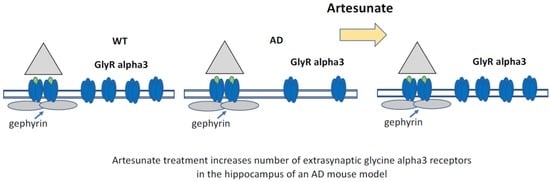
Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate
Abstract
1. Introduction
2. Results
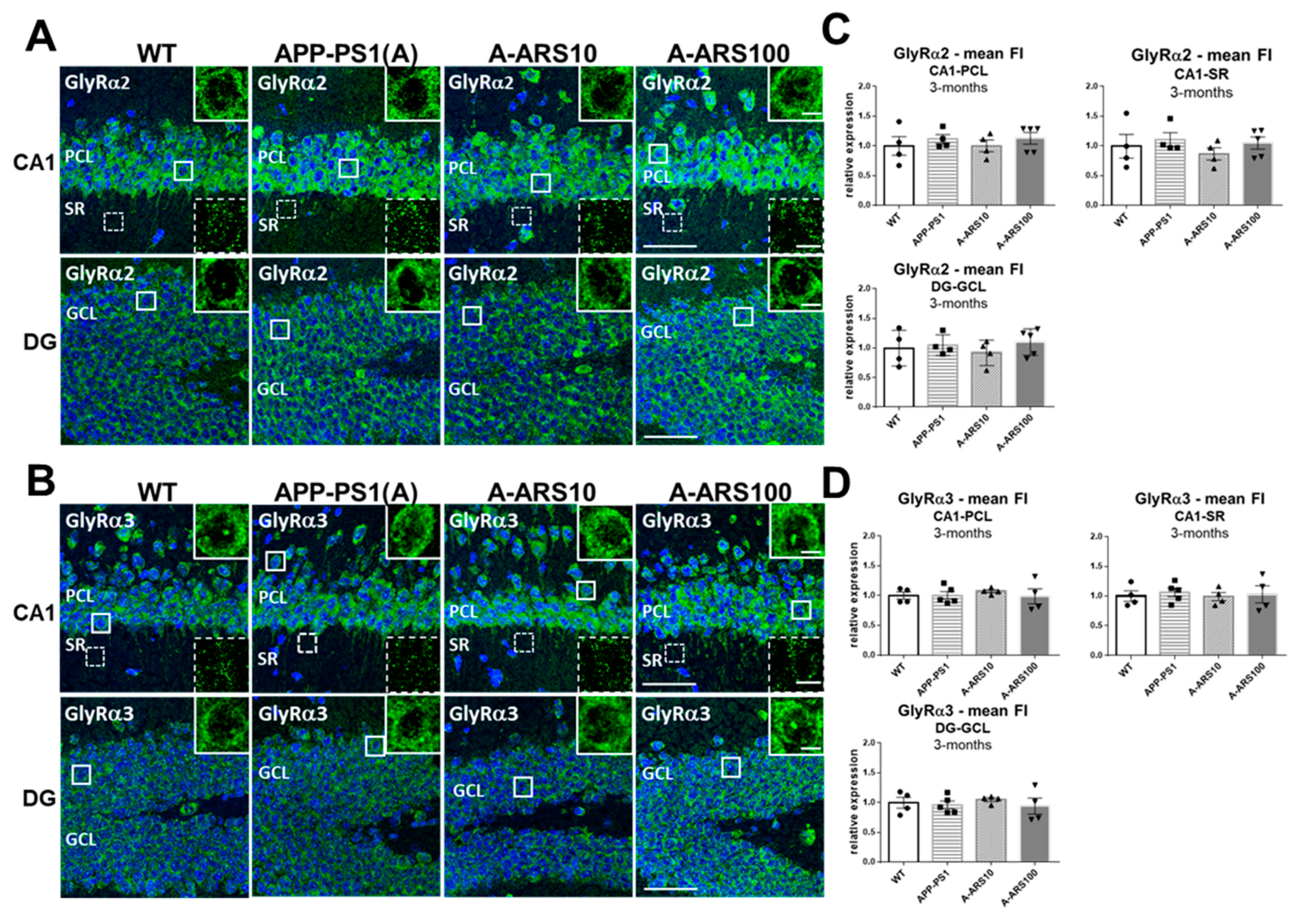
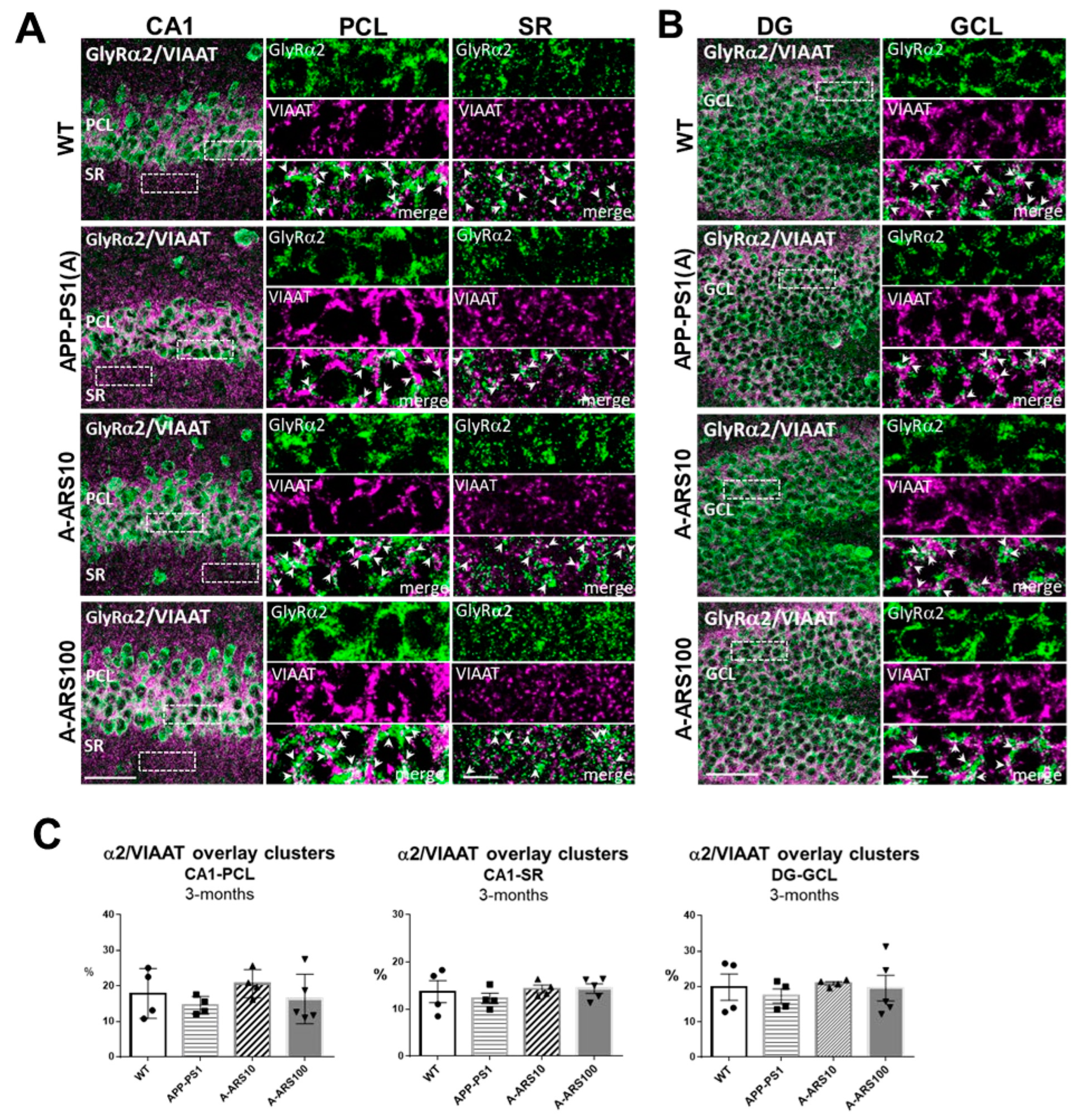
2.1. Protein Levels and Distribution of the α2 and α3 Subunits of GlyRs in the Hippocampus of 3-Month-Old Mice
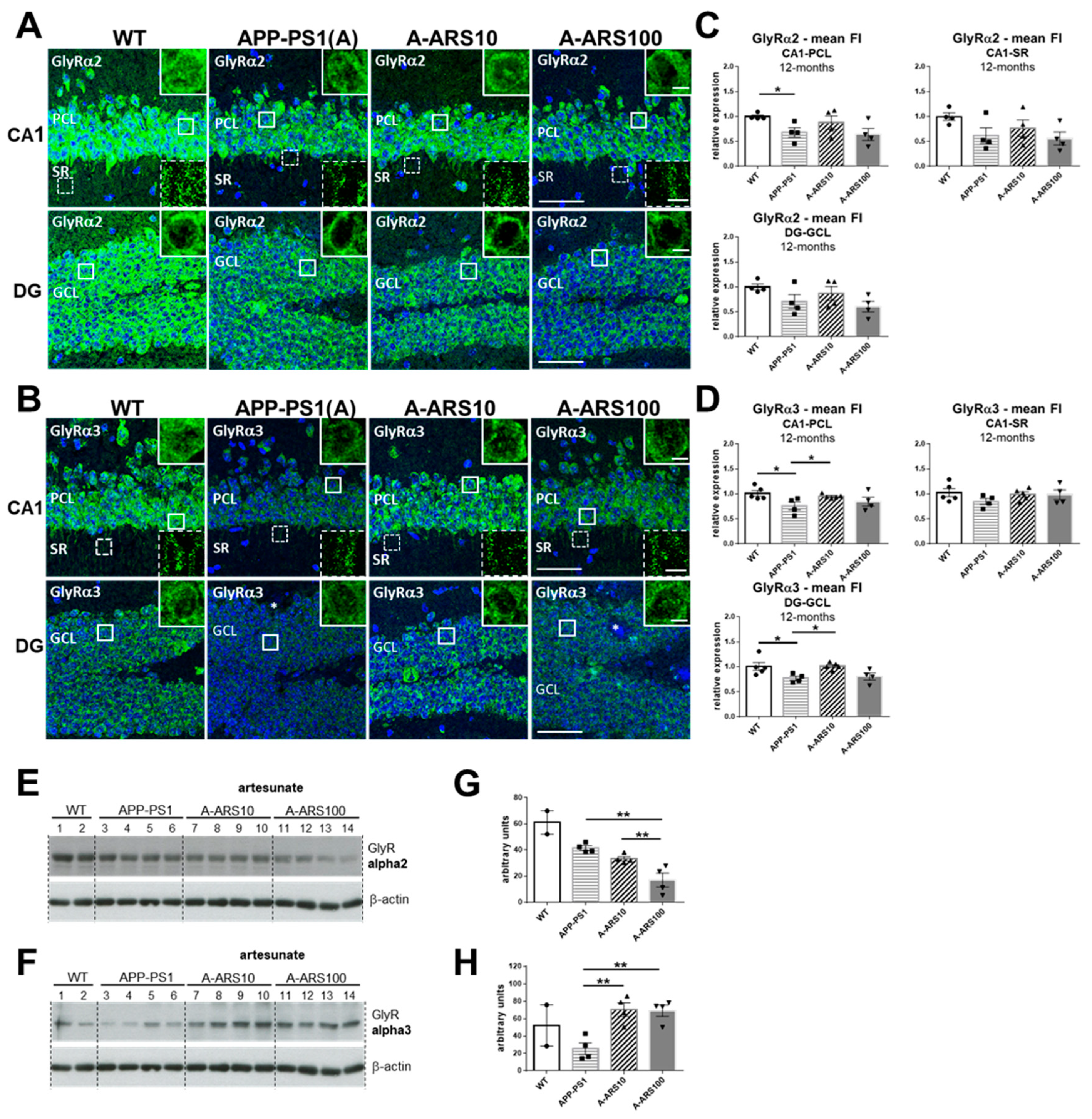
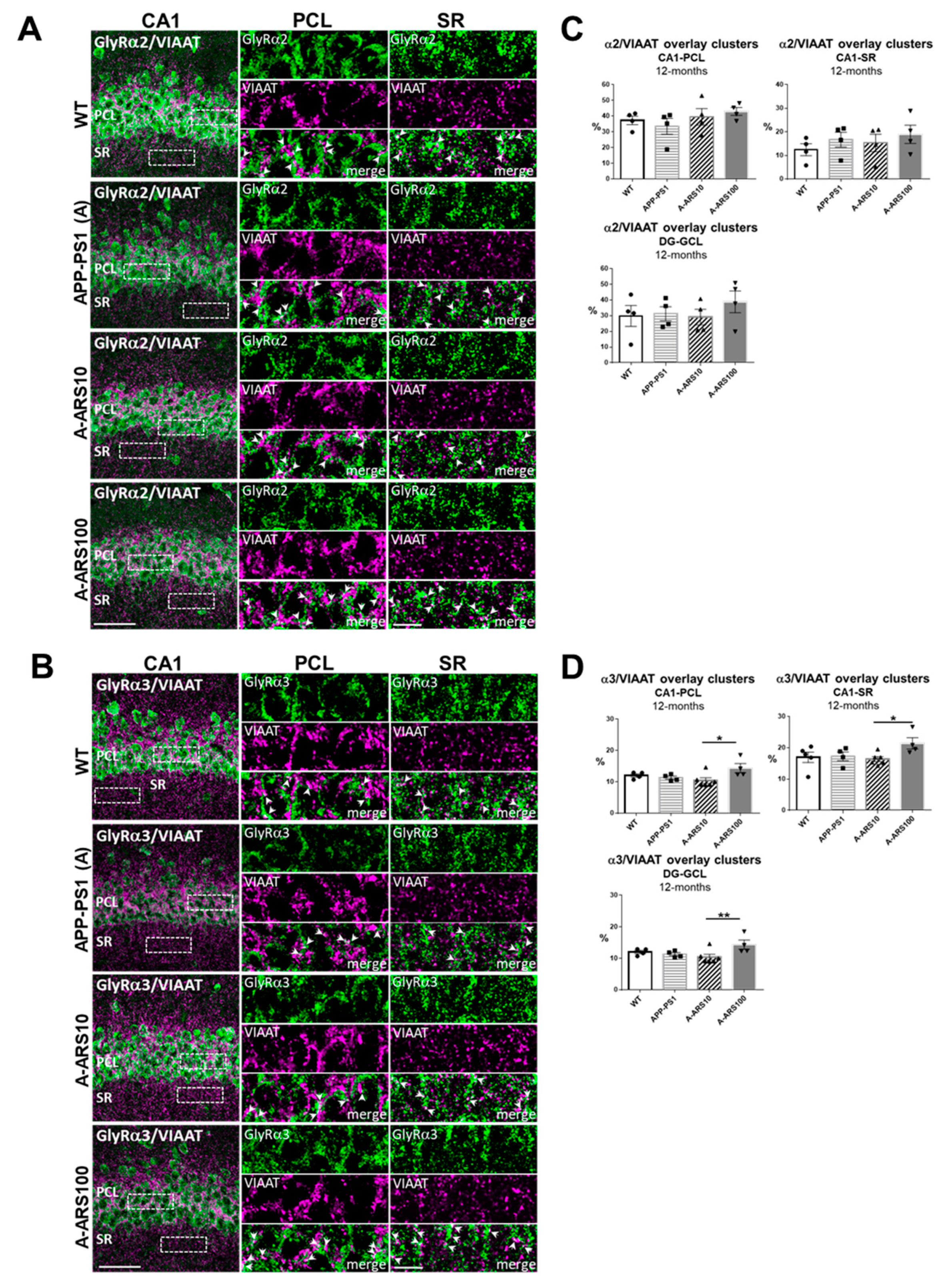
2.2. Protein Levels and Distribution of the α2 and α3 Subunits of GlyRs in the Hippocampus of 12 Month-Old Mice
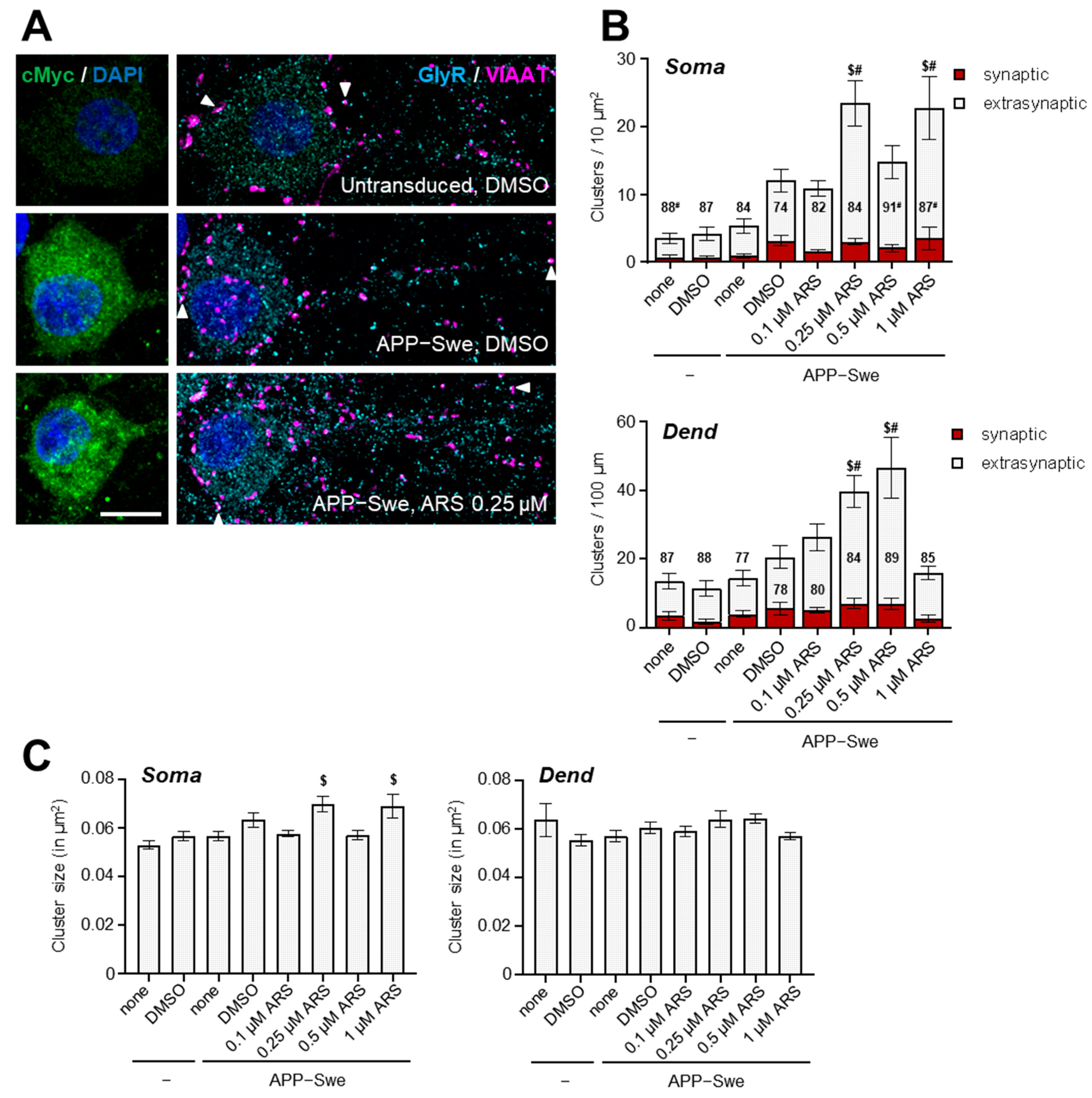
2.3. Distribution of GlyRs in hAPPswe Expressing Cultured Hippocampal Neurons
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Preparation
4.2. Immunofluorescence and Confocal Microscopy of Tissue Sections
4.3. Protein Extracts and Immunoblot Analysis
4.4. Hippocampal Cell Cultures
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Kirsch, J.; Betz, H.; Langosch, D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron 1995, 15, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Tintrup, H.; Kirsch, J.; Nichol, M.C.; Kuhse, J.; Betz, H.; Sanes, J.R. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 1998, 282, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Haddrill, J.L.; Lynch, J.W. A single beta subunit M2 domain residue controls the picrotoxin sensitivity of alphabeta heteromeric glycine receptor chloride channels. J. Neurochem. 2001, 76, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Grudzinska, J.; Schemm, R.; Haeger, S.; Nicke, A.; Schmalzing, G.; Betz, H.; Laube, B. The Beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 2005, 45, 727–739. [Google Scholar] [CrossRef]
- Zhu, H.; Gouaux, E. Architecture and assembly mechanism of native glycine receptors. Nature 2021, 599, 513–517. [Google Scholar] [CrossRef]
- McCracken, L.M.; Lowes, D.C.; Salling, M.C.; Carreau-Vollmer, C.; Odean, N.N.; Blednov, Y.A.; Betz, H.; Harris, R.A.; Harrison, N.L. Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proc. Natl. Acad. Sci. USA 2017, 114, E7179–E7186. [Google Scholar] [CrossRef]
- Nys, M.; Kesters, D.; Ulens, C. Structural insights into Cys-loop receptor function and ligand recognition. Biochem. Pharmacol. 2013, 86, 1042–1053. [Google Scholar] [CrossRef]
- Legendre, P.; Förstera, B.; Jüttner, R.; Meier, J.C. Glycine Receptors Caught between Genome and Proteome—Functional Implications of RNA Editing and Splicing. Front. Mol. Neurosci. 2009, 2, 23. [Google Scholar] [CrossRef]
- Malosio, M.L.; Marquèze-Pouey, B.; Kuhse, J.; Betz, H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991, 10, 2401–2409. [Google Scholar] [CrossRef]
- Sato, K.; Kiyama, H.; Tohyama, M. Regional distribution of cells expressing glycine receptor alpha 2 subunit mRNA in the rat brain. Brain Res. 1992, 590, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.L.; Gong, N. Glycine and glycine receptor signaling in hippocampal neurons: Diversity, function and regulation. Prog. Neurobiol. 2010, 91, 349–361. [Google Scholar] [CrossRef]
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, R.I.; Ribeiro, J.A.; Sebastião, A.M.; Valente, C.A. Age-related changes of glycine receptor at the rat hippocampus: From the embryo to the adult. J. Neurochem. 2011, 118, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, M.; Zhao, C.; Schmieden, V.; Braunewell, K.H. Cellular and subcellular localization of the inhibitory glycine receptor in hippocampal neurons. Biochem. Biophys. Res. Commun. 2004, 324, 1137–1142. [Google Scholar] [CrossRef]
- Danglot, L.; Rostaing, P.; Triller, A.; Bessis, A. Morphologically identified glycinergic synapses in the hippocampus. Mol. Cell. Neurosci. 2004, 27, 394–403. [Google Scholar] [CrossRef]
- Kuhse, J.; Schmieden, V.; Betz, H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 1990, 265, 22317–22320. [Google Scholar] [CrossRef]
- Haverkamp, S. Glycine Receptor Diversity in the Mammalian Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Zhang, L.H.; Gong, N.; Fei, D.; Xu, L.; Xu, T.L. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology 2008, 33, 701–711. [Google Scholar] [CrossRef]
- Chattipakorn, S.C.; McMahon, L.L. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J. Neurophysiol. 2003, 89, 1339–1342. [Google Scholar] [CrossRef]
- Song, W.; Chattipakorn, S.C.; McMahon, L.L. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J. Neurophysiol. 2006, 95, 2366–2379. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Mecca, A.P.; O’Dell, R.S.; Sharp, E.S.; Banks, E.R.; Bartlett, H.H.; Zhao, W.; Lipior, S.; Diepenbrock, N.G.; Chen, M.K.; Naganawa, M.; et al. Synaptic density and cognitive performance in Alzheimer’s disease: A PET imaging study with [11C]UCB-J. Alzheimer’s Dement. 2022, 10, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Govindpani, K.; Calvo-Flores Guzmán, B.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef]
- Martínez-Serra, R.; Alonso-Nanclares, L.; Cho, K.; Giese, K.P. Emerging insights into synapse dysregulation in Alzheimer’s disease. Brain Commun. 2022, 4, fcac083. [Google Scholar] [CrossRef]
- Agarwal, S.; Tannenberg, R.K.; Dodd, P.R. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 14, 313–321. [Google Scholar] [CrossRef]
- Hales, C.M.; Rees, H.; Seyfried, N.T.; Dammer, E.B.; Duong, D.M.; Gearing, M.; Montine, T.J.; Troncoso, J.C.; Thambisetty, M.; Levey, A.I.; et al. Abnormal gephyrin immunoreactivity associated with Alzheimer disease pathologic changes. J. Neuropathol. Exp. Neurol. 2013, 72, 1009–1015. [Google Scholar] [CrossRef]
- Calvo-Flores Guzmán, B.; Kim, S.; Chawdhary, B.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.; Montgomery, J.; Kwakowsky, A. Amyloid-Beta1-42-Induced Increase in GABAergic Tonic Conductance in Mouse Hippocampal CA1 Pyramidal Cells. Molecules 2020, 25, 693. [Google Scholar] [CrossRef]
- Kiss, E.; Gorgas, K.; Schlicksupp, A.; Groß, D.; Kins, S.; Kirsch, J.; Kuhse, J. Biphasic Alteration of the Inhibitory Synapse Scaffold Protein Gephyrin in Early and Late Stages of an Alzheimer Disease Model. Am. J. Pathol. 2016, 186, 2279–2291. [Google Scholar] [CrossRef]
- Hollnagel, J.O.; Elzoheiry, S.; Gorgas, K.; Kins, S.; Beretta, C.A.; Kirsch, J.; Kuhse, J.; Kann, O.; Kiss, E. Early alterations in hippocampal perisomatic GABAergic synapses and network oscillations in a mouse model of Alzheimer’s disease amyloidosis. PLoS ONE 2019, 14, e0209228. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kins, S.; Zöller, Y.; Schilling, S.; Gorgas, K.; Groß, D.; Schlicksupp, A.; Rosner, R.; Kirsch, J.; Kuhse, J. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and C-terminal fragments (CTFs) of the amyloid precursor protein in an AD-mouse model. Mol. Cell. Neurosci. 2021, 113, 103624. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Ahmed, T.; Batra, V.; Paliwal, J. Pharmacokinetics and pharmacodyamics of endoperoxide antimalarials. Curr. Drug Metab. 2009, 10, 289–306. [Google Scholar] [CrossRef]
- Puri, J.; Bellinger, L.L.; Kramer, P.R. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. J. Cell. Physiol. 2011, 226, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Acuña, M.A.; Yévenes, G.E.; Ralvenius, W.T.; Benke, D.; Di Lio, A.; Lara, C.O.; Muñoz, B.; Burgos, C.F.; Moraga-Cid, G.; Corringer, P.J.; et al. Phosphorylation state-dependent modulation of spinal glycine receptors alleviates inflammatory pain. J. Clin. Investig. 2016, 126, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jäggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Van der Pluijm, R.W.; Tripura, R.; Hoglund, R.M.; Phyo, A.P.; Lek, D.; Islam, A.U.; Anvikar, A.R.; Satpathi, P.; Satpathi, S.; Behera, P.K.; et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: A multicentre, open-label, randomised clinical trial. Lancet 2020, 395, 1345–1360. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. Biomed. Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, F.; Xie, Z.; Song, X.; Zhu, Q.; Wei, J.; Tan, S.; Liang, L.; Gong, B. Clinical study of artesunate in the treatment of coronavirus disease. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 32, 417–420. [Google Scholar]
- Sailike, B.; Omarova, Z.; Jenis, J.; Adilbayev, A.; Akbay, B.; Askarova, S.; Jin, W.L.; Tokay, T. Neuroprotective and anti-epileptic potentials of genus Artemisia L. Front. Pharmacol. 2022, 13, 1021501. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Q.; Zhang, C.C.; Sun, X.L.; Cheng, X.X.; Wang, J.B.; Zhang, Y.D.; Xu, J.; Zou, H.Q. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci. Ther. 2013, 19, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Cai, W.; Yang, Q.; Yang, L.; Dai, Y.; Zhao, Z.; Yin, J.; Li, Y.; Li, Q.; Wang, Y.; et al. Artemisinin B Improves Learning and Memory Impairment in AD Dementia Mice by Suppressing Neuroinflammation. Neuroscience 2018, 395, 1–12. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Gaur, U.; Zheng, W. Artemisinin Improved Neuronal Functions in Alzheimer’s Disease Animal Model 3xtg Mice and Neuronal Cells via Stimulating the ERK/CREB Signaling Pathway. Aging Dis. 2020, 11, 801–819. [Google Scholar] [CrossRef]
- Qin, Y.-R.; Ma, C.-Q.; Jiang, J.-H.; Wang, D.-P.; Zhang, Q.-Q.; Liu, M.-R.; Zhao, H.-R.; Fang, Q.; Liu, Y. Artesunate restores mitochondrial fusion-fission dynamics and alleviates neuronal injury in Alzheimer’s disease models. J. Neurochem. 2022, 162, 290–304. [Google Scholar] [CrossRef]
- Kasaragod, V.B.; Hausrat, T.J.; Schaefer, N.; Kuhn, M.; Christensen, N.R.; Tessmer, I.; Maric, H.M.; Madsen, K.L.; Sotriffer, C.; Villmann, C.; et al. Elucidating the Molecular Basis for Inhibitory Neurotransmission Regulation by Artemisinins. Neuron 2019, 101, 673–689.e11. [Google Scholar] [CrossRef]
- Kasaragod, V.B.; Pacios-Michelena, A.; Schaefer, N.; Zheng, F.; Bader, N.; Alzheimer, C.; Villmann, C.; Schindelin, H. Pyridoxal kinase inhibition by artemisinins down-regulates inhibitory neurotransmission. Proc. Natl. Acad. Sci. USA 2020, 117, 33235–33245. [Google Scholar] [CrossRef]
- Pacios-Michelena, A.; Kasaragod, V.B.; Schindelin, H. Artemisinins and their impact on inhibitory neurotransmission. Curr. Opin. Pharmacol. 2021, 59, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Schrader, N.; Smolinsky, B.; Bedet, C.; Vannier, C.; Schwarz, G.; Schindelin, H. Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J. 2006, 25, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honoré, C.; Courtney, M.; Huber, K.V.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Cell 2017, 168, 86–100.e15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shan, L.; Tong, P.; Efferth, T. Cardiotoxicity and Cardioprotection by Artesunate in Larval Zebrafish. Dose-Response 2020, 18, 1559. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Groeneweg, F.; Gorgas, K.; Schlicksupp, A.; Kins, S.; Kirsch, J.; Kuhse, J. Amyloid-β Fosters p35/CDK5 Signaling Contributing to Changes of Inhibitory Synapses in Early Stages of Cerebral Amyloidosis. J. Alzheimer’s Dis. 2020, 74, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kins, S.; Gorgas, K.; Orlik, M.; Fischer, C.; Endres, K.; Schlicksupp, A.; Kirsch, J.; Kuhse, J. Artemisinin-treatment in pre-symptomatic APP-PS1 mice increases gephyrin phosphorylation at Ser270: A modification regulating postsynaptic GABAAR density. Biol. Chem. 2021, 403, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, E.J.; Gallegos, S.; Armijo-Weingart, L.; Araya, A.; Riffo-Lepe, N.O.; Cayuman, F.; Aguayo, L.G. Changes in neuronal excitability and synaptic transmission in nucleus accumbens in a transgenic Alzheimer’s disease mouse model. Sci. Rep. 2020, 10, 19606. [Google Scholar] [CrossRef]
- Bukanova, J.V.; Sharonova, I.N.; Skrebitsky, V.G. Functional modulation of strychnine-sensitive glycine receptors in rat hippocampal pyramidal neurons by amyloid-β protein (1–42). Brain Res. 2016, 1651, 61–72. [Google Scholar] [CrossRef]
- Eichler, S.A.; Förstera, B.; Smolinsky, B.; Jüttner, R.; Lehmann, T.N.; Fähling, M.; Meier, J.C. Splice-specific roles of glycine receptor alpha3 in the hippocampus. Eur. J. Neurosci. 2009, 30, 1077–1091. [Google Scholar] [CrossRef]
- Kuenzel, K.; Mofrad, S.A.; Gilbert, D.F. Phenotyping cellular viability by functional analysis of ion channels: GlyR-targeted screening in NT2-N cells. Methods Mol. Biol. 2017, 1601, 205–214. [Google Scholar]
- Lin, M.S.; Xiong, W.C.; Li, S.J.; Gong, Z.; Cao, X.; Kuang, X.J.; Zhang, Y.; Gao, T.M.; Mechawar, N.; Liu, C.; et al. α2-glycine receptors modulate adult hippocampal neurogenesis and spatial memory. Dev. Neurobiol. 2017, 77, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, C.; Tang, Y.; Chen, J.; Dong, M.; Song, T.; Zhang, X.; Zhao, J. Clozapine inhibits strychnine-sensitive glycine receptors in rat hippocampal neurons. Brain Res. 2009, 1278, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Lo, A.; Knox, K.M.; Barker-Haliski, M. Alzheimer’s Disease and Epilepsy: A Perspective on the Opportunities for Overlapping Therapeutic Innovation. Neurochem. Res. 2021, 46, 1895–1912. [Google Scholar] [CrossRef] [PubMed]
- Gengler, S.; Hamilton, A.; Hölscher, C. Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer’s disease is impaired in old but not young mice. PLoS ONE 2010, 5, e9764. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, F.; Puller, C.; O’Sullivan, G.A.; Paarmann, I.; Betz, H. Distribution of the glycine receptor β-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J. Comp. Neurol. 2012, 520, 3962–3981. [Google Scholar] [CrossRef] [PubMed]
- Tienari, P.J.; De Strooper, B.; Ikonen, E.; Simons, M.; Weidemann, A.; Czech, C.; Hartmann, T.; Ida, N.; Multhaup, G.; Masters, C.L.; et al. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 1996, 15, 5218–5229. [Google Scholar] [CrossRef]
- Lois, C.; Hong, E.J.; Pease, S.; Brown, E.J.; Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002, 295, 868–872. [Google Scholar] [CrossRef]
- Dresbach, T.; Hempelmann, A.; Spilker, C.; Dieck, S.T.; Altrock, W.D.; Zuschratter, W.; Garner, C.C.; Gundelfinger, E.D. Functional regions of the presynaptic cytomatrix protein bassoon: Significance for synaptic targeting and cytomatrix anchoring. Mol. Cell. Neurosci. 2003, 23, 279–291. [Google Scholar] [CrossRef]
- Kalbouneh, H.; Schlicksupp, A.; Kirsch, J.; Kuhse, J. Cyclin-dependent kinase 5 is involved in the phosphorylation of gephyrin and clustering of GABAA receptors at inhibitory synapses of hippocampal neurons. PLoS ONE 2014, 9, e104256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhse, J.; Groeneweg, F.; Kins, S.; Gorgas, K.; Nawrotzki, R.; Kirsch, J.; Kiss, E. Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate. Int. J. Mol. Sci. 2023, 24, 4623. https://doi.org/10.3390/ijms24054623
Kuhse J, Groeneweg F, Kins S, Gorgas K, Nawrotzki R, Kirsch J, Kiss E. Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate. International Journal of Molecular Sciences. 2023; 24(5):4623. https://doi.org/10.3390/ijms24054623
Chicago/Turabian StyleKuhse, Jochen, Femke Groeneweg, Stefan Kins, Karin Gorgas, Ralph Nawrotzki, Joachim Kirsch, and Eva Kiss. 2023. "Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate" International Journal of Molecular Sciences 24, no. 5: 4623. https://doi.org/10.3390/ijms24054623
APA StyleKuhse, J., Groeneweg, F., Kins, S., Gorgas, K., Nawrotzki, R., Kirsch, J., & Kiss, E. (2023). Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate. International Journal of Molecular Sciences, 24(5), 4623. https://doi.org/10.3390/ijms24054623