Isocorydine Ameliorates IL-6 Expression in Bone Marrow-Derived Macrophages and Acute Lung Injury Induced by Lipopolysaccharide
Abstract
1. Introduction
2. Results
2.1. ICD Alleviates LPS-Induced Acute Lung Injury in Mice
2.2. ICD Inhibits IL-6 Expression in LPS-Induced Macrophage Activation and Acute Lung Injury Model
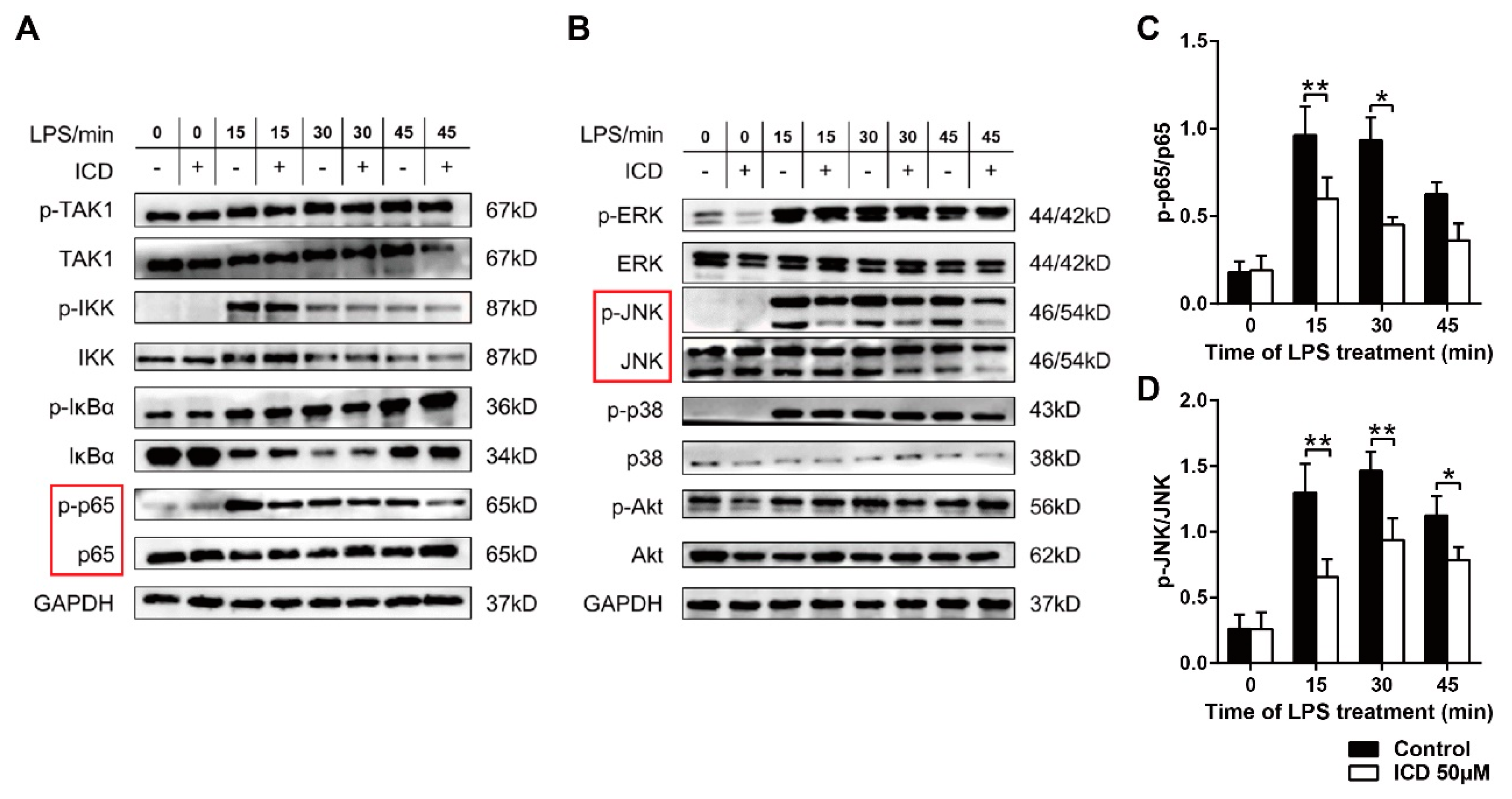
2.3. ICD Suppresses Inflammatory Pathways in LPS-Activated Macrophages
2.4. ICD Reduces the MAPK and NF-κB Pathways Activated by LPS in BMDMs
3. Discussion
4. Materials and Methods
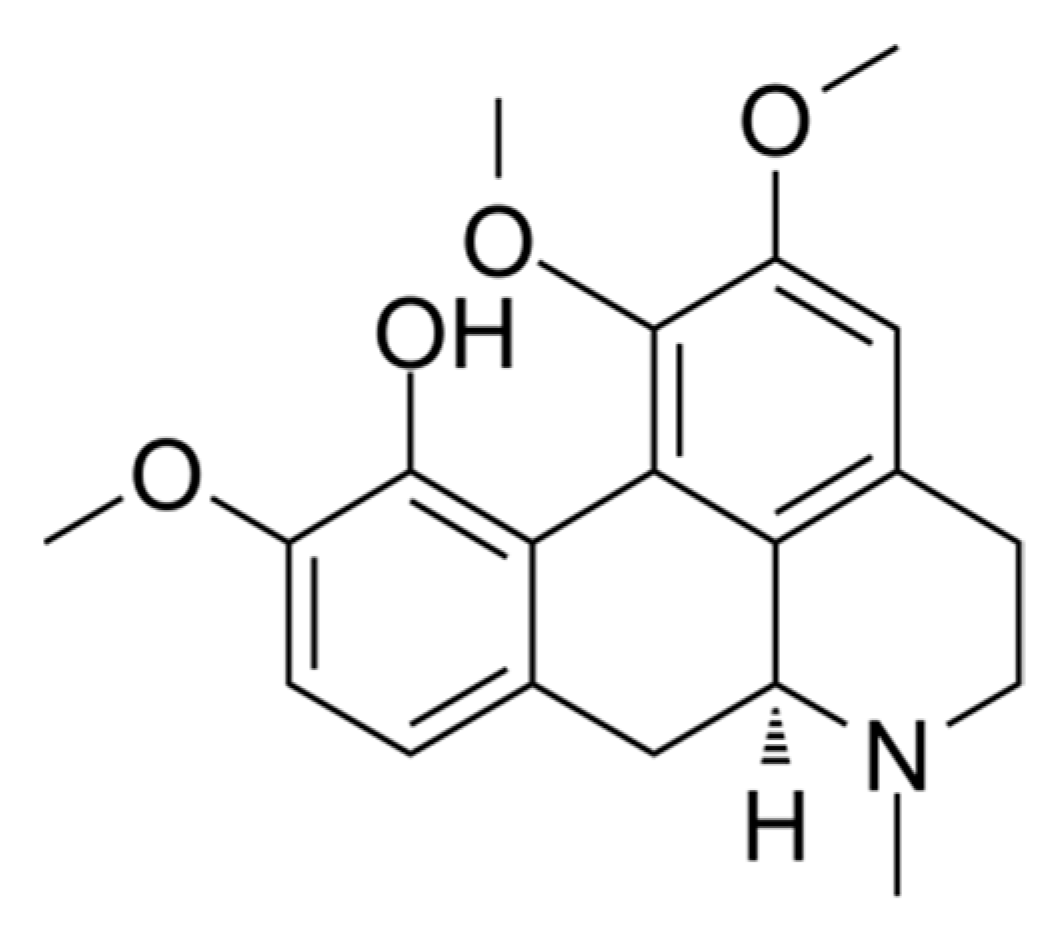
4.1. Isocorydine
4.2. Animals
4.3. Cell Preparation and Culture
4.4. Drug Preparation
4.5. Cell Viability Assay
4.6. Cell Apoptosis Assay
4.7. Establishment of Mouse Acute Lung Injury Model
4.8. H&E Staining of Lung Tissues
4.9. Lung Wet/Dry Ratios
4.10. Real-Time Quantitative Polymerase Chain Reaction
- Gapdh-RTF, CTGAGTATGTCGTGGAGTCT;
- Gapdh-RTR, GTGGATGCAGGGATGATGTT;
- Il6-RTF, AGTTGCCTTCTTGGGACTGA;
- Il6-RTR, TCCACGATTTCCCAGAGAAC;
- Il1a-RTF, GTTCTGCCATTGACCATCTC;
- Il1a-RTR, CAGAATCTTCCCGTTGCTTG;
- Ccl2-RTF, GTGTCCCAAAGAAGCTGTAG;
- Ccl2-RTR, CACATTCAAAGGTGCTGAAGA;
- Ptgs2-RTF, GCGACATACTCAAGCAGGAGCA;
- Ptgs2-RTR, AGTGGTAACCGCTCAGGTGTTG.
4.11. Enzyme-Linked Immunosorbent Assay
4.12. RNA-Seq and Bioinformatics Analysis
4.13. Western Blot
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Nabavi, S.M.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R. Arglabin could target inflammasome-induced ARDS and cytokine storm associated with COVID-19. Mol. Biol. Rep. 2021, 48, 8221–8225. [Google Scholar] [CrossRef] [PubMed]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug. Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Fan, E.K.Y.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018, 19, 50. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, Q.; Zhong, M.; Zhao, Q.; Liu, J.; Di, D.; Liu, J. Study on pharmacokinetics and tissue distribution of the isocorydine derivative (AICD) in rats by HPLC-DAD method. Acta Pharm. Sin. B 2015, 5, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hou, H.; Lu, P.; Zhang, L.; Zhao, F.; Ge, C.; Wang, T.; Yao, M.; Li, J. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/m cell cycle arrest and apoptosis. PLoS ONE 2012, 7, e36808. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, Y.; Liu, J.; Di, D.; Xu, M.; Yang, Y.; Li, W.; Chen, Y.; Liu, J. Isocorydine derivatives and their anticancer activities. Molecules 2014, 19, 12099–12115. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, W.; Liu, B.; Zhou, L.; Deng, L.; Niu, W.; Bao, D.; Cheng, C.; Li, D.; Liu, S.; et al. MiR-200c Inhibits the Tumor Progression of Glioma via Targeting Moesin. Theranostics 2017, 7, 1663–1673. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Chepurnova, D.A.; Samoilova, E.V.; Anisimov, A.A.; Verin, A.D.; Korotaeva, A.A. Compounds of IL-6 Receptor Complex during Acute Lung Injury. Bull. Exp. Biol. Med. 2018, 164, 609–611. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar] [CrossRef]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) inhibitors: Future therapeutic strategies for epilepsy management. J. Neuroinflamm. 2019, 16, 197. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, D.J.; Batistatou, A.; Sykiotis, G.P.; Varakis, I.; Papavassiliou, A.G. Activation of the JNK-AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone 2003, 32, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Raghavendran, K.; Napolitano, L.M. Definition of ALI/ARDS. Crit. Care Clin. 2011, 27, 429–437. [Google Scholar] [CrossRef]
- Luo, J.; Wang, N.; Hua, L.; Deng, F.; Liu, D.; Zhou, J.; Yuan, Y.; Ouyang, F.; Chen, X.; Long, S.; et al. The Anti-Sepsis Effect of Isocorydine Screened from Guizhou Ethnic Medicine is Closely Related to Upregulation of Vitamin D Receptor Expression and Inhibition of NFκB p65 Translocation into the Nucleus. J. Inflamm. Res. 2022, 15, 5649–5664. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Ko, J.H.; Yoon, S.O.; Lee, H.J.; Oh, J.Y. Rapamycin regulates macrophage activation by inhibiting NLRP3 inflammasome-p38 MAPK-NFκB pathways in autophagy- and p62-dependent manners. Oncotarget 2017, 8, 40817–40831. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, Z.; Zhao, H.; Luo, Y. MAPK: A Key Player in the Development and Progression of Stroke. CNS Neurol. Disord. Drug Targets 2020, 19, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Zhou, L.; Xue, Z.; Zhou, L.; Luo, Y.; Lin, F.; Liu, X.; Hong, S.; Li, W.; Wang, D.; et al. Cxxc Finger Protein 1 Positively Regulates GM-CSF-Derived Macrophage Phagocytosis Through Csf2rα-Mediated Signaling. Front. Immunol. 2018, 9, 1885. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Winn, R.K.; Jonas, M.; Chi, E.Y.; Martin, T.R.; Liles, W.C. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: Implications for acute pulmonary inflammation. Am. J. Pathol. 2001, 158, 153–161. [Google Scholar] [CrossRef]
- Smith, K.M.; Mrozek, J.D.; Simonton, S.C.; Bing, D.R.; Meyers, P.A.; Connett, J.E.; Mammel, M.C. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: Gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit. Care Med. 1997, 25, 1888–1897. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.; Li, X.; Fu, Y.; Chen, Y.; Fang, H.; Li, Y.; Gu, Y.; Zhang, J. Isocorydine Ameliorates IL-6 Expression in Bone Marrow-Derived Macrophages and Acute Lung Injury Induced by Lipopolysaccharide. Int. J. Mol. Sci. 2023, 24, 4629. https://doi.org/10.3390/ijms24054629
Tu Y, Li X, Fu Y, Chen Y, Fang H, Li Y, Gu Y, Zhang J. Isocorydine Ameliorates IL-6 Expression in Bone Marrow-Derived Macrophages and Acute Lung Injury Induced by Lipopolysaccharide. International Journal of Molecular Sciences. 2023; 24(5):4629. https://doi.org/10.3390/ijms24054629
Chicago/Turabian StyleTu, Yifan, Xiaodong Li, Yuanzheng Fu, Yunyun Chen, Hui Fang, Yuan Li, Ying Gu, and Jiawei Zhang. 2023. "Isocorydine Ameliorates IL-6 Expression in Bone Marrow-Derived Macrophages and Acute Lung Injury Induced by Lipopolysaccharide" International Journal of Molecular Sciences 24, no. 5: 4629. https://doi.org/10.3390/ijms24054629
APA StyleTu, Y., Li, X., Fu, Y., Chen, Y., Fang, H., Li, Y., Gu, Y., & Zhang, J. (2023). Isocorydine Ameliorates IL-6 Expression in Bone Marrow-Derived Macrophages and Acute Lung Injury Induced by Lipopolysaccharide. International Journal of Molecular Sciences, 24(5), 4629. https://doi.org/10.3390/ijms24054629






