiR-330-5p/MTGR1 Axis. J. Exp. Clin. Cancer Res. 2020, 39, 94. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Lin, J.; Xiao, J.; Tian, Y. LncRNA CCAT1 Promotes Bladder Cancer Cell Proliferation, Migration and Invasion. Int. Braz. J. Urol. 2019, 45, 549–559. [Google Scholar] [CrossRef]
- Petrella, G.; Ciufolini, G.; Vago, R.; Cicero, D.O. The Interplay between Oxidative Phosphorylation and Glycolysis as a Potential Marker of Bladder Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8107. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Zhu, W.; Han, J.; Yang, X.; Zhou, R.; Lu, H.-C.; Yu, H.; Yuan, W.-B.; Li, P.-C.; Tao, J.; et al. The Role of the HIF-1α/ALYREF/PKM2 Axis in Glycolysis and Tumorigenesis of Bladder Cancer. Cancer Commun. 2021, 41, 560–575. [Google Scholar] [CrossRef]
- Mamouni, K.; Kim, J.; Lokeshwar, B.L.; Kallifatidis, G. ARRB1 Regulates Metabolic Reprogramming to Promote Glycolysis in Stem Cell-Like Bladder Cancer Cells. Cancers 2021, 13, 1809. [Google Scholar] [CrossRef]
- Logotheti, S.; Marquardt, S.; Gupta, S.K.; Richter, C.; Edelhäuser, B.A.H.; Engelmann, D.; Brenmoehl, J.; Söhnchen, C.; Murr, N.; Alpers, M.; et al. LncRNA-SLC16A1-AS1 Induces Metabolic Reprogramming during Bladder Cancer Progression as Target and Co-Activator of E2F1. Theranostics 2020, 10, 9620–9643. [Google Scholar] [CrossRef]
- Zhong, X.; Long, Z.; Wu, S.; Xiao, M.; Hu, W. LncRNA-SNHG7 Regulates Proliferation, Apoptosis and Invasion of Bladder Cancer Cells Assurance Guidelines. J. BUON 2018, 23, 776–781. [Google Scholar]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C. LncRNA ZEB2-AS1 Promotes Bladder Cancer Cell Proliferation and Inhibits Apoptosis by Regulating MiR-27b. Biomed. Pharm. 2017, 96, 299–304. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Zhang, Y.; Wang, H.; Sun, H.; Feng, X.; Hou, M.; Chen, G.; Tang, Q.; Ji, M. ASF1B Promotes Cervical Cancer Progression through Stabilization of CDK9. Cell Death Dis. 2020, 11, 705. [Google Scholar] [CrossRef]
- Rui, X.; Wang, L.; Pan, H.; Gu, T.; Shao, S.; Leng, J. LncRNA GAS6-AS2 Promotes Bladder Cancer Proliferation and Metastasis via GAS6-AS2/MiR-298/CDK9 Axis. J. Cell. Mol. Med. 2019, 23, 865–876. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, D.; Wang, C.; Zhu, Y. Tumor Suppressing Effects of Tristetraprolin and Its Small Double-Stranded RNAs in Bladder Cancer. Cancer Med. 2021, 10, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, T.; Xiao, E.; Zhao, H.; Li, Y.; Fu, S.; Gan, L.; Wang, Z.; Zheng, Q.; Wang, Z. Licochalcone B Inhibits Growth of Bladder Cancer Cells by Arresting Cell Cycle Progression and Inducing Apoptosis. Food Chem. Toxicol. 2014, 65, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Cao, S.; Li, C.; Xu, M.; Wei, H.; Yang, H.; Sun, Q.; Ren, Q.; Zhang, L. LncRNA PVT1 Regulates Growth, Migration, and Invasion of Bladder Cancer by MiR-31/CDK1. J. Cell. Physiol. 2019, 234, 4799–4811. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Dai, H.; Zhang, Y.; Li, Q.; Zhao, M.; Yue, T. LncRNA NCK1-AS1 Promotes Cancer Cell Proliferation and Increase Cell Stemness in Urinary Bladder Cancer Patients by Downregulating MiR-143. Cancer Manag. Res. 2020, 12, 1661–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of Tumor-Derived Exosomes in Cancer Metastasis. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 12–19. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as Nucleic Acid Nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Schey, K.L.; Luther, J.M.; Rose, K.L. Proteomics Characterization of Exosome Cargo. Methods 2015, 87, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Puhka, M.; Takatalo, M.; Nordberg, M.-E.; Valkonen, S.; Nandania, J.; Aatonen, M.; Yliperttula, M.; Laitinen, S.; Velagapudi, V.; Mirtti, T.; et al. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics 2017, 7, 3824–3841. [Google Scholar] [CrossRef]
- Weng, J.; Xiang, X.; Ding, L.; Wong, A.L.-A.; Zeng, Q.; Sethi, G.; Wang, L.; Lee, S.C.; Goh, B.C. Extracellular Vesicles, the Cornerstone of next-Generation Cancer Diagnosis? Semin. Cancer Biol. 2021, 74, 105–120. [Google Scholar] [CrossRef]
- Kok, V.C.; Yu, C.-C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Zheng, R.; Du, M.; Wang, X.; Xu, W.; Liang, J.; Wang, W.; Lv, Q.; Qin, C.; Chu, H.; Wang, M.; et al. Exosome–Transmitted Long Non-Coding RNA PTENP1 Suppresses Bladder Cancer Progression. Mol. Cancer 2018, 17, 143. [Google Scholar] [CrossRef]
- Yu, W.-D.; Wang, H.; He, Q.-F.; Xu, Y.; Wang, X.-C. Long Noncoding RNAs in Cancer-Immunity Cycle. J. Cell. Physiol. 2018, 233, 6518–6523. [Google Scholar] [CrossRef]
- Abildgaard, C.; Do Canto, L.M.; Steffensen, K.D.; Rogatto, S.R. Long Non-Coding RNAs Involved in Resistance to Chemotherapy in Ovarian Cancer. Front. Oncol. 2019, 9, 1549. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, K.S.; Raza, A.; Karedath, T.; Raza, S.S.; Fathima, H.; Ahmed, E.I.; Kuttikrishnan, S.; Therachiyil, L.; Kulinski, M.; Dermime, S.; et al. Non-Coding RNAs as Regulators and Markers for Targeting of Breast Cancer and Cancer Stem Cells. Cancers 2020, 12, 351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, L.; Zhan, Y.; Wang, J.; Zhu, Z.; Zhang, X. Identification of Immune-Related LncRNA Signature to Predict Prognosis and Immunotherapeutic Efficiency in Bladder Cancer. Front. Oncol. 2020, 10, 542140. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Zhao, H.; Li, Q.; Hu, R.; Wang, H. Integrin Β8 Facilitates Tumor Growth and Drug Resistance through a Y-Box Binding Protein 1-Dependent Signaling Pathway in Bladder Cancer. Cancer Sci. 2020, 111, 2423–2430. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, T.; Xia, W.; Li, Q.; Chen, X.; Liu, X.; Wei, P.; Xu, W.; Lv, M. Oblongifolin C Reverses GEM Resistance via Suppressing Autophagy Flux in Bladder Cancer Cells. Exp. Ther. Med. 2020, 20, 1431–1440. [Google Scholar] [CrossRef]
- Xiong, Y.; Ju, L.; Yuan, L.; Chen, L.; Wang, G.; Xu, H.; Peng, T.; Luo, Y.; Xiao, Y.; Wang, X. KNSTRN Promotes Tumorigenesis and Gemcitabine Resistance by Activating AKT in Bladder Cancer. Oncogene 2021, 40, 1595–1608. [Google Scholar] [CrossRef]
- Feng, S.Q.; Zhang, X.Y.; Fan, H.T.; Sun, Q.J.; Zhang, M. Up-Regulation of LncRNA MEG3 Inhibits Cell Migration and Invasion and Enhances Cisplatin Chemosensitivity in Bladder Cancer Cells. Neoplasma 2018, 65, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Long Non-Coding RNA DLEU1 Promotes Cell Proliferation, Invasion, and Confers Cisplatin Resistance in Bladder Cancer by Regulating the MiR-99b/HS3ST3B1 Axis. Front. Genet. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zeng, H.; He, X.; Chen, J. Sirtuin 3 Alleviates Diabetic Cardiomyopathy by Regulating TIGAR and Cardiomyocyte Metabolism. J. Am. Heart Assoc. 2021, 10, e018913. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Cao, L. LncRNA MST1P2/MiR-133b Axis Affects the Chemoresistance of Bladder Cancer to Cisplatin-Based Therapy via Sirt1/P53 Signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22452. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. Long Non-Coding RNA UCA1 Increases Chemoresistance of Bladder Cancer Cells by Regulating Wnt Signaling. FEBS J. 2014, 281, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Wu, W.; Xue, M.; Hou, H.; Zhai, W.; Chen, W. Long Non-Coding RNA UCA1 Promotes Cisplatin/Gemcitabine Resistance through CREB Modulating MiR-196a-5p in Bladder Cancer Cells. Cancer Lett. 2016, 382, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Elkin, M.; Shevelev, A.; Schulze, E.; Tykocinsky, M.; Cooper, M.; Ariel, I.; Pode, D.; Kopf, E.; de Groot, N.; Hochberg, A. The Expression of the Imprinted H19 and IGF-2 Genes in Human Bladder Carcinoma. FEBS Lett. 1995, 374, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Ohana, P.; Kopf, E.; Bibi, O.; Ayesh, S.; Schneider, T.; Laster, M.; Tykocinski, M.; de Groot, N.; Hochberg, A. The Expression of the H19 Gene and Its Function in Human Bladder Carcinoma Cell Lines. FEBS Lett. 1999, 454, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Ariel, I.; Sughayer, M.; Fellig, Y.; Pizov, G.; Ayesh, S.; Podeh, D.; Libdeh, B.A.; Levy, C.; Birman, T.; Tykocinski, M.L.; et al. The Imprinted H19 Gene Is a Marker of Early Recurrence in Human Bladder Carcinoma. Mol. Pathol. 2000, 53, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Long Non-Coding RNA H19 Increases Bladder Cancer Metastasis by Associating with EZH2 and Inhibiting E-Cadherin Expression. Cancer Lett. 2013, 333, 213–221. [Google Scholar] [CrossRef]
- Chen, L.-H.; Hsu, W.-L.; Tseng, Y.-J.; Liu, D.-W.; Weng, C.-F. Involvement of DNMT 3B Promotes Epithelial-Mesenchymal Transition and Gene Expression Profile of Invasive Head and Neck Squamous Cell Carcinomas Cell Lines. BMC Cancer 2016, 16, 431. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yu, Z.; Chen, S.-S.; Li, F.; Lei, C.-Y.; Chen, X.-X.; Bao, J.-M.; Luo, Y.; Lin, G.-Z.; Pang, S.-Y.; et al. The YAP1 Oncogene Contributes to Bladder Cancer Cell Proliferation and Migration by Regulating the H19 Long Noncoding RNA. Urol. Oncol. 2015, 33, 427.e1–427.e10. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Nie, L.; Gui, Y.; Cai, Z. Inducing Cell Proliferation Inhibition, Apoptosis, and Motility Reduction by Silencing Long Noncoding Ribonucleic Acid Metastasis-Associated Lung Adenocarcinoma Transcript 1 in Urothelial Carcinoma of the Bladder. Urology 2013, 81, 209.e1–209.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ding, L.; Wang, L.; Zhao, Y.; Sun, Z.; Karnes, R.J.; Zhang, J.; Huang, H. LncRNA MALAT1 Enhances Oncogenic Activities of EZH2 in Castration-Resistant Prostate Cancer. Oncotarget 2015, 6, 41045–41055. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. TGF-β-Induced Upregulation of Malat1 Promotes Bladder Cancer Metastasis by Associating with Suz12. Clin. Cancer Res. 2014, 20, 1531–1541. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Chen, Q.; Wang, Y.; Zhou, Z.; Huang, Y.; Qiu, F. Upregulated MALAT-1 Contributes to Bladder Cancer Cell Migration by Inducing Epithelial-to-Mesenchymal Transition. Mol. Biosyst. 2012, 8, 2289–2294. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Liu, Y.-L.; Fei, K.-L.; Li, P. Long Non-Coding RNA HOTAIR Modulates the Progression of Preeclampsia through Inhibiting MiR-106 in an EZH2-Dependent Manner. Life Sci. 2020, 253, 117668. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Gholami, M.H.; Mirzaei, S.; Zabolian, A.; Haddadi, A.; Farahani, M.V.; Kashani, S.H.; Hushmandi, K.; Najafi, M.; Zarrabi, A.; et al. Dual Relationship between Long Non-Coding RNAs and STAT3 Signaling in Different Cancers: New Insight to Proliferation and Metastasis. Life Sci. 2021, 270, 119006. [Google Scholar] [CrossRef]
- Terreri, S.; Durso, M.; Colonna, V.; Romanelli, A.; Terracciano, D.; Ferro, M.; Perdonà, S.; Castaldo, L.; Febbraio, F.; de Nigris, F.; et al. New Cross-Talk Layer between Ultraconserved Non-Coding RNAs, MicroRNAs and Polycomb Protein YY1 in Bladder Cancer. Genes 2016, 7, 127. [Google Scholar] [CrossRef]
- Olivieri, M.; Ferro, M.; Terreri, S.; Durso, M.; Romanelli, A.; Avitabile, C.; De Cobelli, O.; Messere, A.; Bruzzese, D.; Vannini, I.; et al. Long Non-Coding RNA Containing Ultraconserved Genomic Region 8 Promotes Bladder Cancer Tumorigenesis. Oncotarget 2016, 7, 20636–20654. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zou, H.; Slaughter, C.; Wang, X. DFF, a Heterodimeric Protein That Functions Downstream of Caspase-3 to Trigger DNA Fragmentation during Apoptosis. Cell 1997, 89, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Terreri, S.; Mancinelli, S.; Ferro, M.; Vitale, M.C.; Perdonà, S.; Castaldo, L.; Gigantino, V.; Mercadante, V.; Cecio, R.D.; Aquino, G.; et al. Subcellular Localization of Uc.8+ as a Prognostic Biomarker in Bladder Cancer Tissue. Cancers 2021, 13, 681. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, L.; Li, Y.; Chen, M.; He, W.; Qi, L. The Long Non-Coding RNA XIST Interacted with MiR-124 to Modulate Bladder Cancer Growth, Invasion and Migration by Targeting Androgen Receptor (AR). Cell. Physiol. Biochem. 2017, 43, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, S.; Paskeh, M.D.A.; Hashemi, F.; Zabolian, A.; Hashemi, M.; Entezari, M.; Tabari, T.; Ashrafizadeh, M.; Raee, P.; Aghamiri, S.; et al. Long Non-Coding RNAs as New Players in Bladder Cancer: Lessons from Pre-Clinical and Clinical Studies. Life Sci. 2022, 288, 119948. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, K.; Montazeri, J. Erdafitinib for the treatment of metastatic bladder cancer. Expert Rev Clin Pharm. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Folkvaljon, Y.; Makarov, D.V.; Bratt, O.; Bill-Axelson, A.; Stattin, P. Five-Year Nationwide Follow-up Study of Active Surveillance for Prostate Cancer. Eur. Urol. 2015, 67, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Gillessen, S.; Omlin, A.; Attard, G.; de Bono, J.S.; Efstathiou, E.; Fizazi, K.; Halabi, S.; Nelson, P.S.; Sartor, O.; Smith, M.R.; et al. Management of Patients with Advanced Prostate Cancer: Recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann. Oncol. 2015, 26, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular Determinants of Resistance to Antiandrogen Therapy. Nat. Med. 2004, 10, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging Mechanisms of Resistance to Androgen Receptor Inhibitors in Prostate Cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the Castration-Resistant Prostate Cancer Population: A Systematic Review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone Acetate for Treatment of Metastatic Castration-Resistant Prostate Cancer: Final Overall Survival Analysis of the COU-AA-301 Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.A.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone Acetate plus Prednisone versus Placebo plus Prednisone in Chemotherapy-Naive Men with Metastatic Castration-Resistant Prostate Cancer (COU-AA-302): Final Overall Survival Analysis of a Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.-S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Sawyers, C.L. Biology of Progressive, Castration-Resistant Prostate Cancer: Directed Therapies Targeting the Androgen-Receptor Signaling Axis. J. Clin. Oncol. 2005, 23, 8253–8261. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Iczkowski, K.A.; Kilari, D.; See, W.; Nevalainen, M.T. Androgen Receptor-Dependent and -Independent Mechanisms Driving Prostate Cancer Progression: Opportunities for Therapeutic Targeting from Multiple Angles. Oncotarget 2016, 8, 3724–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Qi, M.; Zhang, J.; Sun, X.; Guo, H.; Pang, Y.; Zhang, Q.; Chen, X.; Zhang, R.; Liu, Z.; et al. Differential Response to Neoadjuvant Hormonal Therapy in Prostate Cancer: Predictive Morphological Parameters and Molecular Markers. Prostate 2019, 79, 709–719. [Google Scholar] [CrossRef] [PubMed]
- De Winter, J.A.R.; Trapman, J.; Brinkmann, A.O.; Boersma, W.J.A.; Mulder, E.; Schroeder, F.H.; Claassen, E.; Van Der Kwast, T.H. Androgen Receptor Heterogeneity in Human Prostatic Carcinomas Visualized by Immunohistochemistry. J. Pathol. 1990, 160, 329–332. [Google Scholar] [CrossRef]
- Choucair, K.; Ejdelman, J.; Brimo, F.; Aprikian, A.; Chevalier, S.; Lapointe, J. PTENgenomic Deletion Predicts Prostate Cancer Recurrence and Is Associated with Low AR Expression and Transcriptional Activity. BMC Cancer 2012, 12, 543. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Goto, Y.; Naya, Y. The Roles of MicroRNAs in the Progression of Castration-Resistant Prostate Cancer. J. Hum. Genet. 2017, 62, 25–31. [Google Scholar] [CrossRef]
- Shih, J.-W.; Wang, L.-Y.; Hung, C.-L.; Kung, H.-J.; Hsieh, C.-L. Non-Coding RNAs in Castration-Resistant Prostate Cancer: Regulation of Androgen Receptor Signaling and Cancer Metabolism. Int. J. Mol. Sci. 2015, 16, 28943–28978. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Wang, R.; Shen, D.; Cheng, S.; Wang, H.; Lu, Z.; Zheng, Q.; Wang, L.; Xia, L.; Li, G. Role of Noncoding RNA in Drug Resistance of Prostate Cancer. Cell Death Dis. 2021, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Ramnarine, V.R.; Kobelev, M.; Gibb, E.A.; Nouri, M.; Lin, D.; Wang, Y.; Buttyan, R.; Davicioni, E.; Zoubeidi, A.; Collins, C.C. The Evolution of Long Noncoding RNA Acceptance in Prostate Cancer Initiation, Progression, and Its Clinical Utility in Disease Management. Eur. Urol. 2019, 76, 546–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Pitchiaya, S.; Cieślik, M.; Niknafs, Y.S.; Tien, J.C.-Y.; Hosono, Y.; Iyer, M.K.; Yazdani, S.; Subramaniam, S.; Shukla, S.K.; et al. Analysis of the Androgen Receptor–Regulated LncRNA Landscape Identifies a Role for ARLNC1 in Prostate Cancer Progression. Nat. Genet. 2018, 50, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, C.; Jin, C.; Yang, J.C.; Tanasa, B.; Li, W.; Merkurjev, D.; Ohgi, K.A.; Meng, D.; Zhang, J.; et al. LncRNA-Dependent Mechanisms of Androgen-Receptor-Regulated Gene Activation Programs. Nature 2013, 500, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Bardhan, A.; Banerjee, A.; Basu, K.; Pal, D.K.; Ghosh, A. PRNCR1: A Long Non-Coding RNA with a Pivotal Oncogenic Role in Cancer. Hum. Genet. 2022, 141, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Aird, J.; Baird, A.-M.; Lim, M.C.J.; McDermott, R.; Finn, S.P.; Gray, S.G. Carcinogenesis in Prostate Cancer: The Role of Long Non-Coding RNAs. Non-Coding RNA Res. 2018, 3, 29–38. [Google Scholar] [CrossRef]
- Tam, C.; Wong, J.H.; Tsui, S.K.W.; Zuo, T.; Chan, T.F.; Ng, T.B. LncRNAs with MiRNAs in Regulation of Gastric, Liver, and Colorectal Cancers: Updates in Recent Years. Appl. Microbiol. Biotechnol. 2019, 103, 4649–4677. [Google Scholar] [CrossRef]
- Sun, B.; Liu, C.; Li, H.; Zhang, L.; Luo, G.; Liang, S.; Lü, M. Research Progress on the Interactions between Long Non-coding RNAs and MicroRNAs in Human Cancer (Review). Oncol. Lett. 2020, 19, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, T.; Liu, L.; Zhang, W.; Zhao, C.; Li, S.; Li, J.; Rao, N.; Le, T.D. LMSM: A Modular Approach for Identifying LncRNA Related MiRNA Sponge Modules in Breast Cancer. PLoS Comput. Biol. 2020, 16, e1007851. [Google Scholar] [CrossRef] [Green Version]
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-Coding RNAs, Micro-RNAs and MRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Wu, K.-Y.; Lin, T.-Y.; Chen, C.-C. The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance. Int. J. Mol. Sci. 2022, 23, 392. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, H.-Y.; Yuan, T.; Zhou, W.-D.; Xiang, Z.-D.; Jiang, Q.-Q.; Wu, D.-L. Long Non-Coding RNA AFAP1-AS1 Facilitates Prostate Cancer Progression by Regulating MiR-15b/IGF1R Axis. Curr. Pharm. Des. 2021, 27, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liao, Q.; Li, W.; Deng, G.; Jia, M.; Fang, Q.; Ji, H.; Meng, M. The LncRNA PTTG3P Promotes the Progression of CRPC via Upregulating PTTG1. Bull. Cancer 2021, 108, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, H.; Xiao, N.; Duan, J.; Wang, Z.; Wang, S. Long Noncoding RNA SChLAP1 Accelerates the Proliferation and Metastasis of Prostate Cancer via Targeting MiR-198 and Promoting the MAPK1 Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, S.; Jin, G.; Zhou, X.; Li, M.; Ying, X.; Wang, L.; Wu, H.; Zhu, Q. Linc00963: A Novel, Long Non-Coding RNA Involved in the Transition of Prostate Cancer from Androgen-Dependence to Androgen-Independence. Int. J. Oncol. 2014, 44, 2041–2049. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; He, C.; Shi, S.; Wang, M.; Ma, J.; Yang, P.; Dong, Y.; Mou, X.; Han, S. Linc00963 Promote Cell Proliferation and Tumor Growth in Castration-Resistant Prostate Cancer by Modulating MiR-655/TRIM24 Axis. Front. Oncol. 2021, 11, 636965. [Google Scholar] [CrossRef]
- Groner, A.C.; Cato, L.; de Tribolet-Hardy, J.; Bernasocchi, T.; Janouskova, H.; Melchers, D.; Houtman, R.; Cato, A.C.B.; Tschopp, P.; Gu, L.; et al. TRIM24 Is an Oncogenic Transcriptional Activator in Prostate Cancer. Cancer Cell 2016, 29, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Wen, B.; Wu, Q.; Cheng, W.; Lou, J.; Wei, J.; Huang, J.; Yao, X.; Weng, G. Long Noncoding RNA SNHG7 Accelerates Prostate Cancer Proliferation and Cycle Progression through Cyclin D1 by Sponging MiR-503. Biomed. Pharm. 2018, 102, 326–332. [Google Scholar] [CrossRef]
- Long, B.; Li, N.; Xu, X.-X.; Li, X.-X.; Xu, X.-J.; Liu, J.-Y.; Wu, Z.-H. Long Noncoding RNA LOXL1-AS1 Regulates Prostate Cancer Cell Proliferation and Cell Cycle Progression through MiR-541-3p and CCND1. Biochem. Biophys. Res. Commun. 2018, 505, 561–568. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhang, Q.; Wang, F.; Zhang, D. Toll-like Receptors and Prostate Cancer. Front. Immunol. 2014, 5, 352. [Google Scholar] [CrossRef] [Green Version]
- Galli, R.; Starace, D.; Busà, R.; Angelini, D.F.; Paone, A.; De Cesaris, P.; Filippini, A.; Sette, C.; Battistini, L.; Ziparo, E.; et al. TLR Stimulation of Prostate Tumor Cells Induces Chemokine-Mediated Recruitment of Specific Immune Cell Types. J. Immunol. 2010, 184, 6658–6669. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Geng, D.; Li, S.; Chen, Z.; Zhao, W. LncRNA PART1 Modulates Toll-like Receptor Pathways to Influence Cell Proliferation and Apoptosis in Prostate Cancer Cells. Biol. Chem. 2018, 399, 387–395. [Google Scholar] [CrossRef]
- Wu, H.; Tian, X.; Zhu, C. Knockdown of LncRNA PVT1 Inhibits Prostate Cancer Progression In Vitro and in Vivo by the Suppression of KIF23 through Stimulating MiR-15a-5p. Cancer Cell Int. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Akoto, T.; Saini, S. Role of Exosomes in Prostate Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3528. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.T.; Brown, J. Prostate Cancer Bone Metastases Promote Both Osteolytic and Osteoblastic Activity. J. Cell. Biochem. 2004, 91, 718–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretschmer, A.; Tilki, D. Biomarkers in Prostate Cancer—Current Clinical Utility and Future Perspectives. Crit. Rev. Oncol. Hematol 2017, 120, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Saini, S. PSA and beyond: Alternative Prostate Cancer Biomarkers. Cell. Oncol. 2016, 39, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.; Pai, S.K.; Gross, S.C.; Hirota, S.; Hosobe, S.; Miura, K.; Saito, K.; Commes, T.; Hayashi, S.; Watabe, M.; et al. The Drg-1 Gene Suppresses Tumor Metastasis in Prostate Cancer. Cancer Res. 2003, 63, 1731–1736. [Google Scholar]
- Lingadahalli, S.; Jadhao, S.; Sung, Y.Y.; Chen, M.; Hu, L.; Chen, X.; Cheung, E. Novel LncRNA LINC00844 Regulates Prostate Cancer Cell Migration and Invasion through AR Signaling. Mol. Cancer Res. 2018, 16, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma. Front. Pharm. 2018, 9, 365. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharm. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Furusato, B.; Mohamed, A.; Uhlén, M.; Rhim, J.S. CXCR4 and Cancer. Pathol. Int. 2010, 60, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A. New Insights on the Role of CXCR4 in Cancer Metastasis. J. Pathol. 2008, 215, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The Role of CXC Chemokines and Their Receptors in Cancer. Cancer Lett. 2008, 267, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Don-Salu-Hewage, A.S.; Chan, S.Y.; McAndrews, K.M.; Chetram, M.A.; Dawson, M.R.; Bethea, D.A.; Hinton, C.V. Cysteine (C)-X-C Receptor 4 Undergoes Transportin 1-Dependent Nuclear Localization and Remains Functional at the Nucleus of Metastatic Prostate Cancer Cells. PLoS ONE 2013, 8, e57194. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhong, T. The Association of CXCR4 Expression with Clinicopathological Significance and Potential Drug Target in Prostate Cancer: A Meta-Analysis and Literature Review. Drug Des. Dev. Ther. 2015, 9, 5115–5122. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Lu, X.; Yang, F.; Qin, L.; Guo, Z.; Sun, Y.; Wu, J. LncRNA UCA1 Acts as a Sponge of MiR-204 to up-Regulate CXCR4 Expression and Promote Prostate Cancer Progression. Biosci. Rep. 2019, 39, BSR20181465. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Qiu, K.; Huang, W. Long Non-Coding RNA (LncRNA) RAMS11 Promotes Metastatis and Cell Growth of Prostate Cancer by CBX4 Complex Binding to Top2alpha. Cancer Manag. Res. 2021, 13, 913–923. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, W.; Nian, X.; Lu, X.; Li, Y.; Liu, F.; Wang, F.; He, B.; Zhao, L.; Zhu, Y.; et al. The Previously Uncharacterized LncRNA APP Promotes Prostate Cancer Progression by Acting as a Competing Endogenous RNA. Int. J. Cancer 2020, 146, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yi, X.-M.; Tang, C.-P.; Ge, J.-P.; Zhang, Z.-Y.; Zhou, W.-Q. Long Non-Coding RNA ATB Promotes Growth and Epithelial-Mesenchymal Transition and Predicts Poor Prognosis in Human Prostate Carcinoma. Oncol. Rep. 2016, 36, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, Y.; Fang, Z.; He, W.; Li, S. Integrated Characterization of LncRNA-Immune Interactions in Prostate Cancer. Front. Cell Dev. Biol. 2021, 9, 641891. [Google Scholar] [CrossRef]
- Dong, L.; Ding, H.; Li, Y.; Xue, D.; Liu, Y. LncRNA TINCR Is Associated with Clinical Progression and Serves as Tumor Suppressive Role in Prostate Cancer. Cancer Manag. Res. 2018, 10, 2799–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.-Y.; Wu, W.-S.; Lin, L.-C.; Wu, Y.-H.; Chiu, H.-W.; Yeh, Y.-L.; Huang, B.-M.; Wang, Y.-J. Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. Int. J. Mol. Sci. 2019, 20, 5366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; Mao, Y.; Ma, X. The LncRNA PCAT1 Is Correlated with Poor Prognosis and Promotes Cell Proliferation, Invasion, Migration and EMT in Osteosarcoma. OncoTargets Ther. 2018, 11, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Işın, M.; Uysaler, E.; Özgür, E.; Köseoğlu, H.; Şanlı, Ö.; Yücel, Ö.B.; Gezer, U.; Dalay, N. Exosomal LncRNA-P21 Levels May Help to Distinguish Prostate Cancer from Benign Disease. Front. Genet. 2015, 6, 168. [Google Scholar]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural Product-Based Nanoformulations for Cancer Therapy: Opportunities and Challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Hussain, Y.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Khan, H.; Daglia, M. Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance. Cancers 2021, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating LncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef] [Green Version]
- Termini, D.; Den Hartogh, D.J.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Liu, T.; Chi, H.; Chen, J.; Chen, C.; Huang, Y.; Xi, H.; Xue, J.; Si, Y. Curcumin Suppresses Proliferation and In Vitro Invasion of Human Prostate Cancer Stem Cells by CeRNA Effect of MiR-145 and LncRNA-ROR. Gene 2017, 631, 29–38. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Okina, E.; Gholami, M.H.; Hushmandi, K.; Hashemi, M.; Kalu, A.; Zarrabi, A.; Nabavi, N.; Rabiee, N.; et al. Molecular Landscape of LncRNAs in Prostate Cancer: A Focus on Pathways and Therapeutic Targets for Intervention. J. Exp. Clin. Cancer Res. 2022, 41, 214. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Ovarian Carcinomas: Five Distinct Diseases with Different Origins, Genetic Alterations, and Clinicopathological Features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-Year Relative Survival for Epithelial Ovarian Cancer. Obs. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, M.; Yang, H.; Zhu, S.; Cheng, X.; Qing, C. Next-Generation Sequencing-Based Genomic Profiling Analysis Reveals Novel Mutations for Clinical Diagnosis in Chinese Primary Epithelial Ovarian Cancer Patients. J. Ovarian Res. 2019, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Burges, A.; Schmalfeldt, B. Ovarian Cancer: Diagnosis and Treatment. Dtsch. Arztebl. Int. 2011, 108, 635–641. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing Treatment in Recurrent Epithelial Ovarian Cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, Y.K. Regulation of Ovarian Cancer Stem Cells or Tumor-Initiating Cells. Int. J. Mol. Sci. 2013, 14, 6624–6648. [Google Scholar] [CrossRef] [Green Version]
- Meryet-Figuière, M.; Lambert, B.; Gauduchon, P.; Vigneron, N.; Brotin, E.; Poulain, L.; Denoyelle, C. An Overview of Long Non-Coding RNAs in Ovarian Cancers. Oncotarget 2016, 7, 44719–44734. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Feng, Y.; Zhang, D.; Zhao, S.D.; Hu, Z.; Greshock, J.; Zhang, Y.; Yang, L.; Zhong, X.; Wang, L.-P.; et al. A Functional Genomic Approach Identifies FAL1 as an Oncogenic Long Noncoding RNA That Associates with BMI1 and Represses P21 Expression in Cancer. Cancer Cell 2014, 26, 344–357. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Ji, G.; Le, X.; Wang, C.; Xu, L.; Feng, M.; Zhang, Y.; Yang, H.; Xuan, Y.; Yang, Y.; et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017, 77, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Leng, T.; Zhang, Q.; Zhao, Q.; Nie, X.; Yang, L. Sanguinarine Inhibits Epithelial Ovarian Cancer Development via Regulating Long Non-Coding RNA CASC2-EIF4A3 Axis and/or Inhibiting NF-ΚB Signaling or PI3K/AKT/MTOR Pathway. Biomed. Pharm. 2018, 102, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long Non-Coding RNA HOTTIP Enhances IL-6 Expression to Potentiate Immune Escape of Ovarian Cancer Cells by Upregulating the Expression of PD-L1 in Neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, M.A.; Babbs, B.; Cochrane, D.R.; Bitler, B.G.; Richer, J.K. The Long Non-Coding RNA MALAT1 Promotes Ovarian Cancer Progression by Regulating RBFOX2-Mediated Alternative Splicing. Mol. Carcinog. 2019, 58, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-P.; Yang, J.-X.; Cao, D.-Y.; Shen, K. Identification of Differentially Expressed Long Non-Coding RNAs in Human Ovarian Cancer Cells with Different Metastatic Potentials. Cancer Biol. Med. 2013, 10, 138–141. [Google Scholar] [CrossRef]
- Liu, E.; Liu, Z.; Zhou, Y. Carboplatin-Docetaxel-Induced Activity against Ovarian Cancer Is Dependent on up-Regulated LncRNA PVT1. Int. J. Clin. Exp. Pathol. 2015, 8, 3803–3810. [Google Scholar]
- Worku, T.; Bhattarai, D.; Ayers, D.; Wang, K.; Wang, C.; Rehman, Z.U.; Talpur, H.S.; Yang, L. Long Non-Coding RNAs: The New Horizon of Gene Regulation in Ovarian Cancer. Cell. Physiol. Biochem. 2017, 44, 948–966. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Hardin, H.; Zhang, R.; Helein, H.; Buehler, D.; Guo, Z.; Lloyd, R.V. The Evolving Concept of Cancer Stem-like Cells in Thyroid Cancer and Other Solid Tumors. Lab. Investig. 2017, 97, 1142–1151. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wang, F.; Guan, Z.; Ge, Y.; Yang, X.; Cai, J. LncRNAs and Their Role in Cancer Stem Cells. Oncotarget 2017, 8, 110685–110692. [Google Scholar] [CrossRef]
- Adorno-Cruz, V.; Kibria, G.; Liu, X.; Doherty, M.; Junk, D.J.; Guan, D.; Hubert, C.; Venere, M.; Mulkearns-Hubert, E.; Sinyuk, M.; et al. Cancer Stem Cells: Targeting the Roots of Cancer, Seeds of Metastasis, and Sources of Therapy Resistance. Cancer Res. 2015, 75, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T.; Mehta, G. The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccelli, I.; Trumpp, A. The Evolving Concept of Cancer and Metastasis Stem Cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of Screening on Ovarian Cancer Mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Ni, J.; Zhu, Y.; Pang, B.; Graham, P.; Zhang, H.; Li, Y. Liquid Biopsy in Ovarian Cancer: Recent Advances in Circulating Extracellular Vesicle Detection for Early Diagnosis and Monitoring Progression. Theranostics 2019, 9, 4130–4140. [Google Scholar] [CrossRef]
- Redis, R.S.; Vela, L.E.; Lu, W.; Ferreira de Oliveira, J.; Ivan, C.; Rodriguez-Aguayo, C.; Adamoski, D.; Pasculli, B.; Taguchi, A.; Chen, Y.; et al. Allele-Specific Reprogramming of Cancer Metabolism by the Long Non-Coding RNA CCAT2. Mol. Cell 2016, 61, 520–534. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.-J.; Lin, Y.-Y.; Ding, J.-X.; Feng, W.-W.; Jin, H.-Y.; Hua, K.-Q. Long Non-Coding RNA ANRIL Predicts Poor Prognosis and Promotes Invasion/Metastasis in Serous Ovarian Cancer. Int. J. Oncol. 2015, 46, 2497–2505. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Gao, G.; Cao, Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis. Mrk. 2016, 2016, 9085195. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Liu, X.-F.; Wang, Y.-C.; Li, N.-D.; Liao, S.-J.; Yu, M.-X.; Liang, C.-Z.; Tu, J.-C. Prognostic Value of Abnormally Expressed LncRNAs in Ovarian Carcinoma: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 23927–23936. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, H.; Xi, M.; Lu, X. Long Noncoding RNA C17orf91 Is a Potential Prognostic Marker and Functions as an Oncogene in Ovarian Cancer. J. Ovarian Res. 2016, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, H.; Li, Y.; Li, L.; Hou, W.; You, Z. Decreased Expression of Long Non-Coding RNA GAS5 Promotes Cell Proliferation, Migration and Invasion, and Indicates a Poor Prognosis in Ovarian Cancer. Oncol. Rep. 2016, 36, 3241–3250. [Google Scholar] [CrossRef] [Green Version]
- Al-Rugeebah, A.; Alanazi, M.; Parine, N.R. MEG3: An Oncogenic Long Non-Coding RNA in Different Cancers. Pathol. Oncol. Res. 2019, 25, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ma, X.; Yu, H.; Yang, J. SNHG15: A Promising Cancer-Related Long Noncoding RNA. Cancer Manag. Res. 2019, 11, 5961–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, V.; Tan, Y.Q.; Wu, M.M.; Ma, L.; Zhu, T.; Lobie, P.E.; Pandey, V. Long Non-Coding RNAs in Recurrent Ovarian Cancer: Theranostic Perspectives. Cancer Lett. 2021, 502, 97–107. [Google Scholar] [CrossRef]
- Bo, H.; Zhu, F.; Liu, Z.; Deng, Q.; Liu, G.; Li, R.; Zhu, W.; Tan, Y.; Liu, G.; Fan, J.; et al. Integrated Analysis of High-Throughput Sequencing Data Reveals the Key Role of LINC00467 in the Invasion and Metastasis of Testicular Germ Cell Tumors. Cell Death Discov. 2021, 7, 206. [Google Scholar] [CrossRef]
- Shanmugalingam, T.; Soultati, A.; Chowdhury, S.; Rudman, S.; Van Hemelrijck, M. Global Incidence and Outcome of Testicular Cancer. Clin. Epidemiol. 2013, 5, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J.; et al. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef]
- Gashaw, I.; Dushaj, O.; Behr, R.; Biermann, K.; Brehm, R.; Rübben, H.; Grobholz, R.; Schmid, K.W.; Bergmann, M.; Winterhager, E. Novel Germ Cell Markers Characterize Testicular Seminoma and Fetal Testis. Mol. Hum. Reprod. 2007, 13, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, J.; Reuter, V.; Bosl, G.J.; Chaganti, R.S. Aberrant Expression of Cyclin D2 Is an Early Event in Human Male Germ Cell Tumorigenesis. Cell Growth Differ. 1997, 8, 293–299. [Google Scholar]
- Schmidt, B.A.; Rose, A.; Steinhoff, C.; Strohmeyer, T.; Hartmann, M.; Ackermann, R. Up-Regulation of Cyclin-Dependent Kinase 4/Cyclin D2 Expression but down-Regulation of Cyclin-Dependent Kinase 2/Cyclin E in Testicular Germ Cell Tumors. Cancer Res. 2001, 61, 4214–4221. [Google Scholar]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.-Q.; Luo, X.-J.; Zhang, J.; Wang, G.-M.; Guo, J.-M. Crosstalk between Meg3 and MiR-1297 Regulates Growth of Testicular Germ Cell Tumor through PTEN/PI3K/AKT Pathway. Am. J. Transl. Res. 2016, 8, 1091–1099. [Google Scholar] [PubMed]
- Guo, J.; Wang, S.; Jiang, Z.; Tang, L.; Liu, Z.; Cao, J.; Hu, Z.; Chen, X.; Luo, Y.; Bo, H. Long Non-Coding RNA RFPL3S Functions as a Biomarker of Prognostic and Immunotherapeutic Prediction in Testicular Germ Cell Tumor. Front. Immunol. 2022, 13, 859730. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Chieffi, P.; Esposito, F. MiRNAs and Biomarkers in Testicular Germ Cell Tumors: An Update. Int. J. Mol. Sci. 2021, 22, 1380. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Lauritsen, J.; Bandak, M.; Oing, C.; Kier, G.G.; Kreiberg, M.; Rosenvilde, J.; Wagner, T.; Bokemeyer, C.; Daugaard, G. Late Adverse Effects and Quality of Life in Survivors of Testicular Germ Cell Tumour. Nat. Rev. Urol. 2021, 18, 227–245. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, C.; Liu, S.; Tang, J.; Zhang, J.; Bu, H.; Wang, Y.; Liang, C.; Bao, M.; Hou, C.; et al. Clusterin Suppresses Invasion and Metastasis of Testicular Seminoma by Upregulating COL15a1. Mol. Ther. Nucleic Acids 2021, 26, 1336–1350. [Google Scholar] [CrossRef]
- Andjilani, M.; Droz, J.-P.; Benahmed, M.; Tabone, E. Down-Regulation of FAK and IAPs by Laminin during Cisplatin-Induced Apoptosis in Testicular Germ Cell Tumors. Int. J. Oncol. 2006, 28, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in Anticancer Combination Therapies. Nat. Rev. Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef]
- Li, Q.; Sun, M.; Wang, M.; Feng, M.; Yang, F.; Li, L.; Zhao, J.; Chang, C.; Dong, H.; Xie, T.; et al. Dysregulation of Wnt/β-Catenin Signaling by Protein Kinases in Hepatocellular Carcinoma and Its Therapeutic Application. Cancer Sci. 2021, 112, 1695–1706. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-C.; Wang, J.; Shao, G.-G.; Wang, Q.; Qu, X.; Wang, B.; Moy, C.; Fan, Y.; Albertyn, Z.; Huang, X.; et al. Comprehensive Genomic and Immunological Characterization of Chinese Non-Small Cell Lung Cancer Patients. Nat. Commun. 2019, 10, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stampouloglou, E.; Cheng, N.; Federico, A.; Slaby, E.; Monti, S.; Szeto, G.L.; Varelas, X. Yap Suppresses T-Cell Function and Infiltration in the Tumor Microenvironment. PLoS Biol. 2020, 18, e3000591. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, F.; Shi, Y.; Zhang, Q.; Xu, S.; Yao, Y.; Jiang, R. RP11-323N12.5 Promotes the Malignancy and Immunosuppression of Human Gastric Cancer by Increasing YAP1 Transcription. Gastric Cancer 2021, 24, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ren, D.; Lei, X.; Shi, R.; Zhu, S.; Zhou, N.; Zu, L.; Mello, R.A.D.; Chen, J.; Xu, S. Mutations Associated with No Durable Clinical Benefit to Immune Checkpoint Blockade in Non-S-Cell Lung Cancer. Cancers 2021, 13, 1397. [Google Scholar] [CrossRef]
- Nachankar, A.; Krishnatry, R.; Joshi, A.; Noronha, V.; Agarwal, J.P. Primary Mediastinal Seminoma; Resistance and Relapse: An Aggressive Entity. Indian J. Med. Paediatr. Oncol. 2013, 34, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.; Honecker, F. Cisplatin Resistance in Germ Cell Tumours: Models and Mechanisms. Andrology 2015, 3, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wermann, H.; Stoop, H.; Gillis, A.J.M.; Honecker, F.; van Gurp, R.J.H.L.M.; Ammerpohl, O.; Richter, J.; Oosterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H.J. Global DNA Methylation in Fetal Human Germ Cells and Germ Cell Tumours: Association with Differentiation and Cisplatin Resistance. J. Pathol. 2010, 221, 433–442. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, Y.; Tan, Z.; Zhou, J.; Kitazawa, R.; Jiang, X.; Tang, Y.; Yang, J. TDRG1 Regulates Chemosensitivity of Seminoma TCam-2 Cells to Cisplatin via PI3K/Akt/MTOR Signaling Pathway and Mitochondria-Mediated Apoptotic Pathway. Cancer Biol. Ther. 2016, 17, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Gan, Y.; Peng, D.; Jiang, X.; Kitazawa, R.; Xiang, Y.; Dai, Y.; Tang, Y.; Yang, J. Long Non-Coding RNA H19 Promotes TDRG1 Expression and Cisplatin Resistance by Sequestering MiRNA-106b-5p in Seminoma. Cancer Med. 2018, 7, 6247–6257. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Nwagwu, C.; Anyim, O.; Ekweremadu, C.; Kim, S. COVID-19 and Cancer: From Basic Mechanisms to Vaccine Development Using Nanotechnology. Int. Immunopharmacol. 2021, 90, 107247. [Google Scholar] [CrossRef] [PubMed]

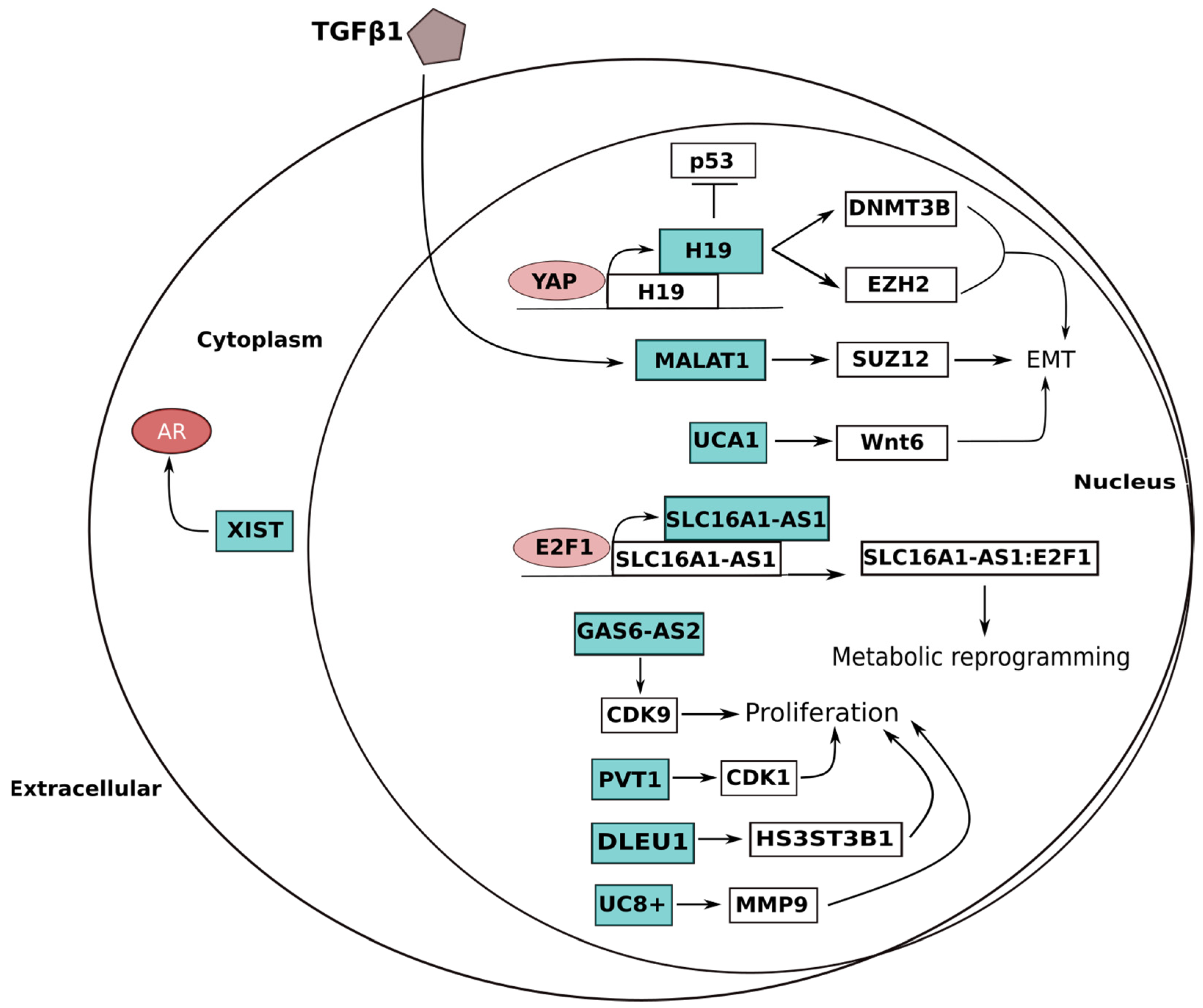
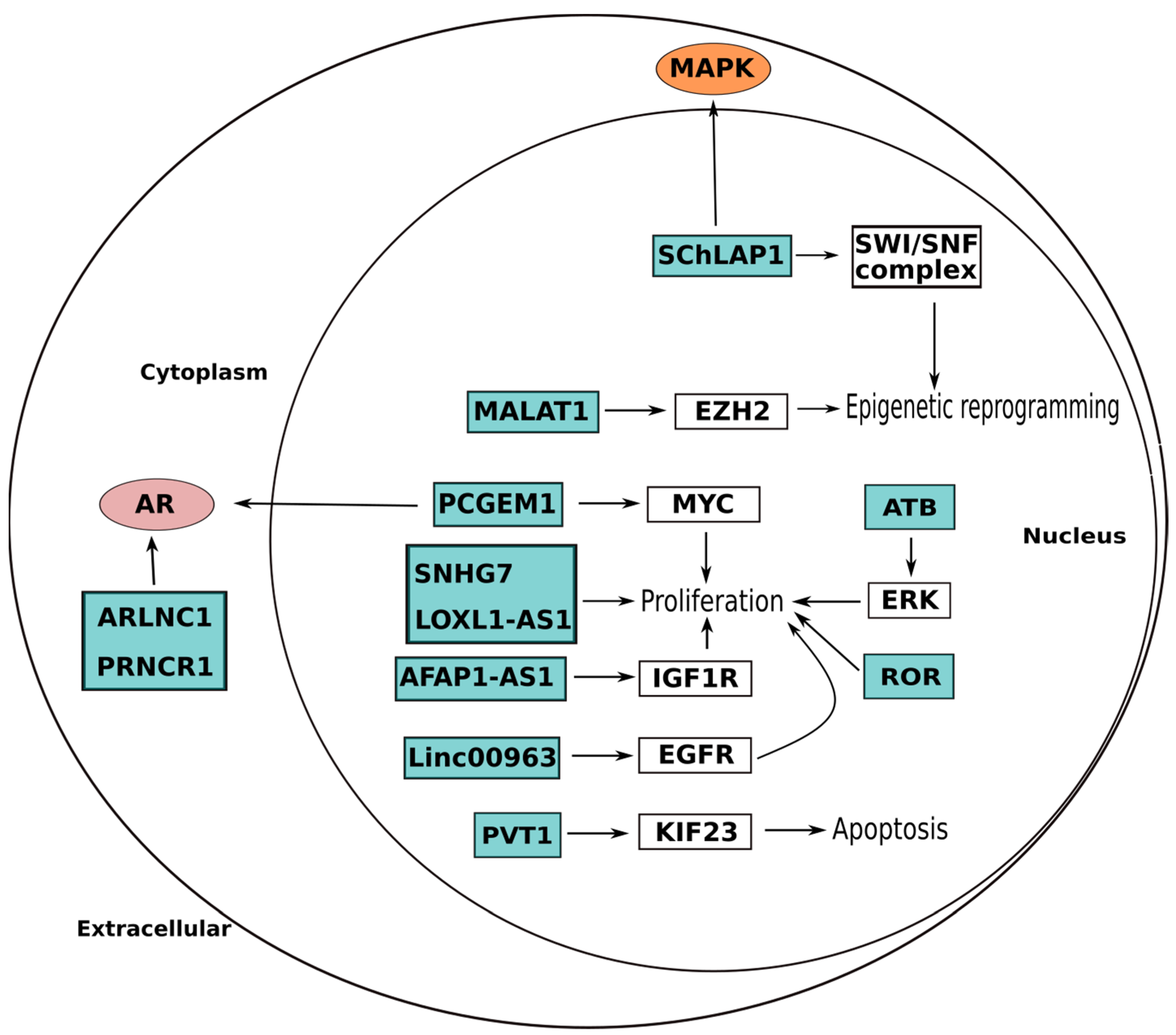
| LncRNA | Chr Location | Oncogenic Mechanisms | Specified Actions | Supposed Role | Expression Pattern * | Refs. |
|---|---|---|---|---|---|---|
| MALAT1 | 11q13 | EMT | (i) Inhibition of ZEB2 suppression by ceRNA; (ii) EZH2-mediated epigenetic reprogramming. | Oncogene | UP | [12,14] |
| ZFAS1 | 20q13 | Proliferation | (i) Control of miR-10a/SKA1 pathway; (ii) Control of miR-150-5P/HMGA2 molecular axis. | Oncogene | UP | [15] |
| CYTOR | 2p11.2 | Proliferation | (i) Control of miR-136-5-p/MAT2B/BAG3 axis; (ii) Control of miR-3679-5p/MACC1 axis. | Oncogene | UP | [16] |
| ROR | 8q21.31 | Proliferation | Control of miR-206/VEGF axis | Oncogene | UP | [24] |
| UCA1 | 9p13.12 | Proliferation | Control of miR-182-5p/DLL4 axis | Oncogene | UP | [25] |
| H19 | 11p15 | Proliferation | ceRNA preventing E2F1 repression | Oncogene | UP | [28,29] |
| HOTAIR | 12q13 | Proliferation Angiogenesis | (i) Action as a ceRNA preventing HIF1α repression; (ii) Epigenetic reprogramming by EZH2; (iii) IGFBP 2 expression’s upregulation. | Oncogene | UP | [31] |
| CADM1-AS1 | 11q23 | Adhesion | Upregulation of CADM1 expression | Suppressor | DOWN | [36] |
| GAS5 | 1q25 | Apoptosis | Promotion of P53-dependent and P53- independent apoptosis | Oncogene | UP | [37,38,39,40] |
| LncRNA | Chr Location | Oncogenic Mechanisms | Specified Actions | Supposed Role | Expression Pattern * | Refs. |
|---|---|---|---|---|---|---|
| MEG3 | 14q32 | Apoptosis | (i) Reduction of MMP2 and MMP9 expression; (ii) Reduction of Bcl-2 expression | Suppressor | DOWN | [101] |
| UCA1 | 19p13 | EMT Metabolism Cell cycle | (i) Induction of Wnt6 expression; (ii) CREB recruitment to enhance miRNA-196a-5p expression | Oncogene | UP | [105,106] |
| SLC16A1-AS1 | 1p13 | Proliferation Metabolism | (i) Stimulation of glycolysis and mitochondrial respiration; (ii) Increased β-oxidation of fatty acids | Oncogene | UP | [76] |
| GAS6-AS2 | 13q34 | Proliferation Cell cycle | CDK9 overexpression | Oncogene | UP | [80] |
| PVT1 | 8q24 | Proliferation | CDK1 upregulation | Oncogene | UP | [83] |
| PTENP1 | 9p13 | Apoptosis | (i) Sponge of miR-103a; (ii) Regulation of miR-103a/PDCD4 axis | Suppressor | DOWN | [93] |
| DLEU1 | 13q14 | Proliferation | Increase of HS3ST3B1 expression by inhibiting miRNA-99b | Oncogene | UP | [102] |
| MST1P2 | 1p36 | Increased chemoresistance | Enhancing of SIRT3 expression by inhibiting miRNA-133b | Oncogene | UP | [104] |
| H19 | 11p15 | EMT Apoptosis | (i) β-catenin upregulation by EZH2 binding; (ii) Action as a ceRNA preventing DNMT3B degradation | Oncogene | UP | [107,108,109,110] |
| MALAT1 | 11q13 | EMT | (i) Direct binding and activation of SUZ12; (ii) ZEB1, ZEB2 and SNAI2 upregulation | Oncogene | UP | [115] |
| XIST | Xq13 | AR pathway | ceRNA preventing AR suppression by miR-124 | Oncogene | UP | [123] |
| UC8+ | 1p36.22 | Proliferation | Sponge for miR-596, increasing the MMP9 expression | Oncogene | UP | [120,121,122] |
| LncRNA | Chr Location | Oncogenic Mechanisms | Specified Actions | Supposed Role | Expression Pattern * | Refs. |
|---|---|---|---|---|---|---|
| ARLNC1 | 16q23 | AR pathway | Stabilization of AR miRNA | Oncogene | UP | [153] |
| PRNCR1 | 8q24 | AR pathway | Direct AR binding and activation | Oncogene | UP | [155] |
| PCGEM1 | 2q32 | AR pathway Proliferation Invasion | (i) Direct AR binding and activation; (ii) Activation of MYC | Oncogene | UP | [154,156] |
| MALAT1 | 11q13 | Proliferation Invasion | Downregulation of multiple tumor suppressor genes through EZH2 binding | Oncogene | UP | [198] |
| UCA1 | 19p13 | Proliferation Invasion | Promotion of expression level of CXCR4 by sponging miR-204 | Oncogene | UP | [187] |
| SChLAP1 | 2q31 | MAPK signaling | (i) SWI/SNF transcriptional regulator complex activation; (ii) MAPK1 inhibitor miR-198 repression | Oncogene | UP | [164] |
| AFAP1-AS1 | 4p16 | Proliferation | Regulation of miR-15b/IGF1R axis | Oncogene | UP | [162] |
| Linc00963 | 9q34.11 | Proliferation Invasion | (i) By taking part in the transactivation of EGFR, prostate cancer promotion can change from an androgen-dependent mode to an androgen-independent mode; (ii) binding of EZH2 and suppression of p21 expression | Oncogene | UP | [166] |
| SNHG7 | 9q34.3 | Proliferation Cell cycle | Regulation of miR-503 and cyclin D | Oncogene | UP | [168] |
| LOXL1-AS1 | 15q24 | Proliferation Cell cycle | Expression modulation of miR-541-3p and of cyclin D | Oncogene | UP | [169] |
| PVT1 | 8q24 | Apoptosis | KIF23 expression promotion by reducing miR-15a-5p expression | Oncogene | UP | [173] |
| ATB | 14q11.2 | Proliferation Invasion Cell cycle EMT | (i) Increase of cyclin E and cyclin D1 expression levels; (ii) Regulation of PI3K-AKT-mTOR and ERK signaling pathways | Oncogene | UP | [190] |
| ROR | 18q21.31 | Proliferation Invasion Migration | (i) ceRNA reducing miRNA-145; (ii) Regulation of PI3K\Akt pathway | Oncogene | UP | [200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortora, F.; La Civita, E.; Trivedi, P.; Febbraio, F.; Terracciano, D.; Cimmino, A. Emerging RNA-Based Therapeutic and Diagnostic Options: Recent Advances and Future Challenges in Genitourinary Cancers. Int. J. Mol. Sci. 2023, 24, 4601. https://doi.org/10.3390/ijms24054601
Tortora F, La Civita E, Trivedi P, Febbraio F, Terracciano D, Cimmino A. Emerging RNA-Based Therapeutic and Diagnostic Options: Recent Advances and Future Challenges in Genitourinary Cancers. International Journal of Molecular Sciences. 2023; 24(5):4601. https://doi.org/10.3390/ijms24054601
Chicago/Turabian StyleTortora, Fabiana, Evelina La Civita, Pankaj Trivedi, Ferdinando Febbraio, Daniela Terracciano, and Amelia Cimmino. 2023. "Emerging RNA-Based Therapeutic and Diagnostic Options: Recent Advances and Future Challenges in Genitourinary Cancers" International Journal of Molecular Sciences 24, no. 5: 4601. https://doi.org/10.3390/ijms24054601
APA StyleTortora, F., La Civita, E., Trivedi, P., Febbraio, F., Terracciano, D., & Cimmino, A. (2023). Emerging RNA-Based Therapeutic and Diagnostic Options: Recent Advances and Future Challenges in Genitourinary Cancers. International Journal of Molecular Sciences, 24(5), 4601. https://doi.org/10.3390/ijms24054601









