Abstract
Periodontal diseases are oral inflammatory diseases affecting the tissues supporting and surrounding the teeth and include gingivitis and periodontitis. Oral pathogens may lead to microbial products spreading into the systemic circulation and reaching distant organs, while periodontal diseases have been related to low-grade systemic inflammation. Gut and oral microbiota alterations might play a role in the pathogenesis of several autoimmune and inflammatory diseases including arthritis, considering the role of the gut–joint axis in the regulation of molecular pathways involved in the pathogenesis of these conditions. In this scenario, it is hypothesized that probiotics might contribute to the oral and intestinal micro-ecological balance and could reduce low-grade inflammation typical of periodontal diseases and arthritis. This literature overview aims to summarize state-of-the-art ideas about linkages among oral–gut microbiota, periodontal diseases, and arthritis, while investigating the role of probiotics as a potential therapeutic intervention for the management of both oral diseases and musculoskeletal disorders.
1. Introduction
Periodontal diseases are oral inflammatory diseases affecting the tissues supporting and surrounding the teeth and include gingivitis and periodontitis [1,2]. Furthermore, gingivitis is associated with bleeding, swollen gums, and pain, whereas periodontitis is related to the loss of periodontal attachment and supporting bone [3]. The latter is often considered a “silent disease” due to the absence of symptoms that characterize the clinical presentation. However, if untreated, the inflammatory condition of periodontitis can lead to tooth loss, with a consequent impairment in mastication function, esthetics, self-confidence, and quality of life [4,5]. The prevalence of periodontal diseases is estimated to range from 20% to 50% worldwide, emerging as the 11th most prevalent condition in the world as reported by the Global Burden of Disease Study of 2016 [6]. Therefore, this detrimental condition is currently considered as a global health problem [7,8].
In this scenario, the first step is understanding the mechanisms underpinning the etiopathogenesis of periodontal diseases, considering that the local inflammatory response might be perpetuated by several oral pathogens (e.g., A. actinomycetemcomitans, P. intermedia, P. gingivalis, T. denticola, F. nucleatum, and T. forsyth) [9,10,11].
Periodontal diseases have been shown to be related to low-grade systemic inflammation, potentially driven by several inflammatory mediators [9,10,12,13,14]. In this context, recent studies showed that patients with periodontal diseases might be characterized by higher circulating levels of C-reactive protein (CRP), fibrinogen, neutrophils, and indirect systemic inflammatory markers, such as tumor necrosis factor (TNF) and Interleukin (IL) 1, 6, and 8 [15,16,17].
High levels of bacteria present in the dysbiotic biofilm in periodontitis might also play a role in the pathogenesis of autoimmune diseases [18,19,20]. In this context, recent studies reported a link between periodontitis and rheumatoid arthritis (RA), considering the higher prevalence of RA in these patients and the correlation between the severity of arthritis and periodontitis [19]. Indeed, it was reported that P. gingivalis has been implicated in the generation of anticyclic citrullinated peptide antibodies (ACPAs), which are recognized as diagnostic and prognostic biomarkers for RA patients [20]. Moreover, Zhou et al. [19] suggested that the downregulation of IL-10 could represent the key mechanism by which periodontitis may promote RA.
Several scientific studies showed that gut and oral microbiota alterations might play a role in the pathogenesis of several autoimmune and inflammatory diseases [21,22,23,24].
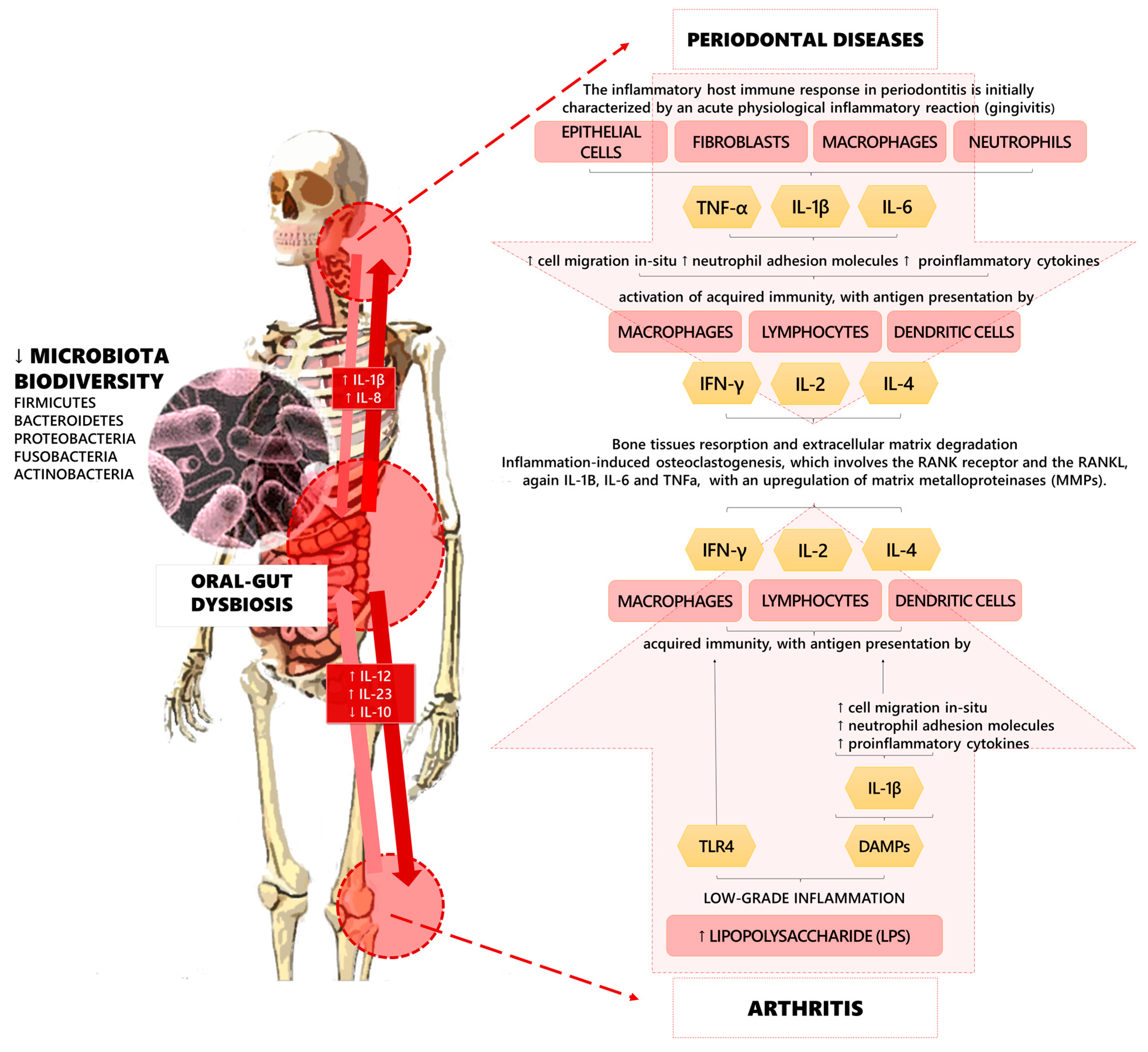
However, recent research has focused on the implications of the microbiota on musculoskeletal health, highlighting the role of the “gut–bone axis” and the “gut–joint axis” in the regulation of molecular pathways involved in the pathogenesis of these detrimental conditions [25,26,27,28]. Furthermore, gut microbial dysbiosis might promote musculoskeletal disorders through the intestinal absorption of vitamin K, calcium, pyridoxal phosphate, pantothenic acid (B5), cobalamin (B12), biotin (B7), folate (B9), thiamine (B1), niacin (B3), and tetrahydrofolate. Moreover, it has been proposed that osteoclasts activity might be indirectly stimulated by gut microbiota via serum levels of insulin-like growth factor 1 (IGF-1) [29,30,31] (see Figure 1 for further details).
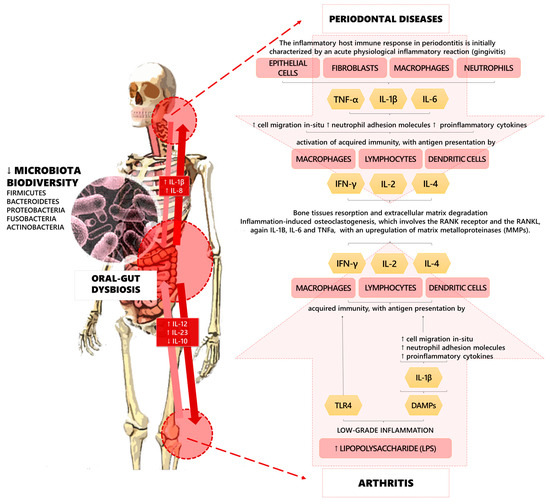
Figure 1.
Pathogenic pathways of the linkages among oral–gut dysbiosis, periodontal diseases, and arthritis.
Although the pathophysiological mechanisms underpinning the interactions among oral–gut microbiota have still not been characterized in detail, it has been hypothesized that dietary supplements including probiotics could contribute to the oral and intestinal micro-ecological balance [26,32,33,34]. However, to date, there is still a large gap of knowledge about the optimal management of oral and gut dysbiosis.
Therefore, the present literature overview aims to summarize the current scientific evidence on correlations among oral–gut microbiota, periodontal diseases, and arthritis, exploring the role that probiotics might play as a therapeutic intervention for oral diseases and musculoskeletal disorders.
2. Oral–Gut Microbiome and Periodontal Diseases
Microbiota includes microbial communities colonizing the mucosae (such as the intestinal tract, reproductive organs, and the respiratory tract) and the skin, for a total of more than 100 trillion microbial cells that encode 100-fold more unique genes than the human genome [35,36,37]. Microbiota colonization appears in the early years of life and changes rapidly, until it becomes unique for each person, then remains relatively stable in adulthood [38]. The oral cavity is one of the most complex ecosystems in the body due to its repeated interaction with the external environment, as well as containing several different microbial habitats, both hard tissues (i.e., the teeth) and soft tissues (i.e., the buccal mucosa, the tongue, the soft and hard palates, and the gingiva) and their respective interfaces (i.e., the supragingival and subgingival margins) [39].
The oral microbiome is estimated to be the second most divergent and abundant after the gut microbiota, considering that it is mainly composed of bacteria, viruses, fungi, protozoa, and archaea; indeed, in the oral cavity of humans, 700 bacterial species, belonging to 185 genera and 12 phyla, have been identified [39]. The oral microbiome includes different phyla consisting of Firmicutes (including Streptococcus), Bacteroidetes (strongly represented by Prevotella), Proteobacteria, Fusobacteria, and Actinobacteria [40]. These bacteria commonly coexist and thrive by forming a biofilm and living in a symbiotic state of co-aggregation, thus maintaining the homeostasis of the oral ecosystem; furthermore, it should be noted that the plaque biofilm can create an adequate balance among the pathogens and commensals and is highly resistant to the environmental stimuli [41,42,43].
Furthermore, fungi are an integral part of a healthy oral microbiota, where commensal fungi entertain a multitude of synergic or antagonistic interactions with bacteria. It is estimated that more than 100 species of fungi colonize the oral cavity (e.g., Candida species, Cladosporium, Aureobasidium, Saccharomyces, Aspergillus, Fusarium, and Cryptococcus), and only in immunocompromised subjects or specific conditions (especially drug abuse) can they become opportunistic pathogens [44,45].
Interestingly, the oral microbiome might be affected not only by the overall health condition of the host but also by environmental and behavioral factors including oral hygiene, nutrition, smoking, and mechanical stress [46]. In particular, the regular consumption of beverages and food with elevated levels of polyphenols (e.g., tea, cranberry, and almond) have been shown to inhibit some oral pathogenic bacteria [47,48]. Meanwhile, Esberg et al. reported that some species, including Actinomyces, Bifidobacterium, Veillonella, and Streptococcus (e.g., S. wiggsiae, S. mutans, and S. sobrinus) were frequently associated with high sucrose intake [49].
The gut microbiota shows several differences compared to the oral one, including pH and O2 tension, host secretions, substrate availability, and digest flow rates [50]. It should be noted that the gastric tract (median pH 1.4) is mainly colonized by Actinobacteria, Bacteroidetes, Firmicutes (including Streptococcus), and Proteobacteria (which include Helicobacter pylori) in healthy subjects [51]. The large intestine hosts the most abundant microbial community, probably because of the slow flow rates and the neutral-to-mildly-acidic pH [52], while the main gut bacterial phyla are the Firmicutes (including Clostridium, Enterococcus, Lactobacillus, and Ruminococcus genera), Bacteroidetes (including Prevotella genera), Actinobacteria, Proteobacteria, and Fusobacteria [37]. However, several pathological conditions might occur in response to the loss of the balance within a human-associated gut microbiota; such gut dysbiosis might be closely related to inflammatory bowel disorders (e.g., Crohn’s disease), esophagitis, Barrett’s esophagus, vaginitis, type 2 diabetes, arthritis, autism, neurodegenerative diseases, and cancer [24,35,53,54,55]. Furthermore, gut microorganisms may stimulate regulatory cells of the immune system to inhibit inflammation and provide a natural defense against pathogenic species through competition [56,57].
Poor oral hygiene is strictly related to oral microbiota modifications, especially in subgingival communities. In this context, Gram-negative species (e.g., Prevotella, Selenomonas, and F. nucleatum) can significantly increase after 2–3 weeks of plaque accumulation, and clinical inflammation of the gingiva is a common clinical presentation of this condition [58]. On the other hand, the depletion of Gram-positive species (e.g., R. dentocariosa, Propionibacterium, and S. maltophila) has negative implications for oral health [58].
Furthermore, the development of periodontitis has been associated with the accumulation of different Gram-negative species compared to gingivitis. In 1998, Socransky et al. [59] identified “the red complex” including three different bacteria species (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) closely related to the arousal of clinical signs and symptoms of periodontitis, and strictly associated with the severity of the disease. In recent years, periodontitis has been correlated with several pathogens, such as F. alocis, Porphyromonas, Synergistetes, Peptostreptococcaceae, and A. actinomycetemcomitans, associated with aggressive periodontitis [60,61,62,63].
Moreover, a strict relationship has been hypothesized among members of the oral microbiome, which shows both antagonistic and synergistic interactions. For instance, Fusobacterium nucleatum was shown to increase the survivability of the periodontal pathogen, P. gingivalis [64], while T. denticola seems to benefit from the succinate produced by P. gingivalis [65]. Furthermore, T. denticola and P. gingivalis concentrations increase significantly in co-culture; indeed, alterations to glycine and glutamate catabolism by T. denticola, as well as changes to thiamine pyrophosphate and fatty acid synthesis by P. gingivalis, have been observed [63,66].
Thus, the recent scientific literature suggests potential correlations among oral microbiota and systemic diseases, probably due to the dissemination of pro-inflammatory, invasive, anaerobic, and oral pathogens into the gut [67,68,69,70,71,72].
3. Gut-Microbiota and Musculoskeletal Health: “Gut–Joint Axis”
Recent evidence has focused on the impact of gut microbiota on musculoskeletal health, highlighting the role of the “gut–joint axis” in the regulation of the pathogenic pathways of musculoskeletal conditions [25,26,73,74]. However, to date, the longevity has been negatively correlated with increased alpha diversity in gut microbiota, with recent research focusing on “leaky gut syndrome”, an aged-related condition characterized by increased gut permeability, resulting in microbial products spreading in the bloodstream and an increase in inflammatory states [74,75,76].
Although several questions are still open about the role of the leaky gut syndrome in the development and progression of osteoarthritis (OA), serum levels of bacterial metabolites might be correlated with joint degeneration in OA patients [23]. Inflammation serum markers in patients with OA are positively associated with bacterially produced lipopolysaccharides (LPS), supporting the hypothesis of a role of microbiota-induced systemic inflammation in several pathways underpinning the development of OA [77,78]. Therefore, these findings suggested that gut dysbiosis, especially in the elderly, might be strictly linked to OA pathogenesis, and the gut–joint axis might be a potentially modifiable cofactor to be targeted by a comprehensive therapeutic intervention [21,22,23,79]. In line with these findings, the expert consensus of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) has recently supported the hypothesis that gut microbiota alterations might be considered as hidden risk factors for the development and progression of OA [79]. In this context, it has been proposed that gut microbiota modulation might have positive effects on OA [25]. Furthermore, a recent study on mice underlined that probiotic administration might significantly reduce pro-inflammatory cytokine production in knee cartilage [80]. Similarly, a probiotic diet might be effective in modulating prostaglandin-endoperoxide synthase 2 (PTGS2) and transforming growth factor-beta (TGF-β), with intriguing implications for targeting low-grade systemic inflammation promoted by dysbiosis in patients with OA [80]. In accordance with these findings, gut microbiota might be crucially affected by dietary supplements, with several studies underlining the positive effects of nutraceuticals in promoting health status in older adults, particularly in the case of early diagnoses [23,81].
The main linking factor between gut microbiota and OA seems to be represented by low-grade chronic inflammation, supporting a new OA phenotype called “metabolic OA”, where several pro-inflammatory stimuli are associated with drastic changes in the composition of the intestinal microbiota [22]. Thus, aging might play a key role in intestinal microbiota composition, inducing reduced phyla diversity, a greater proportion of Bacteroides spp., and a distinct abundance of Clostridium groups [82].
In the last decade, a growing literature showed that the alteration of gut and oral cavity microbiota could have a detrimental impact on the pathogenesis of autoimmune and inflammatory joint diseases, such as OA and rheumatoid arthritis (RA) [21,22,23]. Increased inflammation is a relevant pathophysiological mechanism in RA and the potential correlation between serum levels of bacterial metabolites and joint degeneration is a crucial issue for future investigation [83,84].
Furthermore, several components of intestinal microbiota might affect host immunity, particularly in patients with autoimmune diseases such as RA [85,86,87]. The correlation between intestinal immune cell activation and arthritis is based on the potential migration of gut-derived immune cells to the joints, provoking an impairment in terms of differentiation of T cell types (i.e., Treg cells) involved in the pathogenesis of RA [86,87,88,89].
Specifically, it has been hypothesized that gut dysbiosis might be a mediator for inflammation in the temporomandibular joint (TMJ) by regulating the microglial activation in the trigeminal nociceptive system [90,91]. Moreover, it should be noted that TMJ inflammation commonly leads to temporomandibular disorders (TMDs), which are considered a sub-classification of musculoskeletal disorders commonly treated with conservative approaches [91,92,93]. Furthermore, RA might affect TMJ by causing disease-related symptoms, with a correlation between laboratory values of various inflammatory biomarkers causing rheumatic diseases and the progression of TMD [94,95,96,97,98,99].
Scher et al. [100] compared the composition of subgingival microbiota in patients with RA against controls and revealed that Prevotella and Leptotrichia species might characterize patients with RA. Meanwhile, distinct subgingival microbiota was found in RA patients without periodontal diseases, suggesting that changes in oral microbiota might be RA-specific [101,102,103,104]. Furthermore, it has been demonstrated that serum antibodies against P. gingivalis could increase during the preclinical phase, becoming stable after the diagnosis of RA [105,106]. Thus, an association between periodontal bacteria exposure and RA autoantibody development might represent an emerging research topic in the future [106].
A growing literature now seems to support the role of oral–gut microbiota in inflammatory conditions (i.e., OA, RA, and TMJ arthritis); however, several questions are still open in this field and future studies are needed to better characterize this concept.
4. Impact of Probiotics on Oral Microbiota and Periodontal Diseases
Probiotics are defined as living microorganisms that can have beneficial effects on the host when taken in sufficient doses [107]. They are available in several food products, such as yogurts, milk-based foods, powders, capsules, and oral solutions [108].
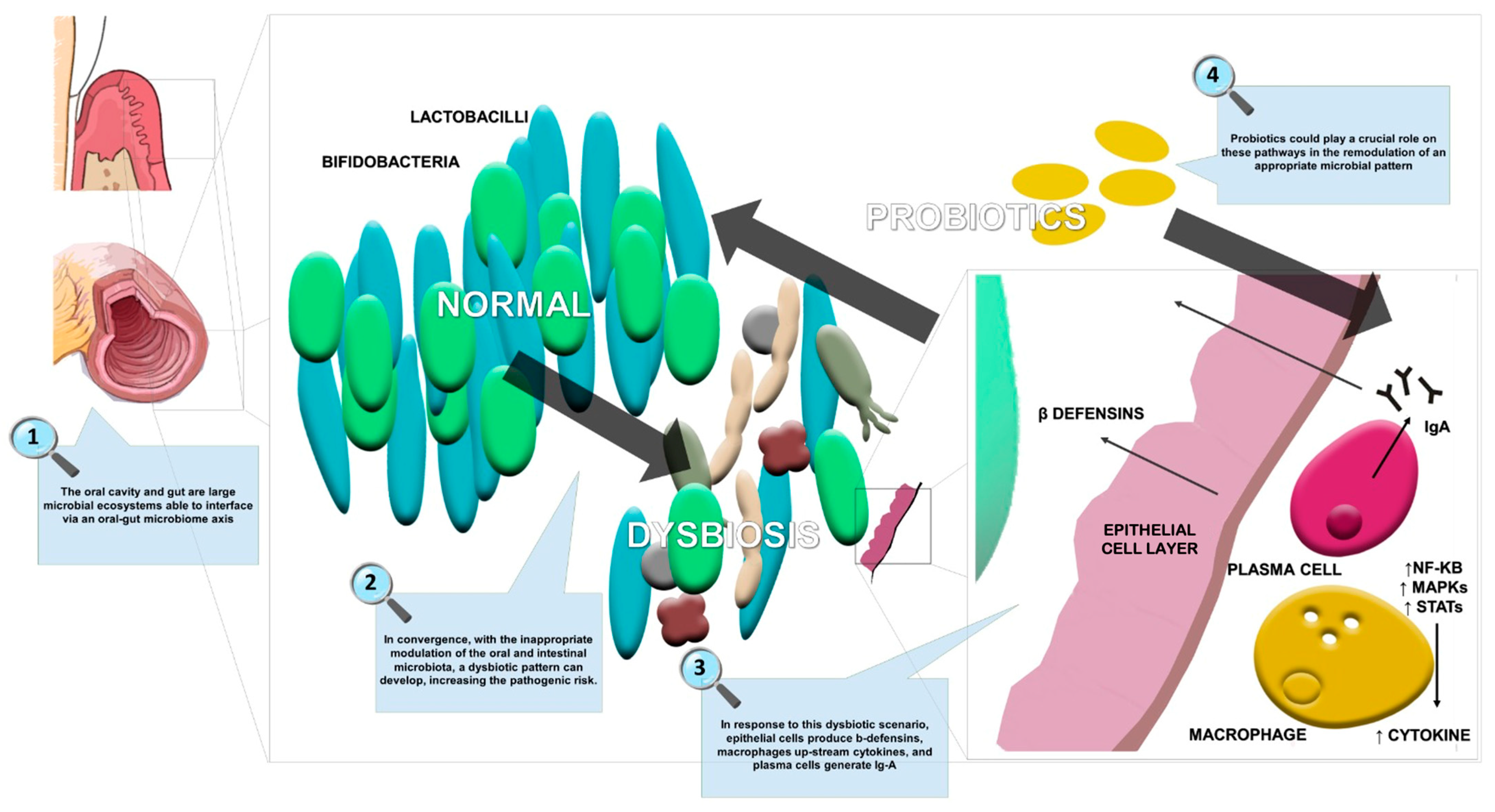
Moreover, the scientific literature is focused on the use of probiotic strains (e.g., Lactobacillus, Bifidobacterium, Escherichia, Enterococcus, and B. subtilis) and yeasts (e.g., Saccharomyces) in the maintenance of gastrointestinal microbiota balance. Figure 2 summarizes the effects of probiotics in oral and gut dysbiosis management, including restoration of the epithelial membrane resulting in a reduction of systemic inflammation.
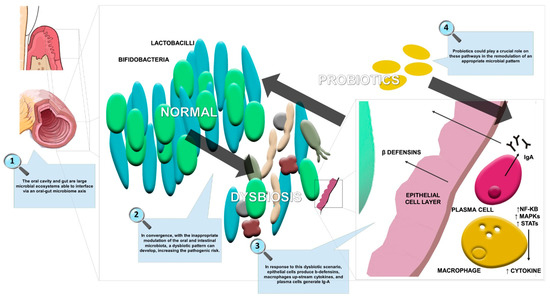
Figure 2.
The effects of probiotics in oral and gut dysbiosis.
Probiotic supplements with a concentration of 107–108 cells per gram could play a role in the treatment of inflammatory chronic diseases [107,108,109]. Indeed, recent evidence showed that probiotics might reinforce the epithelial barrier, thus allowing fibroblastic activity and epithelial cell migration [109,110].
Interestingly, probiotics that have a role in oral health are concentrated in the genera Streptococcus, Lactobacillus, Bifidobacterium, Weissella, B. subtilis, and S. cerevisiae, with their therapeutic use in dentistry growing significantly in recent years [111].
Several microorganisms isolated from the oral cavity are commercially produced as oral health-promoting probiotics, including L. reuteri, L. brevis, and S. salivarius, and their effectiveness is shown in the management of dental caries, oral candida infection, halitosis, and periodontal diseases [107,112,113,114,115].
Recently, Liu et al. [116] performed a systematic review and meta-analysis of randomized controlled trials (RCTs) on the effects of probiotics on gingival inflammation and oral microbiota composition in patients suffering from plaque-induced gingivitis. The authors included 11 RCTs with a total of 554 patients, reporting that the oral probiotics had no significant improvement in the Gingival Index (GI), Plaque Index (PI), and bleeding on probing (BOP) in patients affected by plaque-induced gingivitis. Moreover, no significant differences were found in the amount of P. gingivalis, A. actinomycetemcomitans, P. intermedia, and F. nucleatum between the probiotic group and the placebo group. Their findings were in line with another systematic review and meta-analysis by Hardan et al. [117] on the use of probiotics as an adjuvant therapy within clinical periodontal parameters. The authors showed that the use of probiotics did not improve the PI (p = 0.16). However, the systematic review also assessed the efficacy of probiotics as an adjuvant therapy in the treatment of periodontitis, and showed significant improvement in terms of PPD, CAL, and BOP (p < 0.001).
In line with these results, the effects of probiotics on the management of periodontal diseases were reported by several other studies [118,119,120]. Tekce et al. [119] evaluated the effectiveness of L. reuteri as an adjuvant treatment for chronic periodontitis patients, evaluating the clinical effects on periodontal tissues. The authors reported that plaque index, gingival index, bleeding on probing, and probing depth were significantly lower (p < 0.05) in the study group compared with controls at all time points [119].
In 2018, Invernici et al. [121] evaluated the effect of Bifidobacterium animalis subsp. lactis HN019 as an adjuvant to scaling and root planing (SRP) in patients with generalized chronic periodontitis (with 30% or more of the sites with probing pocket depth ≥ 4 mm and clinical attachment level ≥ 4 mm, and a minimum of five teeth with at least one site with CAL and PPD ≥ 5 mm). By collecting gingival crevicular fluid they determined the levels of IL-1β, IL-10, and IL-8. Furthermore, they evaluated the microbiota changes after probiotic therapy. The authors showed that subjects who underwent probiotic therapy reported higher levels of IL-10 than those at baseline at 30 days (p < 0.05) and showed greater amounts of Actinomyces naeslundii and Streptococcus mitis, and lower amounts of P. gingivalis, T. denticola, F. nucleatum, C. showae, and E. nodatum in deep periodontal pockets (p < 0.05) [121].
In 2020, the same research group evaluated the effects of Bifidobacterium animalis subsp. lactis HN019 in generalized chronic periodontitis patients [122]. They analyzed the immunocompetence of the gingival tissues by evaluating the expression of beta-defensin (BD)-3, toll-like receptor 4 (TLR4), and cluster of differentiation (CD)-57 and CD-4. Plaque accumulation, gingival bleeding, and the antimicrobial properties of HN019 were analyzed. Their results showed that subjects who underwent probiotic therapy presented with a lower PI at 30 days and had lower marginal gingival bleeding at 90 days (p < 0.05); in addition, increased BD-3, TLR4, and CD-4 expression in periodontal tissues were reported. Lastly, the findings showed a lower mean adhesion of P. gingivalis together with B. lactis HN019 to buccal epithelial cells (p < 0.05).
In this context, as depicted by Table 1, the oral microbiota could represent a potential target for probiotic supplementation to reduce the risk of periodontal diseases.

Table 1.
Impact of probiotics on the oral microbiota in human studies.
5. Role of Probiotics in Patients Affected by Arthritis
Probiotics could exert an immunomodulatory action by regulating intestinal inflammation and immune function and by preventing an increase in intestinal permeability and bacterial translocation. Therefore, probiotics might reduce the production of autoantibodies in the inflamed intestine and reduce the migration of pro-inflammatory immune cells from the gut tissue to the joints [123,124]. In this scenario, probiotics could be a beneficial intervention in the complex treatment of inflammatory joint diseases [125,126]. It should be noted that several studies [127,128,129,130,131,132,133,134,135,136,137] showed that the administration of specific probiotics (E. faecium, L. casei, L. plantarum, B. longum, Bifidobacteria, P. histicola, L. acidophilus, L. helveticus, B. adolescentis, and L. fermentum) may reduce RA symptoms by increasing anti-inflammatory cytokines (i.e., IL-10 and TGF-β) and inhibiting pro-inflammatory cytokines (i.e., IL-1β, IL-2, IL-6, IL-12, IL-17, and NF-κB), thus promoting the differentiation of CD4+ T cells into regulatory T cells (Tregs).
However, the role of probiotics in humans affected by inflammatory joint diseases is still debated in the scientific literature. In 2017, Mohammed et al. [124] performed a systematic review and meta-analysis of randomized or quasi-randomized clinical trials on the effect of probiotics on the treatment of RA. The authors included six randomized and controlled trials and three quasi-RCTs, with a total of 361 patients. Their results showed that oral probiotics lowered the pro-inflammatory cytokine IL-6 (SMD—0.708, 95% CI—1.370 to 0.047, p = 0.036), which is an indicator for joint destruction in RA, but no significant differences were found in disease activity score (DAS) and swollen joint count (SJC) between the probiotic and placebo groups.
Another recent systematic review with a meta-analysis performed by Zeng et al. [123] showed that the use of probiotics did not improve clinical variables such as DAS (p = 0.17) and swollen joint counts (p = 0.71) in patients. However, they assessed the efficacy of probiotics on inflammatory markers in RA and showed significantly lower levels of CRP (SMD −1.57 (−2.98, −0.15; p = 0.03)), highlighting the potential role of curcumin in CRP reduction [123]. Furthermore, Mandel et al. [127] demonstrated that the administration of B. coagulans to RA patients was effective in reducing the patient pain assessment score and the pain scale (p = 0.052 and 0.046, respectively). Moreover, a randomized, double-blind, placebo-controlled clinical trial showed the effects of L. casei on RA activity and inflammatory cytokines in women [128]. The authors demonstrated an improvement in the DAS (p < 0.01) associated with a reduction of serum levels, TNF-α, IL-6, and IL-12 (p < 0.05), and an increase of IL-10 (p < 0.05) in the group supplemented with probiotics [128]. Furthermore, Alipour et al. [129] treated patients with L. casei and found improvements in CRP levels, tender/swollen joint counts, and DAS28 compared to a placebo (p < 0.05). Zamani et al. [130] also demonstrated that patients who received a daily capsule containing three viable and freeze-dried strains (L. acidophilus, L. casei, and B. bifidum) showed an improvement in DAS28 (−0.3 ± 0.4 vs. −0.1 ± 0.4, p = 0.01) and serum high-sensitivity C-reactive protein (hs-CRP) concentrations (−6.66 ± 2.56 vs. +3.07 ± 5.53 mg/L, p < 0.001) compared with a placebo. Moreover, Cannarella et al. [131] demonstrated an exertive role of supplementing with a mixture of probiotics (L. acidophilus, L. casei, L. lactis, B. lactis, and B. bifidus) on TNF-α (p = 0.004) and IL-6 (p < 0.05), but no effect on DAS28 (p > 0.05). On other hand, a pilot study conducted by Hataka et al. [132] demonstrated that Lactobacillus rhamnosus administration did not show a statistically significant difference in the activity of RA in terms of both clinical variables and inflammatory markers. In accordance with this result, Pineda et al. [133] showed a non-significant decreasing trend in serum levels of IL-1α, IL-6, IL-10, IL-12, TNF-α, and Monocyte chemoattractant protein-1 (MCP-1) following L. rhamnosus combined with L. reuteri treatment in RA patients.
Concerning the use of probiotics in patients affected by spondyloarthritis, two studies affirmed no significant decrease in any disease activity markers such as the Bath Ankylosing Spondylitis Functional Index (BASFI) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), ASAS-endorsed core domains, global health status, and CRP after probiotic intervention compared with a placebo (p > 0.05) [134,135]. As mentioned above, OA can be considered a persistent low-grade inflammation of the joints. Thus, over the past few years, there has been a growing interest in the role of the probiotics in OA therapy [136,137]. An important link between gut microbiota and patient’ clinical features highlighted the possibility of positively interfering with disease progression and presentation with microbiota modulation [26]. To date, mouse model studies on the use of probiotics, such as C. butyricum, L. casei, L. acidophilus, L. fermentum, L. paracasei, S. thermophilus, B. longum, B. bifidum, B. breve, L. rhamnosus, L. plantarum, L. helveticus, and L. salivarius, have demonstrated a positive role in the preservation of knee cartilage, synovial membrane, and fibrous tissue. Moreover, this supplementation significantly lowered serum levels of inflammatory and bone metabolism markers (such as metalloproteinases, cyclooxygenase-2, leukotriene B4, and cartilage oligomeric matrix protein) and inflammatory cytokines (such as IL-1β, IL-2, IL-6, IL-12, IL-17, TNF-α, and IFN-γ), while increasing levels of anti-inflammatory cytokines (IL-4 and IL-10) and anti-IFN-γ and glycosaminoglycans [138,139,140,141,142,143]. Meanwhile, a large RCT considered the effects of L. casei Shirota in human patients with knee OA who were asked to ingest either skimmed milk containing the probiotic or the placebo daily for 6 months. The study demonstrated an improvement in The Western Ontario and McMaster Universities Arthritis Index (WOMAC) in the intervention group (p < 0.05) [137].
Taken together, gut microbiome dysbiosis might be considered important in the pathogenic mechanism of inflammatory joint diseases both in terms of onset and progression; moreover, probiotics might play a role in the complex management of such chronic inflammatory diseases.
Accordingly, in Table 2 we describe the evidence for the role of probiotics in patients affected by musculoskeletal disorders.

Table 2.
Probiotics for musculoskeletal disorders in human studies.
6. Conclusions
This literature overview aimed to summarize the evidence on potential correlation among oral–gut microbiota, periodontal diseases, and arthritis, with an interest on the impact of probiotics on low-grade inflammation.
The scientific literature showed that poor oral hygiene can be correlated to oral microbiota modifications (e.g., F. alocis, Porphyromonas, Synergistetes, Peptostreptococcaceae, and A. actinomycetemcomitans) with a linkage between the accumulation of different Gram-negative species and the onset of periodontal diseases. Moreover, considering the role of the gut–joint axis in regulating molecular pathways involved in the pathogenesis of several musculoskeletal conditions (e.g., OA, RA, and TMJ arthritis), the microbiome may influence the pathogenesis of musculoskeletal diseases.
Although the pathophysiological mechanisms underpinning these interactions have not been fully characterized, a growing literature has been supporting the hypothesis of therapeutic action with dietary supplements and probiotics (e.g., E. faecium, L. casei, L. plantarum, B. longum, Bifidobacteria, P. histicola, L. acidophilus, L. helveticus, B. adolescentis, and L. fermentum) for the treatment of chronic inflammatory diseases.
In conclusion, in this paper, we described state-of-the-art findings from the scientific literature on the role of probiotics in the prevention and management of dysbiosis-related disorders. We consider that the gut–oral microbiota could be a new target for patients affected by periodontal diseases and arthritis in future.
However, it should be noted that there is still a gap in the scientific knowledge, not only on the role of oral microbiota in the pathogenesis of inflammation, but also on interactions among microbiota and other systemic conditions. Thus, further observational studies are needed to define, firstly, the specific target and, secondly, the impact of probiotics on patients affected by inflammatory diseases.
Author Contributions
Conceptualization, M.F. and A.d.S.; methodology, A.G., M.I. and A.d.S.; software, N.M.; validation, M.M., F.R., L.F. and A.A.; investigation, M.F., L.L. and D.C.; data curation, M.F., M.M. and R.d.S.; writing—original draft preparation, M.F., L.L. and D.C.; writing—review and editing, A.G., M.I. and A.d.S.; visualization, M.M., F.R., N.M., R.d.S., L.F. and A.A.; supervision, A.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Claudio Curci for his support in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontal. 2018, 45 (Suppl. 20), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Carranza, G. Clinical Periodontology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- World Health Organization. Oral Health; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016 [published correction appears in Lancet. 2017 Oct 28;390,e38]. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- FDI World Dental Federation. Periodontal Health and Disease: A Practical Guide to Reduce the Global Burden of Periodontal Disease; FDI World Dental Federation: Geneva, Switzerland, 2018. [Google Scholar]
- Carrizales-Sepúlvedam, E.F.; Ordaz-Faríasm, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; Pezzotti, F.; Foglio Bonda, P.L.; Calafiore, D.; de Sire, A. Periodontal Disease and Vitamin D Deficiency in Pregnant Women: Which Correlation with Preterm and Low-Weight Birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef]
- de Sire, A.; Baricich, A.; Ferrillo, M.; Migliario, M.; Cisari, C.; Invernizzi, M. Buccal hemineglect: Is it useful to evaluate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors? A cross-sectional study. Top. Stroke Rehabil. 2020, 27, 208–214. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 1, 72–80. [Google Scholar]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral Health in Breast Cancer Women with Vitamin D Deficiency: A Machine Learning Study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76 (Suppl. 11), 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Periodontitis and risk of immune-mediated systemic conditions: A systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zou, F.; Cheng, X.; Huang, Y.; Zou, H.; Niu, Q.; Qiu, Y.; Shan, F.; Luo, A.; Teng, W.; et al. Porphyromonas gin-givalis induces periodontitis, causes immune imbalance, and promotes rheumatoid arthritis. J. Leukoc. Biol. 2021, 110, 461–473. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and Rheumatoid Arthritis: The Same Inflammatory Mediators? Mediators Inflamm. 2019, 2019, 6034546. [Google Scholar] [CrossRef]
- Lian, W.S.; Wang, F.S.; Chen, Y.S.; Tsai, M.H.; Chao, H.R.; Jahr, H.; Wu, R.W.; Ko, J.Y. Gut Microbiota Ecosystem Governance of Host Inflammation, Mitochondrial Respiration and Skeletal Homeostasis. Biomedicines 2022, 10, 860. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Z.; Tu, S.Q.; Wei, J.M.; Hou, Y.L.; Kuang, Z.L.; Kang, X.N.; Ai, H. Role of Interleukin-17A in the Pathomechanisms of Periodontitis and Related Systemic Chronic Inflammatory Diseases. Front. Immunol. 2022, 13, 862415. [Google Scholar] [CrossRef]
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.A.; Schott, E.M.; Gill, S.R.; Zuscik, M.J. The gut microbiome-joint connection: Implications in osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101. [Google Scholar] [CrossRef]
- de Sire, R.; Talocco, C.; Petito, V.; Lopetuso, L.R.; Graziani, C.; Gasbarrini, A.; Scaldaferri, F. Microbiota and inflammatory bowel disease: An update. Recenti Prog. Med. 2018, 109, 570–573. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- de Sire, R.; Rizzatti, G.; Ingravalle, F.; Pizzoferrato, M.; Petito, V.; Lopetuso, L.; Graziani, C.; de Sire, A.; Mentella, M.C.; Mele, M.C.; et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Hathaway-Schrader, J.D.; Carson, M.D.; Gerasco, J.E.; Warner, A.J.; Swanson, B.A.; Aguirre, J.I.; Westwater, C.; Liu, B.; Novince, C.M. Commensal gut bacterium critically regulates alveolar bone homeostasis. Lab. Investig. 2022, 102, 363–375. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef]
- Wang, N.; Hao, Y.; Fu, L. Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway. Nutrients 2022, 14, 3955. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Do Only Calcium and Vitamin D Matter? Micronutrients in the Diet of Inflammatory Bowel Diseases Patients and the Risk of Osteoporosis. Nutrients 2021, 13, 525. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Floch, M.H. Probiotics and Prebiotics. Gastroenterol. Hepatol. 2014, 10, 680–681. [Google Scholar]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Hord, N.G. Eukaryotic-microbiota crosstalk: Potential mechanisms for health benefits of prebiotics and probiotics. Annu. Rev. Nutr. 2008, 28, 215–231. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Koliarakis, I.; Messaritakis, I.; Nikolouzakis, T.K.; Hamilos, G.; Souglakos, J.; Tsiaoussis, J. Oral Bacteria and Intestinal Dysbiosis in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 4146. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis from a Microbial and Host Perspective Relative to Oral Health and Disease. Front. Microbiol. 2021, 12, 617485. [Google Scholar] [CrossRef]
- Krom, B.; Kidwai, S.; Ten Cate, J. Candida and other fungal species: Forgotten players of healthy oral microbiota. J. Dent. Res. 2014, 93, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, H.; Zhang, W.; Ni, L. Salivary Microbiota Shifts under Sustained Consumption of Oolong Tea in Healthy Adults. Nutrients 2020, 12, 966. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Tsou, S.-H.; Hu, S.-W.; Yang, J.-J.; Yan, M.; Lin, Y.-Y. Potential Oral Health Care Agent from Co_ee against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Esberg, A.; Haworth, S.; Hasslöf, P.; Lif Holgerson, P.; Johansson, I. Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes. Nutrients 2020, 12, 681. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Ianiro, G.; Molina-Infante, J.; Gasbarrini, A. Gastric microbiota. Helicobacter 2015, 20 (Suppl. 1), 68–71. [Google Scholar] [CrossRef]
- Cueva, C.; Silva, M.; Pinillos, I.; Bartolomé, B.; Moreno-Arribas, M.V. Interplay between Dietary Polyphenols and Oral and Gut Microbiota in the Development of Colorectal Cancer. Nutrients 2020, 12, 625. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2021, 9, 1. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Pilone, V.; Carinci, F.; Lauritano, D.; Sbordone, L. The Association between Periodontitis and Human Colorectal Cancer: Genetic and Pathogenic Linkage. Life 2020, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S95–S111. [Google Scholar] [CrossRef]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and Complex Bacterial Profiles in Human Periodontitis and Health Revealed by 16S Pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.-K.; Poulsen, K.; Væth, M.; Poulsen, S.; Kilian, M. Risk of Aggressive Periodontitis in Adolescent Carriers of the JP2 Clone of Aggregatibacter (Actinobacillus) Actinomycetemcomitans in Morocco: A Prospective Longitudinal Cohort Study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Role of Fusobacterium Nucleatum and Coaggregation in Anaerobe Survival in Planktonic and Biofilm Oral Microbial Communities During Aeration. Infect. Immun. 1998, 66, 4729–4732. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D. Nutritional Interactions Between Two Suspected Periodontopathogens, Treponema Denticola and Porphyromonas Gingivalis. Infect. Immun. 1992, 60, 5298–5301. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ikegami, A.; Kuramitsu, H.K. Synergistic Biofilm Formation by Treponema Denticola and Porphyromonas Gingivalis. FEMS Microbiol. Lett. 2005, 250, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Vipperla, K.; O’Keefe, S.J. Diet, microbiota, and dysbiosis: A ‘recipe’ for colorectal cancer. Food Funct. 2016, 7, 1731–1740. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Zhu, X.; Jiao, J.; Li, Y.; Li, Y.; Zhao, L. Fusobacterium nucleatum aggravates ulcerative colitis through promoting gut microbiota dysbiosis and dysmetabolism. J. Periodontol. 2022, 11. [Google Scholar] [CrossRef]
- Idrissi Janati, A.; Karp, I.; Latulippe, J.F.; Charlebois, P.; Emami, E. Periodontal disease as a risk factor for sporadic colorectal cancer: Results from COLDENT study. Cancer Causes Control 2022, 33, 463–472. [Google Scholar] [CrossRef]
- Rizzoli, R. Microbiota and Bone Health: The Gut-Musculoskeletal Axis. Calcif. Tissue Int. 2018, 102, 385–386. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [PubMed]
- Graziani, C.; Talocco, C.; de Sire, R.; Petito, V.; Lopetuso, L.R.; Gervasoni, J.; Persichilli, S.; Franceschi, F.; Ojetti, V.; Gasbarrini, A.; et al. Intestinal permeability in physiological and pathological conditions: Major determinants and assessment modalities. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Stabler, T.; Pei, F.X.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef]
- Biver, E.; Berenbaum, F.; Valdes, A.M.; de Carvalho, I.A.; Bindels, L.B.; Brandi, M.L.; Calder, P.C.; Castronovo, V.; Cavalier, E.; Cherubini, A.; et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res. Rev. 2019, 55, 100946. [Google Scholar] [CrossRef]
- Korotkyi, O.H.; Vovk, A.A.; Dranitsina, A.S.; Falalyeyeva, T.M.; Dvorshchenko, K.O.; Fagoonee, S.; Ostapchenko, L.I. The influence of probiotic diet and chondroitin sulfate administration on Ptgs2, Tgfb1 and Col2a1 expression in rat knee cartilage during monoiodoacetate-induced osteoarthritis. Minerva Med. 2019, 110, 419–424. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; de Sire, A.; Migliore, A.; Brandi, M.L.; Piscitelli, P. Early osteoarthritis: How to define, diagnose, and manage. A systematic review. Eur. Geriatr. Med. 2017, 8, 383–396. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin Shi Gai, Z.; Heng, X.; Zhang, C.; Yang, J.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 8, 17126. [Google Scholar] [CrossRef]
- Malfait, A.M. Osteoarthritis year in review 2015: Biology. Osteoarthr. Cartil. 2016, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Dieguez, D.; Miller, L.M.; Young, H.A. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F. Role of Intestinal Microbiota on Gut Homeostasis and Rheumatoid Arthritis. J. Immunol. Res. 2021, 2021, 8167283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Kotschenreuther, K.; Yan, S.; Kofler, D.M. Migration and homeostasis of regulatory T cells in rheumatoid arthritis. Front. Immunol. 2022, 13, 947636. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol alleviates temporomandibular joint inflammatory pain by recovering disturbed gut microbiota. Brain Behav. Immun. 2020, 87, 455–464. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; d’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022, 23, 1–17. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Fortunato, F.; Bindi, M.; Pezzotti, F.; Ammendolia, A.; Giudice, A.; Foglio Bonda, P.L.; de Sire, A. Temporomandibular disorders and neck pain in primary headache patients: A retrospective machine learning study. Acta Odontol. Scand. 2023, 81, 151–157. [Google Scholar] [CrossRef]
- Ferrillo, M.; Giudice, A.; Marotta, N.; Fortunato, F.; Di Venere, D.; Ammendolia, A.; Fiore, P.; de Sire, A. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 12164. [Google Scholar] [CrossRef] [PubMed]
- Moen, K.; Bertelsen, L.T.; Hellem, S.; Jonsson, R.; Brun, J.G. Salivary gland and temporomandibular joint involvement in rheumatoid arthritis: Relation to disease activity. Oral Dis. 2005, 11, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yildizer Keris, E.; Yaman, S.D.; Demirag, M.D.; Haznedaroglu, S. Temporomandibular joint findings in patients with rheumatoid arthritis, ankylosing spondylitis, and primary Sjogren’s syndrome. J. Investig. Clin. Dent. 2016, 8, e12255. [Google Scholar] [CrossRef]
- Shim, J.; Kim, C.; Ryu, J.; Choi, S. Correlation between TM joint disease and rheumatic diseases detected on bone scintigraphy and clinical factors. Sci. Rep. 2020, 10, 4547. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.L.; van de Westerlo, E.M.; Jechorek, R.P.; Feltis, B.A.; Wilkins, T.D.; Erlandsen, S.L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology 1996, 110, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1469–1476. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef]
- Lopez-Oliva, I.; Paropkari, A.D.; Saraswat, S.; Serban, S.; Yonel, Z.; Sharma, P.; de Pablo, P.; Raza, K.; Filer, A.; Chapple, I.; et al. Dysbiotic subgingival microbial communities in periodontally healthy patients with rheumatoid arthritis. Arthritis Rheum. 2018, 70, 1008–1013. [Google Scholar] [CrossRef]
- Arleevskaya, M.I.; Boulygina, E.A.; Larionova, R.; Validov, S.; Kravtsova, O.; Shagimardanova, E.I.; Velo, L.; Hery-Arnaud, G.; Carlé, C.; Renaudineau, Y. Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 12599. [Google Scholar] [CrossRef]
- Quirke, A.M.; Luglim, E.B.; Wegnerm, N.; Hamilton, B.C.; Charles, P.; Chowdhury, M.; Ytterberg, A.J.; Zubarev, R.A.; Potempa, J.; Culshaw, S.; et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: A potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Sherina, N.; Kharlamova, N.; Potempa, B.; Larsson, B.; Israelsson, L.; Potempa, J.; Rantapää-Dahlqvist, S.; Lundberg, K. Concentration of antibodies against Porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Guo, Q. Probiotic Species in the Management of Periodontal Diseases: An Overview. Front. Cell Infect. Microbiol. 2022, 12, 806463. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Morelli, L.; Reid, G.; Sanders, M.; Stanton, C. Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 18 December 2021).
- Youssef, M.; Ahmed, H.Y.; Zongo, A.; Korin, A.; Zhan, F.; Hady, E.; Umair, M.; Shahid Riaz Rajoka, M.; Xiong, Y.; Li, B. Probiotic Supplements: Their Strategies in the Therapeutic and Prophylactic of Human Life-Threatening Diseases. Int. J. Mol. Sci. 2021, 22, 11290. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Araujo, L.D.C.; Furlaneto, F.A.C.; da Silva, L.A.B.; Kapila, Y.L. Use of the Probiotic Bifidobacterium animalis subsp. Lactis HN019 in Oral Diseases. Int. J. Mol. Sci. 2022, 23, 9334. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Mahasneh, S.A.; Mahasneh, A.M. Probiotics: A Promising Role in Dental Health. Dent. J. 2017, 5, 26. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Review of the Role of Probiotic Supplementation in Dental Caries. Probiotics Antimicrob. Proteins 2020, 12, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Shin, I.S.; Jeon, J.G.; Yang, Y.M.; Kim, J.G.; Lee, D.W. The Effect of Probiotics on Halitosis: A Systematic Review and Meta-Analysis. Probiotics Antimicrob. Proteins 2019, 11, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Huang, J.; Tao, R. Effect of probiotics on gingival inflammation and oral microbiota: A meta-analysis. Oral Dis. 2022, 28, 1058–1067. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Flores-Rodríguez, M.; Omaña-Covarrubias, A.; Nicastro, M.; Lazarescu, F.; Zarow, M.; Monteiro, P.; Jakubowicz, N.; et al. The Use of Probiotics as Adjuvant Therapy of Periodontal Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceutics 2022, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan, I.S.; Cakar, G.; Kadir, T.; Yılmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Henrique Soares, K.; Firoozi, P.; Maria de Souza, G.; Beatriz Lopes Martins, O.; Gabriel Moreira Falci, S.; Rocha Dos Santos, C.R. Efficacy of Probiotics Compared to Chlorhexidine Mouthwash in Improving Periodontal Status: A Systematic Review and Meta-Analysis. Int. J. Dent. 2023, 2023, 4013004. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef]
- Invernici, M.M.; Furlaneto, F.A.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis Subsp Lactis HN019 Presents Antimicrobial Potential against Periodontopathogens and Modulates the Immunological Response of Oral Mucosa in Periodontitis Patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Deng, Y.; He, Q.; Yang, K.; Li, J.; Xiang, W.; Liu, H.; Zhu, X.; Chen, H. Safety and efficacy of probiotic supplementation in 8 types of inflammatory arthritis: A systematic review and meta-analysis of 34 randomized controlled trials. Front. Immunol. 2022, 13, 961325. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Joyce Wu, H.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Homayouni-Rad, A.; Alipour, B.; Sharif, S.-K.; Vaghef-Mehrabany, L.; Alipour-Ajiry, S. Effects of probiotics supplementation on oxidative stress indices in women with rheumatoid arthritis: A randomized double-blind clinical trial. J. Am. Coll. Nutr. 2016, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Khalil, A.M.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 6, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; VaghefMehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef]
- Cannarella, L.A.T.; Mari, N.L.; Alcantara, C.C.; Iryioda, T.M.V.; Costa, N.T.; Oliveira, S.R.; Lozovoy, M.A.B.; Reiche, E.M.V.; Dichi, I.; Simão, A.N.C. Mixture of probiotics reduces inflammatory biomarkers and improves the oxidative/nitrosative profile in people with rheumatoid arthritis. Nutrition 2021, 89, 111282. [Google Scholar] [CrossRef]
- Hatakka, K.; Martio, J.; Korpela, M.; Herranen, M.; Poussa, T.; Laasanen, T.; Saxelin, M.; Vapaatalo, H.; Moilanen, E.; Korpela, R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis—A pilot study. Scand. J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef]
- Pineda, M.L.A.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2011, 17, CR347–CR354. [Google Scholar] [CrossRef]
- Jenks, K.; Stebbings, S.; Burton, J.; Schultz, M.; Herbison, P.; Highton, J. Probiotic therapy for the treatment of spondyloarthritis: A randomized controlled trial. J. Rheumatol. 2010, 37, 2118–2125. [Google Scholar] [CrossRef]
- Brophy, S.; Burrows, C.L.; Brooks, C.; Gravenor, M.B.; Siebert, S.; Allen, S.J. Internet-Based Randomised Controlled Trials for the Evaluation of Complementary and Alternative Medicines: Probiotics in Spondyloarthropathy. BMC Musculoskelet. Disord. 2008, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Probiotics and prebiotics in clinical tests: An update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.Y.; Choi, H.J.; Kim, M.G.; Jeong, D.G.; Lee, D.G.; Yoon, J.M.; Kang, D.J.; Park, S.; Ji, J.G.; Joo, I.H.; et al. Effects of ID-CBT5101 in Preventing and Alleviating Osteoarthritis Symptoms in a Monosodium Iodoacetate-Induced Rat Model. J. Microbiol. Biotechnol. 2018, 28, 1199–1208. [Google Scholar] [CrossRef]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, S.H.; Jhun, J.; Choi, J.; Jung, K.; Cho, K.H.; Kim, S.J.; Yang, C.W.; Park, S.H.; Cho, M.L. The Combination of Probiotic Complex, Rosavin, and Zinc Improves Pain and Cartilage Destruction in an Osteoarthritis Rat Model. J. Med. Food 2018, 21, 364–371. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.Y.; Jhun, J.Y.; Jung, K.; Park, S.H.; Yang, C.W.; Cho, Y.; Kim, S.J.; Cho, M.L. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol. Lett. 2018, 203, 6–14. [Google Scholar] [CrossRef]
- Jhun, J.; Cho, K.H.; Lee, D.H.; Kwon, J.Y.; Woo, J.S.; Kim, J.; Na, H.S.; Park, S.H.; Kim, S.J.; Cho, M.L. Oral Administration of Lactobacillus rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef]
- Sophocleous, A.; Azfer, A.; Huesa, C.; Stylianou, E.; Ralston, S.H. Probiotics Inhibit Cartilage Damage and Progression of Osteoarthritis in Mice. Calcif. Tissue Int. 2023, 112, 66–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).