Reproductive Suppression Caused by Spermatogenic Arrest: Transcriptomic Evidence from a Non-Social Animal
Abstract
1. Introduction
2. Results
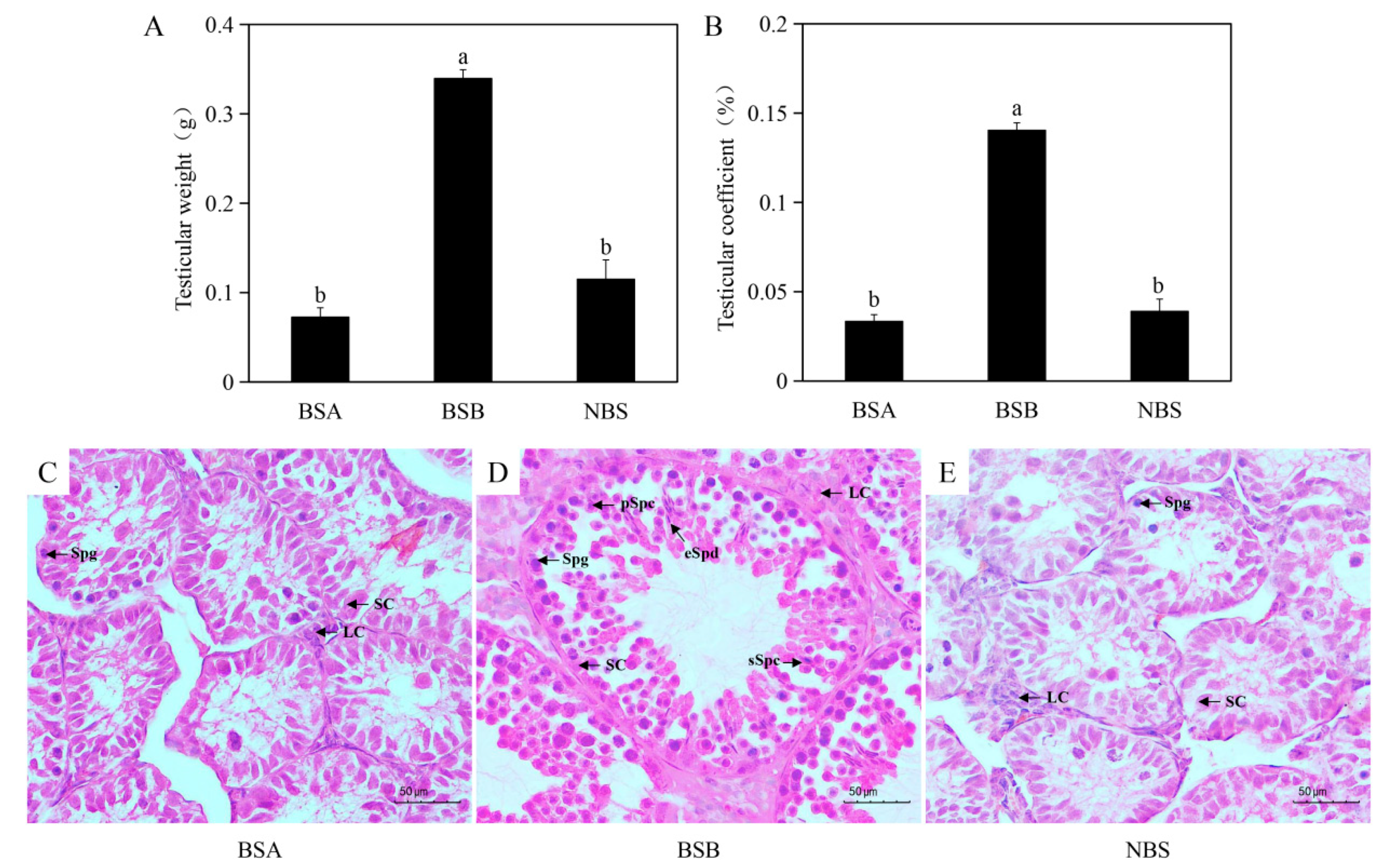
2.1. Morphologic and Histologic Changes in the Testes of Plateau Zokors
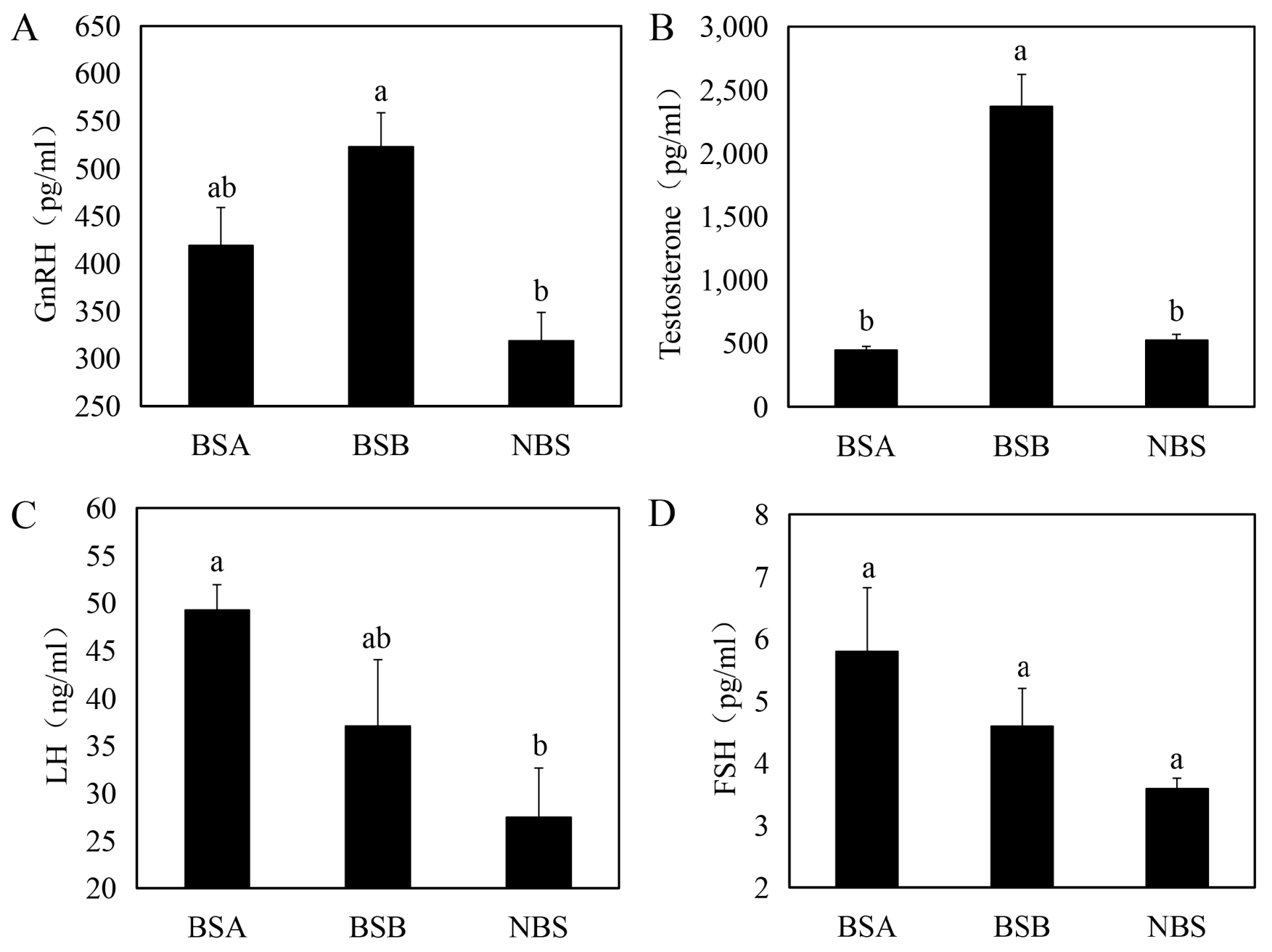
2.2. Serum Hormone Difference of Plateau Zokors
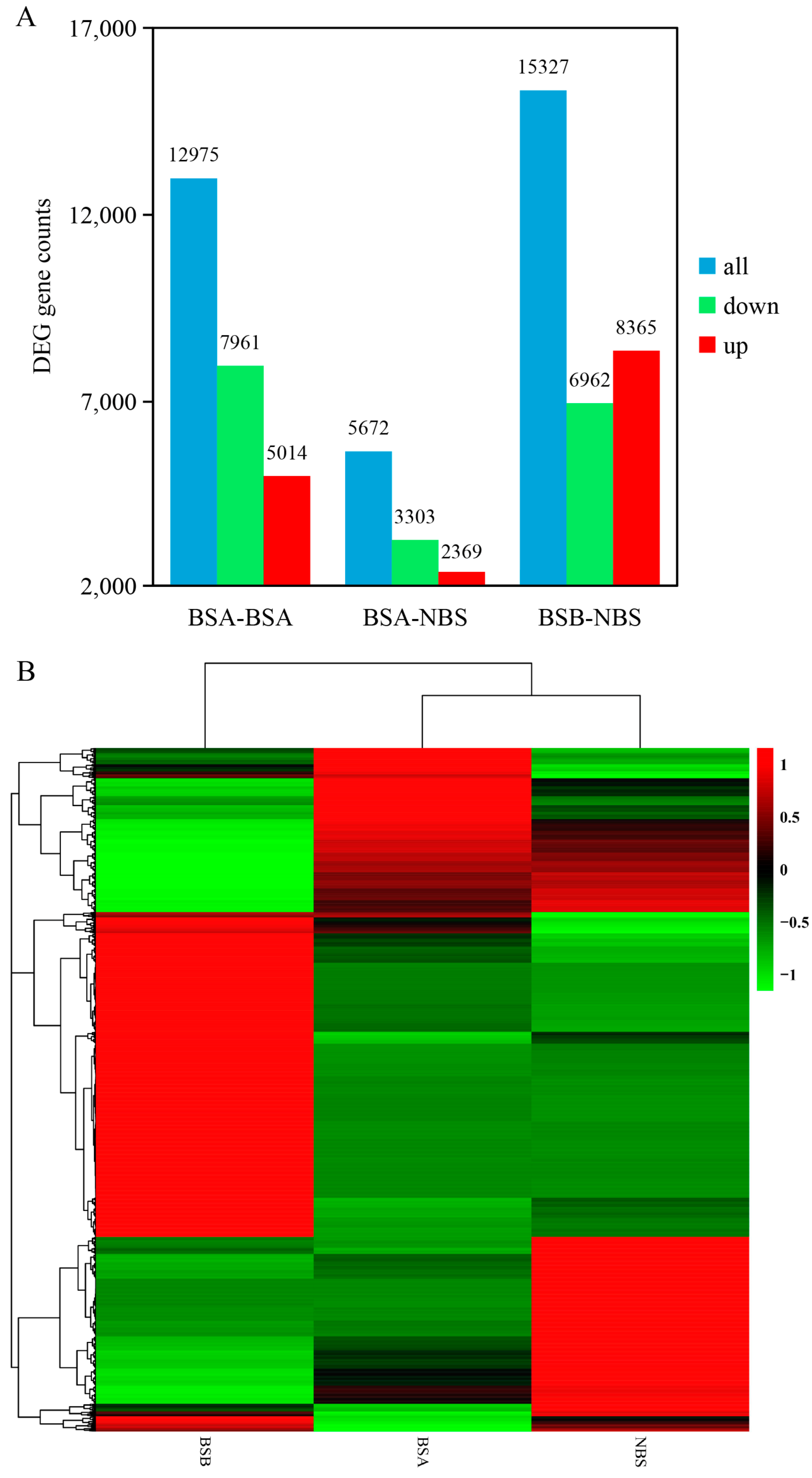
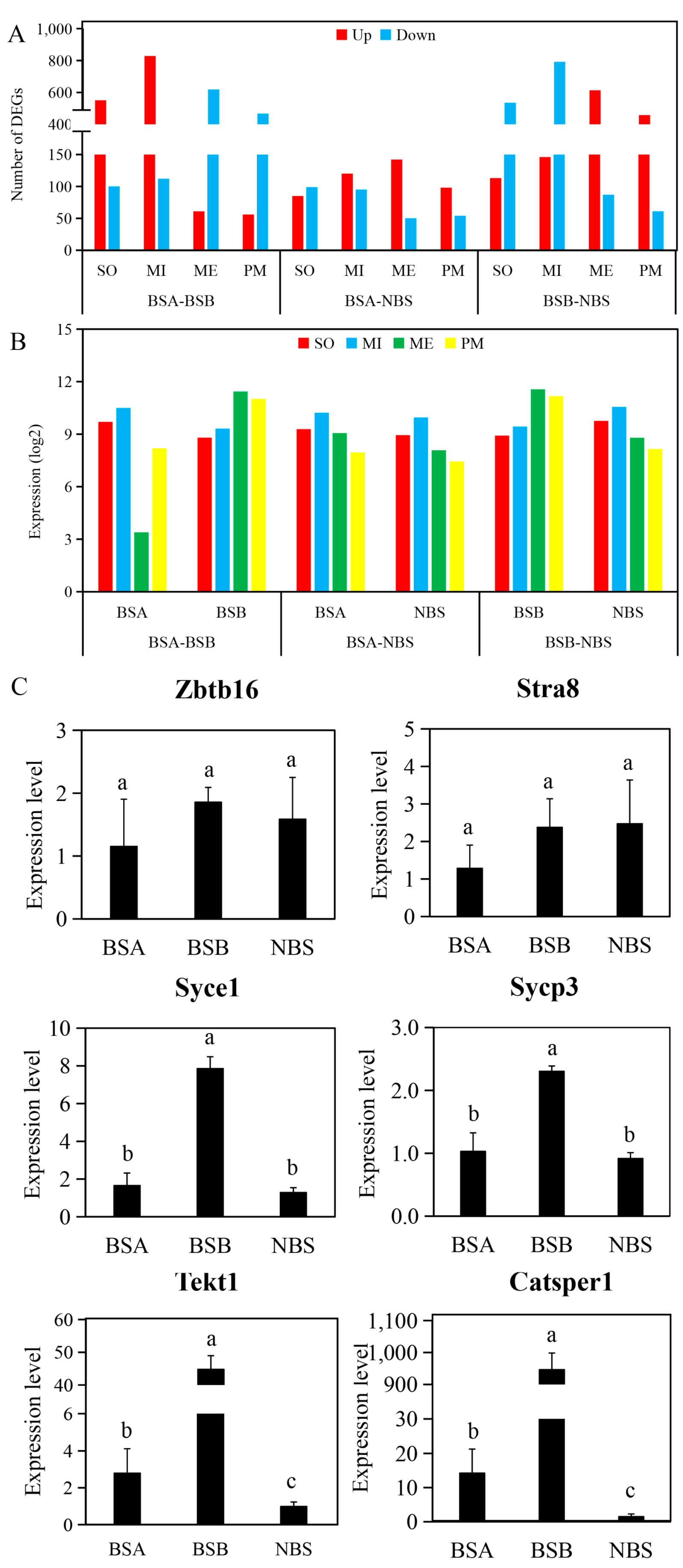
2.3. Differentially Expressed Gene Analysis of the Testes in Plateau Zokors
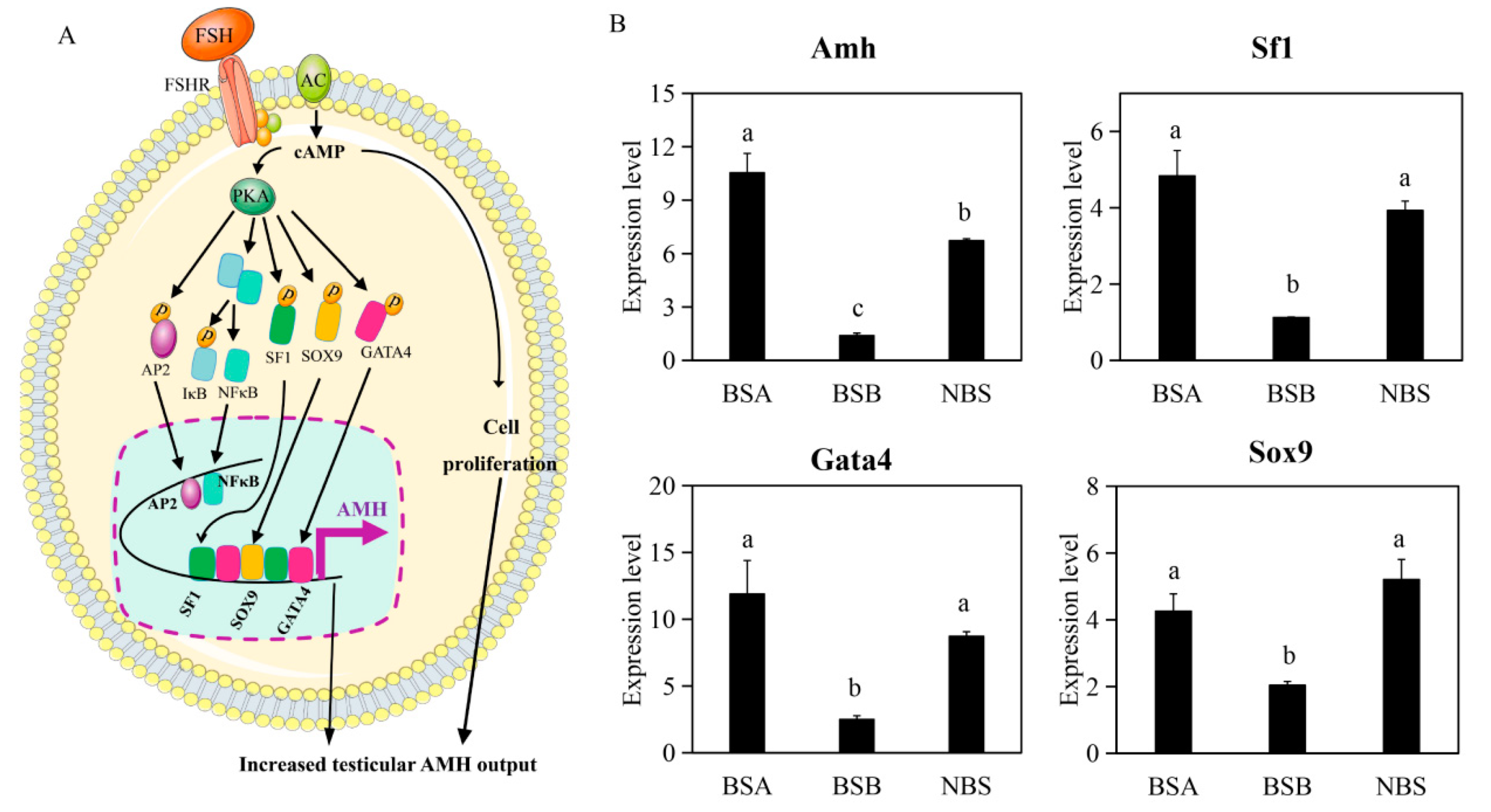
2.4. Differences in Transcription Factors That Regulate AMH (Anti-Müllerian Hormone) Promoter
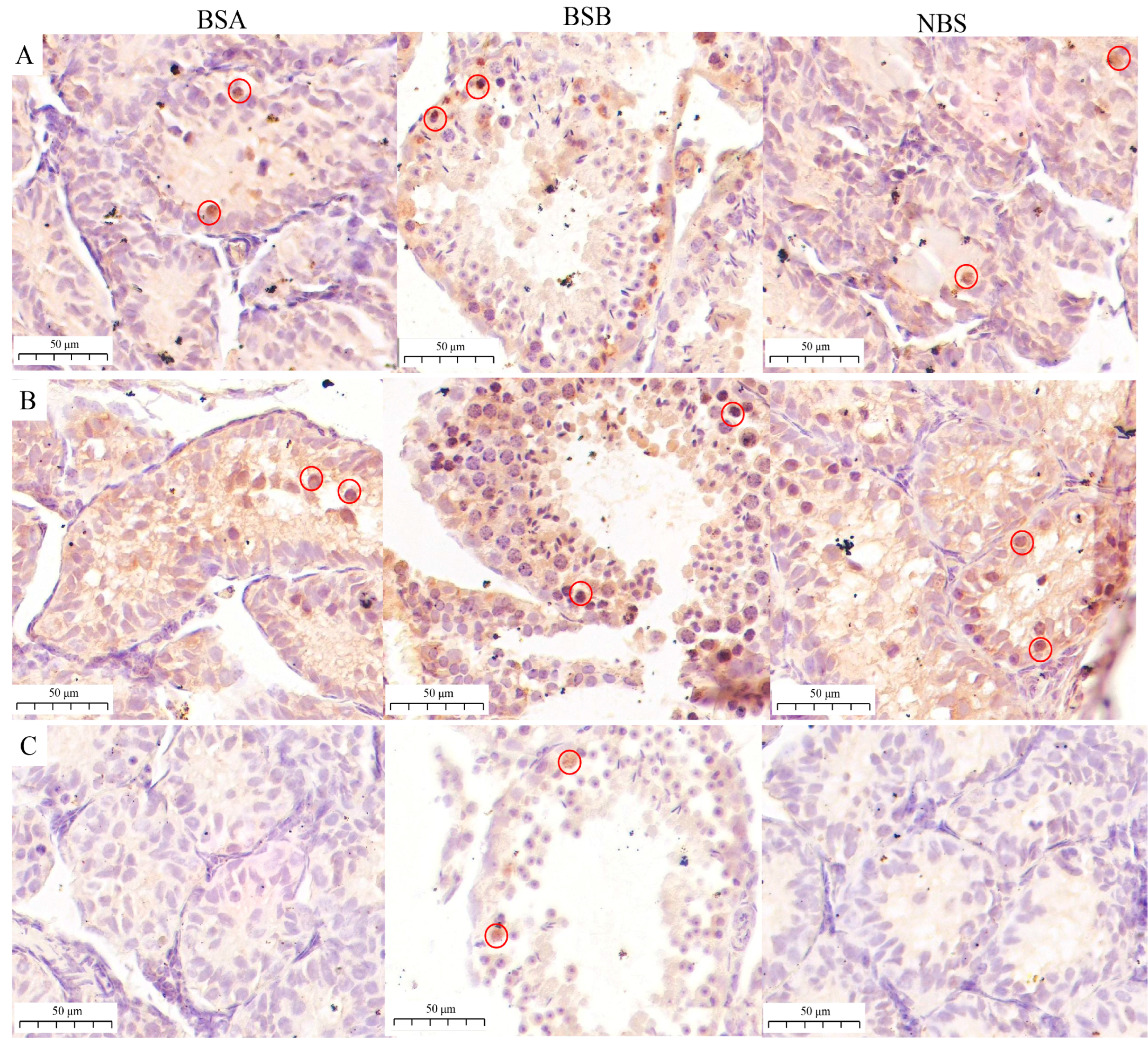
2.5. Differences in Spermatogenic Stages in the Plateau Zokor
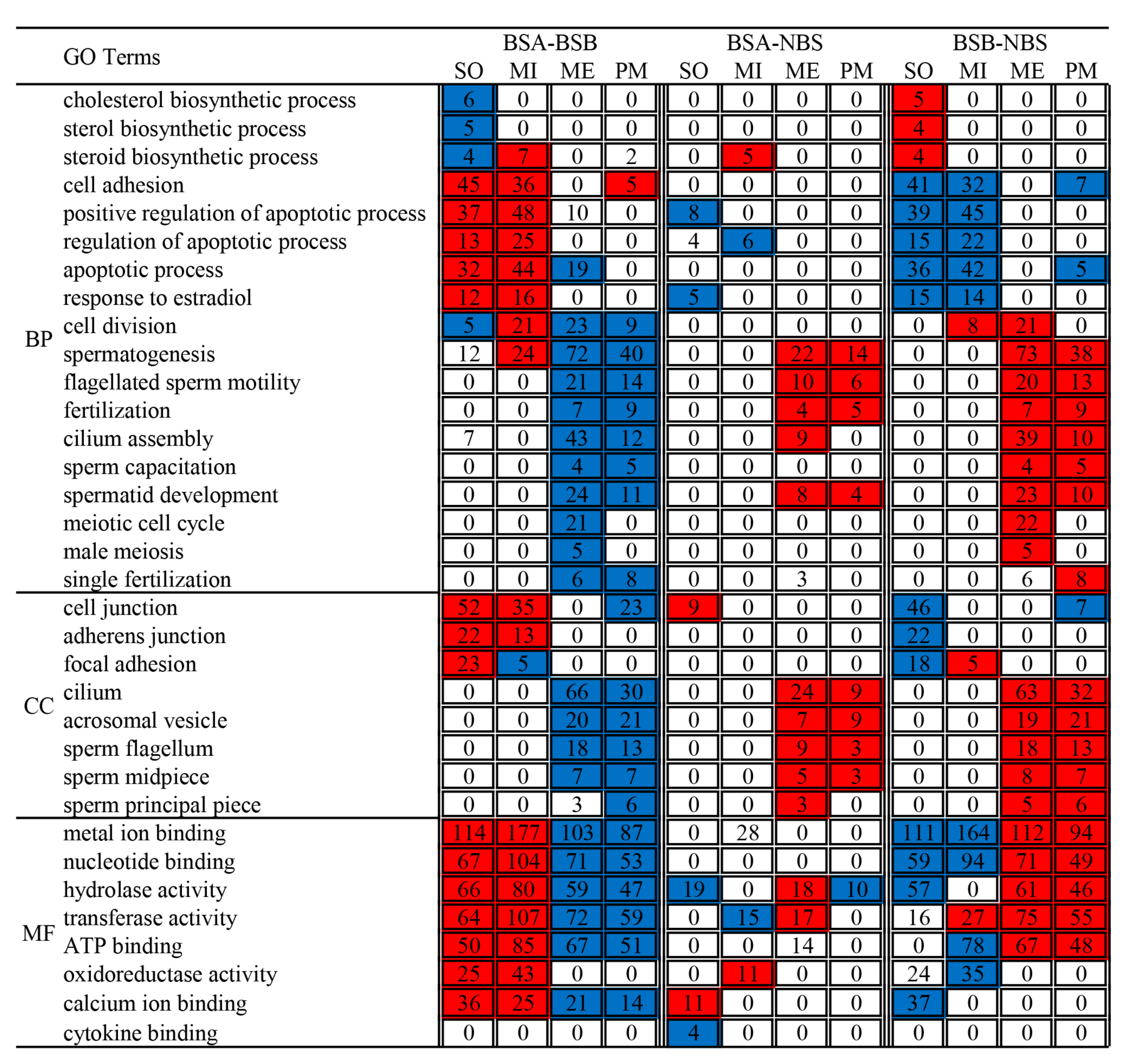
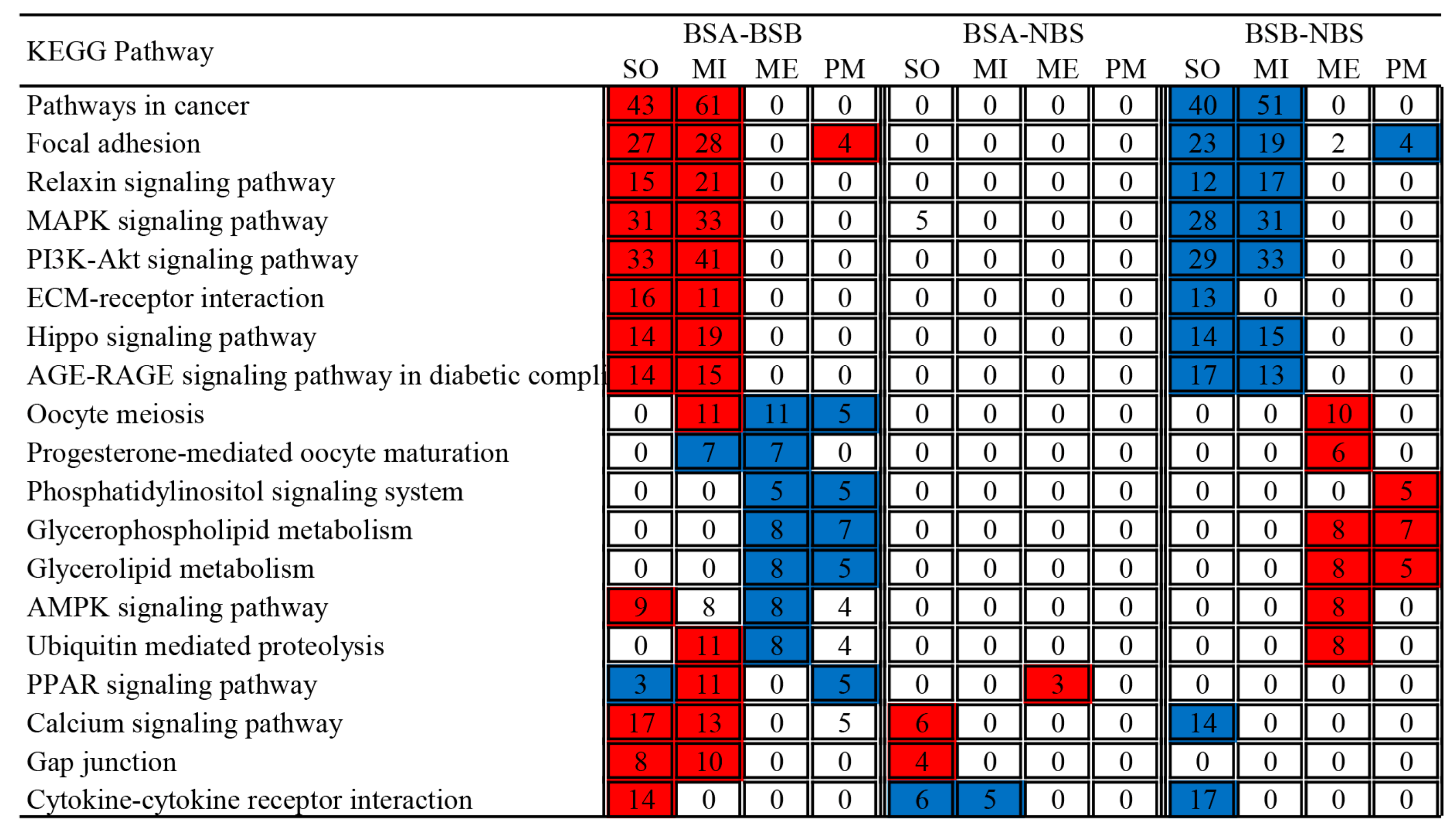
2.6. Functional Enrichment Analyses of Differentially Expressed Genes
2.7. Validation of Gene Expression by qPCR
3. Discussion
3.1. High AMH Levels and Low Testosterone Levels Likely Caused Delayed Testicular Development in Plateau Zokors
3.2. The Spermatogenic Arrest of the Non-Breeders Occurred at the Spermatogonia Stage
3.3. Transcriptional Regulation of Reproductive Suppression in Plateau Zokors
3.4. Management Implications
4. Materials and Methods
4.1. Animals
4.2. Hormone Determination
4.3. H&E Staining and Immunohistochemistry
4.4. RNA-seq
4.5. Total RNA Extraction, Library Preparation, and Sequencing
4.6. Transcriptomic Data Analysis
4.7. GO and KEGG Enrichment Analysis
4.8. Quantitative Real-Time Reverse-Transcription PCR
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beehner, J.C.; Lu, A. Reproductive suppression in female primates: A review. Evol. Anthropol. 2013, 22, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, Z.V.; Jessen, A.; Koene, J.M. Male reproductive suppression: Not a social affair. Curr. Zool. 2017, 63, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.H.; Brotherton, P.N.; Russell, A.F.; O’Riain, M.J.; Gaynor, D.; Kansky, R.; Griffin, A.; Manser, M.; Sharpe, L.; McIlrath, G.M.; et al. Cooperation, control, and concession in meerkat groups. Science 2001, 291, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.A.; Cant, M.A. Reproductive skew and the threat of eviction: A new perspective. Proc. Biol. Sci. 1999, 266, 275. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Sapolsky, R.M. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 2003, 15, 711–724. [Google Scholar] [CrossRef]
- Jeon, J.; Choe, J.C. Reproductive skew and the origin of sterile castes. Am. Nat. 2003, 161, 206–224. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 1998, 13, 288–292. [Google Scholar] [CrossRef]
- Saltzman, W. Reproductive skew, cooperative breeding, and eusociality in vertebrates: Hormones. In Encyclopedia of Animal Behavior Test, 2nd ed.; Choe, J., Ed.; Academic: Oxford, UK, 2019; pp. 448–453. [Google Scholar]
- O’Riain, M.; Bennett, N.; Brotherton, P.; McIlrath, G.; Clutton-Brock, T.H. Reproductive suppression and inbreeding avoidance in wild populations of co-operatively breeding meerkats (Suricata suricatta). Behav. Ecol. Sociobiol. 2000, 48, 471–477. [Google Scholar] [CrossRef]
- Cant, M.A. Social control of reproduction in banded mongooses. Anim. Behav. 2000, 59, 147–158. [Google Scholar] [CrossRef]
- White, S.A.; Nguyen, T.; Fernald, R.D. Social regulation of gonadotropin-releasing hormone. J. Exp. Biol. 2002, 205, 2567–2581. [Google Scholar] [CrossRef]
- Charpentier, M.J.; Tung, J.; Altmann, J.; Alberts, S.C. Age at maturity in wild baboons: Genetic, environmental and demographic influences. Mol. Ecol. 2008, 17, 2026–2040. [Google Scholar] [CrossRef]
- Maggioncalda, A.N.; Czekala, N.M.; Sapolsky, R.M. Male orangutan subadulthood: A new twist on the relationship between chronic stress and developmental arrest. Am. J. Phys. Anthropol. 2002, 118, 25–32. [Google Scholar] [CrossRef]
- Van den Berghe, F.; Paris, D.B.; Van Soom, A.; Rijsselaere, T.; Van der Weyde, L.; Bertschinger, H.J.; Paris, M.C. Reproduction in the endangered African wild dog: Basic physiology, reproductive suppression and possible benefits of artificial insemination. Anim. Reprod. Sci. 2012, 133, 1–9. [Google Scholar] [CrossRef]
- Kruczek, M.; Styrna, J. Semen quantity and quality correlate with bank vole males’ social status. Behav. Process. 2009, 82, 279–285. [Google Scholar] [CrossRef]
- Bennett, N.C. Reproductive suppression in social Cryptomys damarensis colonies—A lifetime of socially-induced sterility in males and females (Rodentia: Bathyergidae). J. Zool. 1994, 234, 25–39. [Google Scholar] [CrossRef]
- Mulugeta, E.; Marion-Poll, L.; Gentien, D.; Ganswindt, S.B.; Ganswindt, A.; Bennett, N.C.; Blackburn, E.G.; Faulkes, C.G.; Heard, E. Molecular insights into the pathways underlying naked mole-rat eusociality. BioRxiv 2017, 209932. [Google Scholar] [CrossRef]
- Bens, M.; Szafranski, K.; Holtze, S.; Sahm, A.; Groth, M.; Kestler, H.A.; Hildebrandt, T.B.; Platzer, M. Naked mole-rat transcriptome signatures of socially suppressed sexual maturation and links of reproduction to aging. BMC Biol. 2018, 16, 77. [Google Scholar] [CrossRef]
- Sahm, A.; Platzer, M.; Koch, P.; Henning, Y.; Bens, M.; Groth, M.; Burda, H.; Begall, S.; Ting, S.; Goetz, M.; et al. Increased longevity due to sexual activity in mole-rats is associated with transcriptional changes in the HPA stress axis. eLife 2021, 10, e57843. [Google Scholar] [CrossRef]
- Norris, R.W.; Zhou, K.; Zhou, C.; Yang, G.; William Kilpatrick, C.; Honeycutt, R.L. The phylogenetic position of the zokors (Myospalacinae) and comments on the families of muroids (Rodentia). Mol. Phylogenet. Evol. 2004, 31, 972–978. [Google Scholar] [CrossRef]
- Zhang, Y. The Biology and Ecology of Plateau Zokors (Eospalax fontanierii). In Subterranean Rodents: News from Underground; Begall, S., Burda, H., Schleich, C.E., Eds.; Springer: Berlin, Germany, 2007; pp. 237–249. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Liu, J. Burrowing rodents as ecosystem engineers: The ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal Rev. 2003, 33, 284–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J. Effects of plateau zokors (Myospalax fontanierii) on plant community and soil in an alpine meadow. J. Mammal. 2003, 84, 644–651. [Google Scholar] [CrossRef]
- Kong, Y.; Xidong, L.; Gaowei, X.; Wei, L.; Meerburg, B.G. Control of Eospalax baileyi (plateau zokor) with arrow traps in western China. Pakistan J. Zool. 2016, 48, 125–129. Available online: http://zsp.com.pk/pdf48/125-129%20(15)%20QPJZ-293-2015%2011-10-15%20Control%20of%20Eospalax%20baileyi.pdf (accessed on 18 December 2022).
- Zhang, Y. Effect of removal on age structure and reprduction of plateau zokor population in alpine meadow. Acta Theriol. Sin. 1999, 3, 44–51. Available online: http://www.mammal.cn/CN/Y1999/V19/I3/204 (accessed on 18 December 2022).
- Faulkes, C.G.; Bennett, N.C. Social evolution in African mole-rats—A comparative overview. Adv. Exp. Med. Biol. 2021, 1319, 1–33. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Grinspon, R.P.; Schteingart, H.F.; Rey, R.A. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int. J. Pediatr. Endocrinol. 2016, 2016, 20. [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.T.; Yeh, S.D.; Xu, Q.; Wang, R.S.; Guillou, F.; Lardy, H.; Yeh, S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 6876–6881. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Worku, T.; Davis, J.S.; Talpur, H.S.; Bhattarai, D.; Kadariya, I.; Hua, G.; Cao, J.; Dad, R.; Farmanullah; et al. Role and mechanism of AMH in the regulation of Sertoli cells in mice. J. Steroid Biochem. Mol. Biol. 2017, 174, 133–140. [Google Scholar] [CrossRef]
- Sriraman, V.; Niu, E.; Matias, J.R.; Donahoe, P.K.; MacLaughlin, D.T.; Hardy, M.P.; Lee, M.M. Müllerian inhibiting substance inhibits testosterone synthesis in adult rats. J. Androl. 2001, 22, 750–758. [Google Scholar] [CrossRef]
- An, X.; Wang, Y.; Li, Y.; Jia, G.; Yang, Q. Morphological features and regulation of seasonal spermatogenesis in plateau zokor (Eospalax baileyi). Acta Theriol. Sin. 2020, 40, 435–445. Available online: http://www.mammal.cn/CN/Y2020/V40/I5/435 (accessed on 18 December 2022).
- Liu, M.; Cao, G.; Zhang, Y.; Qu, J.; Li, W.; Wan, X.; Li, Y.X.; Zhang, Z.; Wang, Y.L.; Gao, F. Changes in the morphology and protein expression of germ cells and Sertoli cells in plateau pikas testes during non-breeding season. Sci. Rep. 2016, 6, 22697. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, Y.; Li, X.Y.; Li, J.; Tang, J.X.; Li, Y.Y.; Deng, S.L.; Cheng, C.Y.; Liu, Y.X. Kruppel-like factor 6 regulates Sertoli cell blood-testis barrier. Front. Biosci. 2019, 24, 1316–1329. [Google Scholar] [CrossRef]
- Tokue, M.; Ikami, K.; Mizuno, S.; Takagi, C.; Miyagi, A.; Takada, R.; Noda, C.; Kitadate, Y.; Hara, K.; Mizuguchi, H.; et al. SHISA6 confers resistance to differentiation-promoting Wnt/β-catenin signaling in mouse spermatogenic Stem Cells. Stem Cell Rep. 2017, 8, 561–675. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Omolaoye, V.A.; Kandasamy, R.K.; Hachim, M.Y.; Du Plessis, S.S. Omics and male infertility: Highlighting the application of transcriptomic data. Life 2022, 12, 280. [Google Scholar] [CrossRef]
- Girault, M.S.; Dupuis, S.; Ialy-Radio, C.; Stouvenel, L.; Viollet, C.; Pierre, R.; Favier, M.; Ziyyat, A.; Barbaux, S. Deletion of the spata3 gene induces sperm alterations and in vitro hypofertility in mice. Int. J. Mol. Sci. 2021, 22, 1959. [Google Scholar] [CrossRef]
- von Bülow, M.; Heid, H.; Hess, H.; Franke, W.W. Molecular nature of calicin, a major basic protein of the mammalian sperm head cytoskeleton. Exp. Cell Res. 1995, 219, 407–413. [Google Scholar] [CrossRef]
- Niemeyer, J.; Mentrup, T.; Heidasch, R.; Müller, S.A.; Biswas, U.; Meyer, R.; Papadopoulou, A.A.; Dederer, V.; Haug-Kröper, M.; Adamski, V.; et al. The intramembrane protease SPPL2c promotes male germ cell development by cleaving phospholamban. EMBO Rep. 2019, 20, e46449. [Google Scholar] [CrossRef]
- Syeda, S.S.; Sánchez, G.; McDermott, J.P.; Hong, K.H.; Blanco, G.; Georg, G.I. The Na+ and K+ transport system of sperm (ATP1A4) is essential for male fertility and an attractive target for male contraception. Biol. Reprod. 2020, 103, 343–356. [Google Scholar] [CrossRef]
- Tanaka, H.; Iguchi, N.; Miyagawa, Y.; Koga, M.; Kohroki, J.; Nishimune, Y. Differential expression of succinyl CoA transferase (SCOT) genes in somatic and germline cells of the mouse testis. Int. J. Androl. 2003, 26, 52–56. [Google Scholar] [CrossRef]
- Bai, M.; Sun, L.; Zhao, J.; Xiang, L.; Cheng, X.; Li, J.; Jia, C.; Jiang, H. Histological analysis and identification of spermatogenesis-related genes in 2-, 6-, and 12-month-old sheep testes. Naturwissenschaften 2017, 104, 84. [Google Scholar] [CrossRef]
- Sharma, S.; Hanukoglu, A.; Hanukoglu, I. Localization of epithelial sodium channel (ENaC) and CFTR in the germinal epithelium of the testis, Sertoli cells, and spermatozoa. J. Mol. Histol. 2018, 49, 195–208. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Yang, Y.; Xu, Y.D.; Yu, H.L. Targeted disruption of the spermatid-specific gene Spata31 causes male infertility. Mol. Reprod. Dev. 2015, 82, 432–440. [Google Scholar] [CrossRef]
- Mutoji, K.; Singh, A.; Nguyen, T.; Gildersleeve, H.; Kaucher, A.V.; Oatley, M.J.; Oatley, J.M.; Velte, E.K.; Geyer, C.B.; Cheng, K.; et al. TSPAN8 expression distinguishes spermatogonial stem cells in the prepubertal mouse testis. Biol. Reprod. 2016, 95, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yeh, H.J.; Zhong, R.; Li, Y.S.; Deuel, T.F. A dominant-negative pleiotrophin mutant introduced by homologous recombination leads to germ-cell apoptosis in male mice. Proc. Natl. Acad. Sci. USA 1999, 96, 6734–6738. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Qian, Y.; Chohan, K.R.; Shirley, C.R.; Amidon, W.; Banerjee, S.; Middleton, F.A.; Conkrite, K.L.; Barcza, M.; Gonchoroff, N.; et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl. Acad. Sci. USA 2006, 103, 14813–14818. [Google Scholar] [CrossRef] [PubMed]
- Charron, Y.; Willert, J.; Lipkowitz, B.; Kusecek, B.; Herrmann, B.G.; Bauer, H. Two isoforms of the RAC-specific guanine nucleotide exchange factor TIAM2 act oppositely on transmission ratio distortion by the mouse t-haplotype. PLoS Genet. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Gibbs, Z.A.; Reza, L.C.; Cheng, C.C.; Westcott, J.M.; McGlynn, K.; Whitehurst, A.W. The testis protein ZNF165 is a SMAD3 cofactor that coordinates oncogenic TGFβ signaling in triple-negative breast cancer. eLife 2020, 9, e57679. [Google Scholar] [CrossRef]
- Zheng, W.; Zou, Z.; Lin, S.; Chen, X.; Wang, F.; Li, X.; Dai, J. Identification and functional analysis of spermatogenesis-associated gene modules in azoospermia by weighted gene coexpression network analysis. J. Cell Biochem. 2019, 120, 3934–3944. [Google Scholar] [CrossRef]
- Cabrillana, M.E.; Bocanegra, V.; Monclus, M.A.; Lancellotti, T.S.; Simón, L.; Funes, A.K.; Colombo, R.; Ruiz, E.M.; Vincenti, A.E.; Oliva, R.; et al. ODF1, sperm flagelar protein is expressed in kidney collecting ducts of rats. Heliyon 2019, 5, e02932. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Hachim, M.Y.; du Plessis, S.S. Using publicly available transcriptomic data to identify mechanistic and diagnostic biomarkers in azoospermia and overall male infertility. Sci. Rep. 2022, 12, 2584. [Google Scholar] [CrossRef]
- Song, W.H.; Zuidema, D.; Yi, Y.J.; Zigo, M.; Zhang, Z.; Sutovsky, M.; Sutovsky, P. Mammalian cell-free system recapitulates the early events of post-fertilization sperm mitophagy. Cells 2021, 10, 2450. [Google Scholar] [CrossRef]
- Wang, X.; Xie, W.; Yao, Y.; Zhu, Y.; Zhou, J.; Cui, Y.; Guo, X.; Yuan, Y.; Zhou, Z.; Liu, M. The heat shock protein family gene Hspa1l in male mice is dispensable for fertility. PeerJ 2020, 8, e8702. [Google Scholar] [CrossRef]
- Xin, A.; Qu, R.; Chen, G.; Zhang, L.; Chen, J.; Tao, C.; Fu, J.; Tang, J.; Ru, Y.; Chen, Y.; et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci. Adv. 2020, 6, eaaz4796. [Google Scholar] [CrossRef]
- Chen, L.; Ouyang, J.; Li, X.; Xiao, X.; Sun, W.; Li, S.; Zhou, L.; Liao, Y.; Zhang, Q. DNAH17 is essential for rat spermatogenesis and fertility. J. Genet. 2021, 100, 14. [Google Scholar] [CrossRef]
- Gytz, H.; Hansen, M.F.; Skovbjerg, S.; Kristensen, A.C.; Hørlyck, S.; Jensen, M.B.; Fredborg, M.; Markert, L.D.; McMillan, N.A.; Christensen, E.I. Apoptotic properties of the type 1 interferon induced family of human mitochondrial membrane ISG12 proteins. Biol. Cell 2017, 109, 94–112. [Google Scholar] [CrossRef]
- Jung, H.; Song, H.; Yoon, M. The KIT is a putative marker for differentiating spermatogonia in stallions. Anim. Reprod. Sci. 2015, 152, 39–46. [Google Scholar] [CrossRef]
- Ma, H.T.; Niu, C.M.; Xia, J.; Shen, X.Y.; Xia, M.M.; Hu, Y.Q.; Zheng, Y. Stimulated by retinoic acid gene 8 (Stra8) plays important roles in many stages of spermatogenesis. Asian J. Androl. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, Q.; Nan, J.; Wang, J.; Zhang, Y.; Zhao, X. Syce1 and Syce3 regulate testosterone and dihydrotestosterone synthesis via steroidogenic pathways in mouse Sertoli and Leydig cells. J. Steroid Biochem. Mol. Biol 2022, 223, 106135. [Google Scholar] [CrossRef]
- Syrjänen, J.L.; Pellegrini, L.; Davies, O.R. A molecular model for the role of SYCP3 in meiotic chromosome organisation. eLife 2014, 3, e02963. [Google Scholar] [CrossRef]
- Larsson, M.; Norrander, J.; Gräslund, S.; Brundell, E.; Linck, R.; Ståhl, S.; Höög, C. The spatial and temporal expression of Tekt1, a mouse tektin C homologue, during spermatogenesis suggest that it is involved in the development of the sperm tail basal body and axoneme. Eur. J. Cell Biol. 2000, 79, 718–725. [Google Scholar] [CrossRef]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Hazra, R.; Corcoran, L.; Robson, M.; McTavish, K.J.; Upton, D.; Handelsman, D.J.; Allan, C.M. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol. Endocrinol. 2013, 27, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, S.; Lee, W.M.; Wong, C.; Lui, W.Y.; Silvestrini, B.; Cheng, C.Y. Myosin VIIa supports spermatid/organelle transport and cell adhesion during spermatogenesis in the rat testis. Endocrinology 2019, 160, 484–503. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Sirianni, R.; Casaburi, I.; Ruggiero, C.; Maggiolini, M.; Andò, S.; Pezzi, V. 17β-Estradiol activates GPER- and ESR1-dependent pathways inducing apoptosis in GC-2 cells, a mouse spermatocyte-derived cell line. Mol. Cell Endocrinol. 2012, 355, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, I.; Rodríguez, A.B.; Pariente, J.A. Apoptosis is a demanding selective tool during the development of fetal male germ cells. Front. Cell Dev. Biol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The commitment to meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Almeida, J.; Conley, A.J.; Ball, B.A. Expression of anti-Müllerian hormone, CDKN1B, connexin 43, androgen receptor and steroidogenic enzymes in the equine cryptorchid testis. Equine. Vet. J. 2013, 45, 538–545. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K. The hippo pathway: Biology and pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Sobarzo, C.; Scarcelli, R.; Denduchis, B.; Lustig, L.; Cigorraga, S.B.; Meroni, S.B. Adenosine regulates Sertoli cell function by activating AMPK. Mol. Cell Endocrinol. 2010, 330, 49–58. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Zhao, Y.; Bai, D.; Du, M.; Song, L.; Liu, Z.; Yin, Z.; Manglai, D. Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse. BMC Genet. 2020, 21, 46. [Google Scholar] [CrossRef]
- Chen, S.X.; Bogerd, J.; Schoonen, N.E.; Martijn, J.; de Waal, P.P.; Schulz, R.W. A progestin (17α,20β-dihydroxy-4-pregnen-3-one) stimulates early stages of spermatogenesis in zebrafish. Gen. Comp. Endocrinol. 2013, 185, 1–9. [Google Scholar] [CrossRef]
- Kang, Y.; Tan, Y.; Wang, C.; Yao, B.; An, K.; Liu, M.; Su, J. Antifertility effects of levonorgestrel, quinestrol, and their mixture (EP-1) on plateau zokor in the Qinghai-Tibetan Plateau. Integr. Zool. 2022, 17, 1002–1016. [Google Scholar] [CrossRef]
- Su, J.; Peng, R.; Nan, Z.; Ji, W.; Cai, Z. Age determination and composition analyses of plateau zokor (Eospalax baileyi) in Gannan meadow. Chinese J. Zool. 2018, 1, 46–54. [Google Scholar] [CrossRef]
- Lardenois, A.; Gattiker, A.; Collin, O.; Chalmel, F.; Primig, M. GermOnline 4.0 is a genomics gateway for germline development, meiosis and the mitotic cell cycle. Database 2010, 2010, baq030. [Google Scholar] [CrossRef]
- Chalmel, F.; Rolland, A.D.; Niederhauser-Wiederkehr, C.; Chung, S.S.; Demougin, P.; Gattiker, A.; Moore, J.; Patard, J.J.; Wolgemuth, D.J.; Jégou, B.; et al. The conserved transcriptome in human and rodent male gametogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 8346–8351. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 3 September 2022).

| Upregulated | Downregulated | |||||
|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | Fold Change | Gene Symbol | Gene Name | Fold Change | |
| BSA–BSB | Cx3cl1 | C-X3-C motif chemokine ligand 1 | 6.52 | Tnp1 | Transition protein 1 | −9.46 |
| Col3a1 | Collagen type III alpha 1 chain | 4.73 | 4933402P03Rik | −9.27 | ||
| Star | Steroidogenic acute regulatory protein | 3.90 | Oxct2a | Oxct2a 3-oxoacid coa transferase 2a | −8.90 | |
| Shisa2 | Shisa family member 2 | 3.73 | Mst1r | Macrophage stimulating 1 receptor | −8.25 | |
| Rprm | Reprimo, tp53 dependent g2 arrest mediator homolog | 3.26 | Spata3 | Spermatogenesis associated 3 | −8.04 | |
| Matn2 | Matrilin 2 | 3.25 | 1700001O22Rik | −7.45 | ||
| Prss35 | Serine protease 35 | 2.98 | Sppl2c | Signal peptide peptidase like 2c | −7.25 | |
| Klf6 | Kruppel like factor 6 | 2.91 | Atp1a4 | Atpase Na+/K+ transporting subunit alpha 4 | −6.00 | |
| Amh | Anti-mullerian hormone | 2.84 | Ccin | Calicin | −5.94 | |
| Tubb5 | Tubulin beta-5 chain | 2.43 | Ace | Angiotensin I converting enzyme | −5.61 | |
| BSA–NBS | Syt13 | Synaptotagmin 13 | 5.64 | Mt-nd6 | Mitochondrially encoded nadh:ubiquinone oxidoreductase core subunit 6 | −13.23 |
| Padi1 | Peptidyl arginine deiminase 1 | 5.59 | Cd38 | Cd38 molecule | −8.05 | |
| Fer1l4 | Fer-1 like family member 4 | 5.45 | Mt-nd3 | Mitochondrially encoded nadh:ubiquinone oxidoreductase core subunit 3 | −7.57 | |
| Scnn1b | Sodium channel epithelial 1 subunit beta | 5.22 | Sptbn5 | Spectrin beta, non-erythrocytic 5 | −6.36 | |
| Spata31d1a | Spermatogenesis-associated protein 31d1 | 4.91 | Gbp7 | Guanylate binding protein 7 | −4.91 | |
| Tspan8 | Tetraspanin 8 | 4.89 | Wfikkn2 | Wap, follistatin/kazal, immunoglobulin, kunitz and netrin domain containing 2 | −4.73 | |
| Dmgdh | Dimethylglycine dehydrogenase | 4.87 | Ephx3 | Epoxide hydrolase 3 | −4.41 | |
| Camk1g | Calcium/calmodulin dependent protein kinase Ig | 4.50 | Pcbd1 | Pterin-4 alpha-carbinolamine dehydratase 1 | −4.14 | |
| Myoc | Myocilin | 4.10 | Fgd2 | Fyve, rhogef and ph domain containing 2 | −4.03 | |
| Ptn | Pleiotrophin | 3.68 | Rrad | Rrad, ras related glycolysis inhibitor and calcium channel regulator | −3.94 | |
| BSB–NBS | Gsg1 | Germ cell associated 1 | 12.95 | Tgfbi | Transforming growth factor beta induced | −5.70 |
| Odf1 | Outer dense fiber of sperm tails 1 | 11.90 | Rrad | Rrad, ras related glycolysis inhibitor and calcium channel regulator | −5.66 | |
| Spata20 | Spermatogenesis associated 20 | 10.01 | Ifi27 | Interferon alpha inducible protein 27 | −4.36 | |
| Hspa1l | Heat shock protein family a (hsp70) member 1 like | 9.51 | Prr5 | Proline rich 5 | −3.73 | |
| Actl7a | Actin-like protein 7a | 9.38 | Slc48a1 | Solute carrier family 48 member 1 | −3.65 | |
| Slc13a5 | Solute carrier family 13 member 5 | 8.76 | Tagln2 | Transgelin 2 | −3.63 | |
| Gm5617 | Chromosome 11 open reading frame 71 | 8.03 | Wipf3 | Was/wasl interacting protein family member 3 | −3.56 | |
| Spata18 | Spermatogenesis associated 18 | 7.61 | Stk10 | Serine/threonine kinase 10 | −3.54 | |
| Kcnt1 | Potassium sodium-activated channel subfamily t member 1 | 6.94 | Ankrd46 | Ankyrin repeat domain 46 | −3.39 | |
| Dnah17 | Dynein axonemal heavy chain 17 | 6.44 | 3632451O06Rik | −3.20 | ||
| Gene Symbol | Primer Sequence (5′→3′) | Product Size (bp) |
|---|---|---|
| SF1 | F: GATTCCCCGCAACAACCTCC | 193 |
| R: TTCCTCGTTCACCATCCCAA | ||
| SOX9 | F: AGTGTAGAGGAGCATTGGTAAGC | 168 |
| R: GCCTTTGCTTGCACTTCGAG | ||
| AMH | F: GTGCGCTGCTTCTGCTAAAA | 124 |
| R: GCGCAAGGTGCTTCCGTTA | ||
| ZBTB16 | F: CCCCGGTGGAGAAGCATTTG | 170 |
| R: CTGAATGAAACCCTGAGGGAGG | ||
| STRA8 | F: GCACACCGTTTTGAATCCCC | 197 |
| R: TTTCCCGCCATCACGACTTT | ||
| SYCE1 | F: CAGGTCATCGGCAACTGGGA | 151 |
| R: GGACTTCAATCCGGGGCTCTA | ||
| SYCP3 | F: TACTGAAGAAAATACTCCAGGTGA | 134 |
| R: TGGCAAGAAGAGCCTTGTTAAT | ||
| TEKT1 | F: GTGCACGACTGTAACCTCCA | 183 |
| R: CCACACCCCTGCAATGAGAT | ||
| CATSPER1 | F: CAGGCCACACCATCTTGACT | 107 |
| R: CACCATGTAAGTGAGGCCGT | ||
| β–Actin | F: TTGTGCGTGACATCAAAGAG | 208 |
| R: ATGCCAGAAGATTCCATACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, B.; An, K.; Kang, Y.; Tan, Y.; Zhang, D.; Su, J. Reproductive Suppression Caused by Spermatogenic Arrest: Transcriptomic Evidence from a Non-Social Animal. Int. J. Mol. Sci. 2023, 24, 4611. https://doi.org/10.3390/ijms24054611
Yao B, An K, Kang Y, Tan Y, Zhang D, Su J. Reproductive Suppression Caused by Spermatogenic Arrest: Transcriptomic Evidence from a Non-Social Animal. International Journal of Molecular Sciences. 2023; 24(5):4611. https://doi.org/10.3390/ijms24054611
Chicago/Turabian StyleYao, Baohui, Kang An, Yukun Kang, Yuchen Tan, Degang Zhang, and Junhu Su. 2023. "Reproductive Suppression Caused by Spermatogenic Arrest: Transcriptomic Evidence from a Non-Social Animal" International Journal of Molecular Sciences 24, no. 5: 4611. https://doi.org/10.3390/ijms24054611
APA StyleYao, B., An, K., Kang, Y., Tan, Y., Zhang, D., & Su, J. (2023). Reproductive Suppression Caused by Spermatogenic Arrest: Transcriptomic Evidence from a Non-Social Animal. International Journal of Molecular Sciences, 24(5), 4611. https://doi.org/10.3390/ijms24054611






