Coarse-Grained Molecular Dynamics of pH-Sensitive Lipids
Abstract
:1. Introduction
2. Results and Discussion
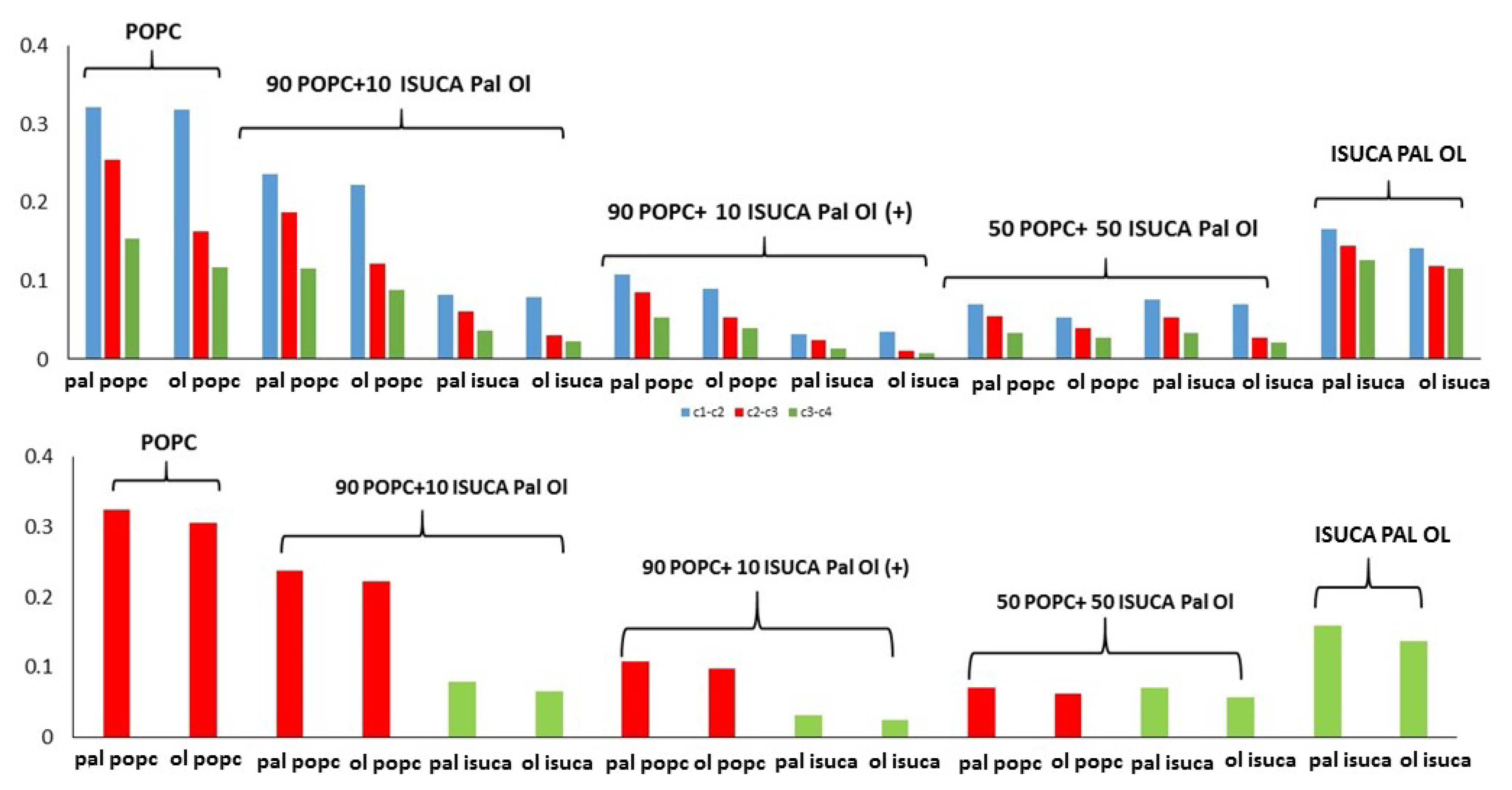
2.1. Structural Properties: Equlilibrium Area per Lipid, Hydrophobic Thickness and Second-Rank Order Parameter
2.2. Dynamic Properties: Diffusion Coefficient
3. Materials and Methods
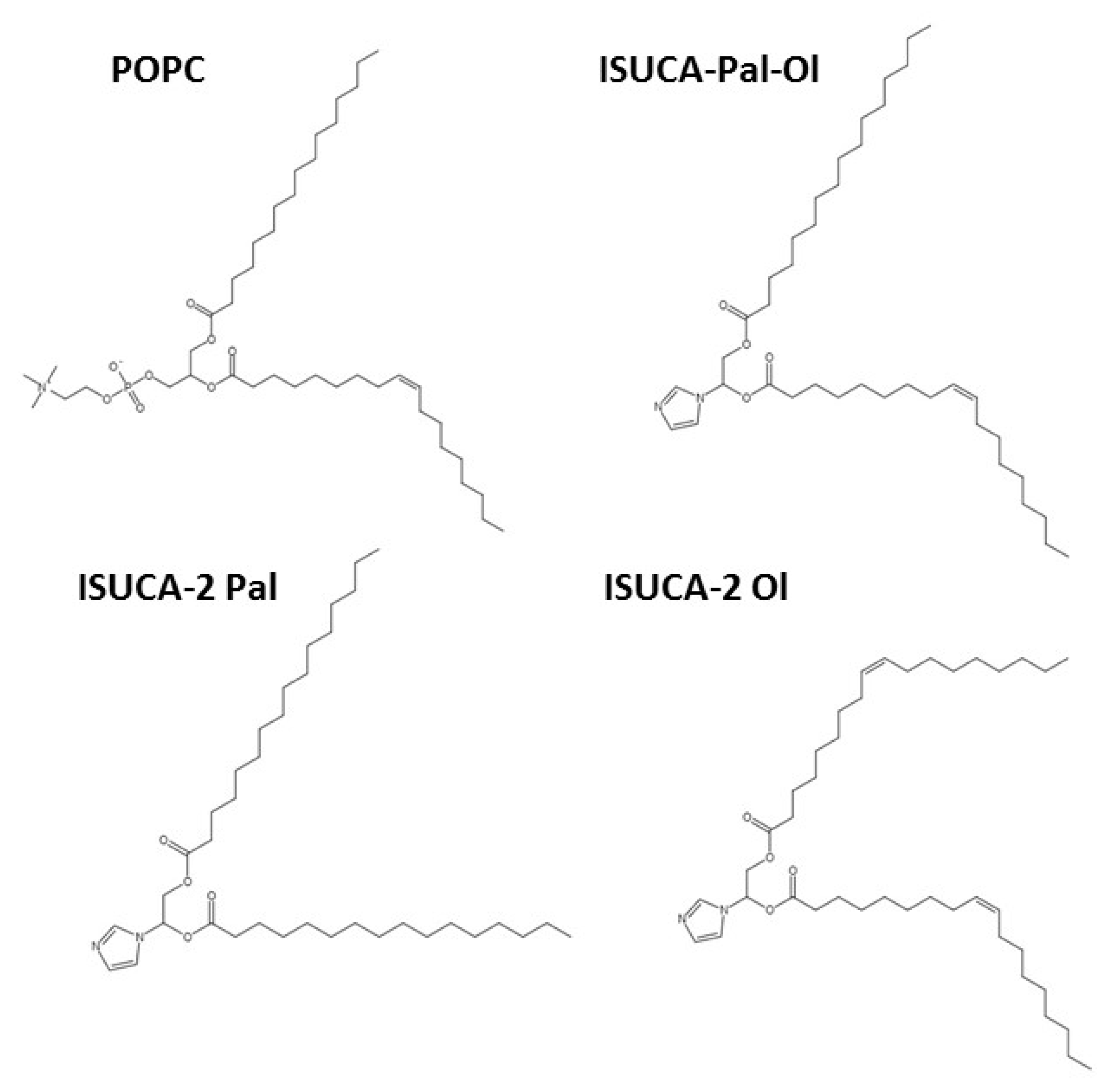
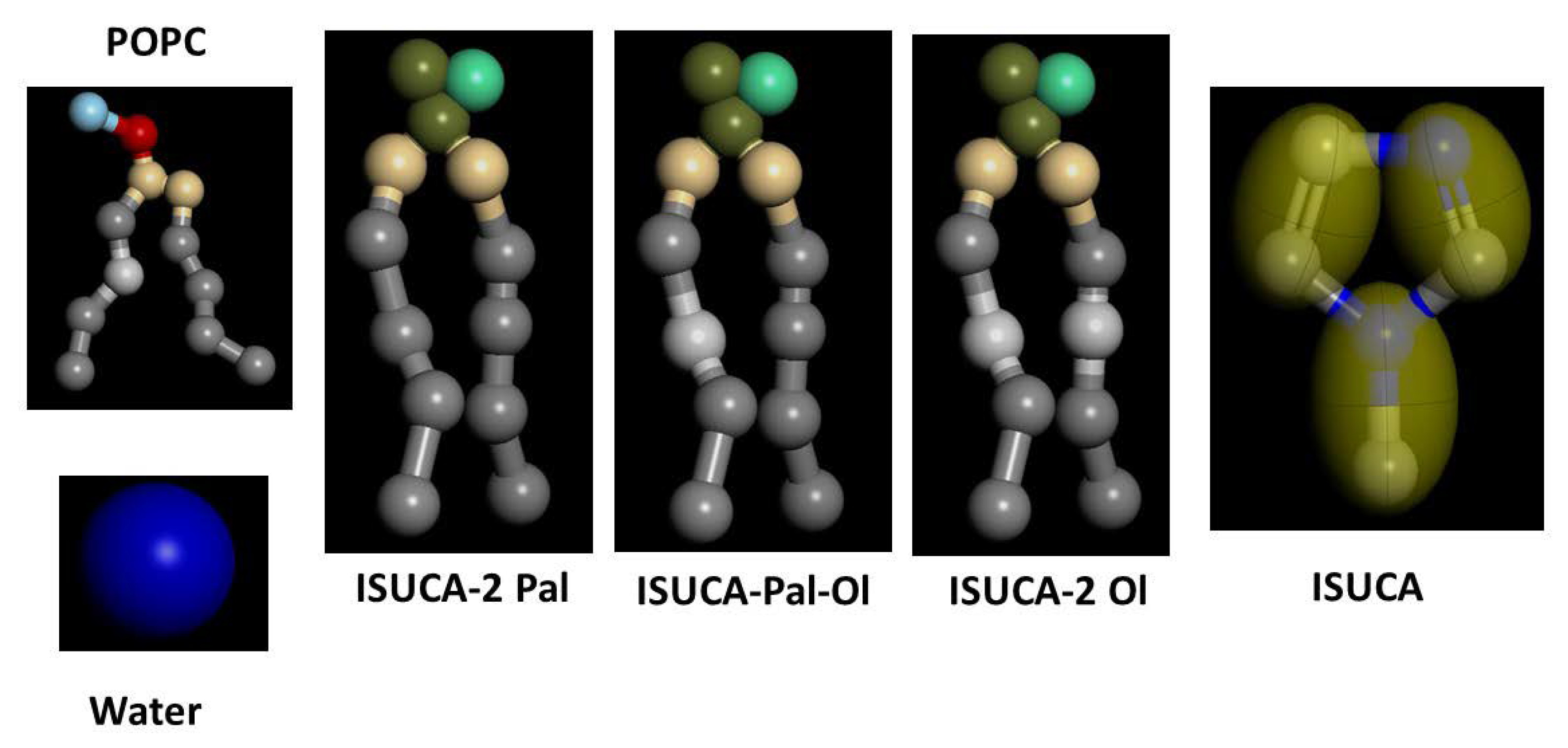
3.1. Models
3.2. Calculation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drummond, D.C.; Zignani, M.; Leroux, J.-C. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000, 39, 409–460. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Vadlamudi, M.; Huang, Y.; Guo, X. Lipid-Coated, pH-Sensitive Magnesium Phosphate Particles for Intracellular Protein Delivery. Pharm. Res. 2019, 36, 81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Samoshina, N.M.; Samoshin, V.V.; Franz, A.H.; Guo, X. Fliposomes: Trans-2-aminocyclohexanol-based amphiphiles as pH-sensitive conformational switches of liposome membrane—A structure-activity relationship study. Chem. Phys. Lipids 2017, 210, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Park, J.-Y.; Wright, A.M.; Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): Effects of the lipid composition (lysolipid and DSPE–PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss. 2013, 161, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar] [CrossRef]
- Chi, Y.; Yin, X.; Sun, K.; Feng, S.; Liu, J.; Chen, D.; Guo, C.; Wu, Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 2017, 261, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Zhou, Z.; Wang, K.; Li, C.; Qiao, H.; Oupicky, D.; Sun, M. Near-infrared light-triggered drug release from a multiple lipid carrier complex using an all-in-one strategy. J. Control. Release 2017, 261, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R. Causes and consequences of acidic pH in tumors: A magnetic resonance study. Adv. Enzym. Regul. 1999, 39, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Löbenberg, R. Liposomal Drug Delivery: A Versatile Platform for Challenging Clinical Applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules 2016, 21, 1284. [Google Scholar] [CrossRef] [Green Version]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Oya, T.A.; Ishiyama, A.A.; Nishikawa, N.A. Imidazole Compound and Liposome Containing Same. European Patent EP 3170812A1, 2017. Available online: https://data.epo.org/publication-server/document?iDocId=5335445&iFormat=0 (accessed on 16 October 2022).
- Huang, R.; Gyanani, V.; Zhao, S.; Lu, Y.; Guo, X. Imidazole-Based pH-Sensitive Convertible Liposomes for Anticancer Drug Delivery. Pharmaceuticals 2022, 15, 306. [Google Scholar] [CrossRef]
- Provent, P.; Benito, M.; Hiba, B.; Farion, R.; López-Larrubia, P.; Ballesteros, P.; Rémy, C.; Segebarth, C.; Cerdán, S.; Coles, J.A.; et al. Serial In vivo Spectroscopic Nuclear Magnetic Resonance Imaging of Lactate and Extracellular pH in Rat Gliomas Shows Redistribution of Protons Away from Sites of Glycolysis. Cancer Res. 2007, 67, 7638–7645. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.Y.; Lee, H. All-atom simulations and free-energy calculations of coiled-coil peptides with lipid bilayers: Binding strength, structural transition, and effect on lipid dynamics. Sci. Rep. 2016, 6, 22299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skjevik, Å.A.; Madej, B.D.; Dickson, C.J.; Lin, C.; Teigen, K.; Walker, R.C.; Gould, I.R. Simulation of lipid bilayer self-assembly using all-atom lipid force fields. Phys. Chem. Chem. Phys. 2016, 18, 10573–10584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakobyan, D.; Heuer, A. Comparing an All-Atom and a Coarse-Grained Description of Lipid Bilayers in Terms of Enthalpies and Entropies: From MD Simulations to 2D Lattice Models. J. Chem. Theory Comput. 2019, 15, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-K.; Yu, Y.; Klauda, J.B. All-Atom Modeling of Complex Cellular Membranes. Langmuir 2021, 38, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Molecular Simulations of PEGylated Biomolecules, Liposomes, and Nanoparticles for Drug Delivery Applications. Pharmaceutics 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Lemaalem, M.; Hadrioui, N.; Derouiche, A.; Ridouane, H. Structure and dynamics of liposomes designed for drug delivery: Coarse-grained molecular dynamics simulations to reveal the role of lipopolymer incorporation. RSC Adv. 2020, 10, 3745–3755. [Google Scholar] [CrossRef]
- Parchekani, J.; Allahverdi, A.; Taghdir, M.; Naderi-Manesh, H. Design and simulation of the liposomal model by using a coarse-grained molecular dynamics approach towards drug delivery goals. Sci. Rep. 2022, 12, 2371. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [Green Version]
- Markvoort, A.J.; Smeijers, A.F.; Pieterse, K.; van Santen, R.A.; Hilbers, P.A.J. Lipid-Based Mechanisms for Vesicle Fission. J. Phys. Chem. B 2007, 111, 5719–5725. [Google Scholar] [CrossRef]
- Shinoda, W.; DeVane, R.; Klein, M.L. Computer simulation studies of self-assembling macromolecules. Curr. Opin. Struct. Biol. 2012, 22, 175–186. [Google Scholar] [CrossRef]
- Van Hoof, B.; Markvoort, A.J.; Van Santen, R.A.; Hilbers, P.A.J. On Protein Crowding and Bilayer Bulging in Spontaneous Vesicle Formation. J. Phys. Chem. B 2012, 116, 12677–12683. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Risselada, H.J.; Marrink, S.J. Curvature effects on lipid packing and dynamics in liposomes revealed by coarse grained molecular dynamics simulations. Phys. Chem. Chem. Phys. 2009, 11, 2056–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünewald, F.; Kroon, P.C.; Souza, P.C.T.; Marrink, S.J. Protocol for Simulations of PEGylated Proteins with Martini 3. Structural Genomics. Methods Mol. Biol. 2021, 2199, 315–335. [Google Scholar] [CrossRef] [PubMed]
- López, C.A.; Rzepiela, A.J.; de Vries, A.H.; Dijkhuizen, L.; Hünenberger, P.H.; Marrink, S.J. Martini Coarse-Grained Force Field: Extension to Carbohydrates. J. Chem. Theory Comput. 2009, 5, 3195–3210. [Google Scholar] [CrossRef]
- Ramadurai, S.; Holt, A.; Schäfer, L.V.; Krasnikov, V.V.; Rijkers, D.T.; Marrink, S.J.; Killian, J.A.; Poolman, B. Influence of Hydrophobic Mismatch and Amino Acid Composition on the Lateral Diffusion of Transmembrane Peptides. Biophys. J. 2010, 99, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Risselada, H.J.; Marrink, S.J. The freezing process of small lipid vesicles at molecular resolution. Soft Matter 2009, 5, 4531–4541. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, Y.G.; Aeffner, S.; Risselada, H.J.; Salditt, T.; Marrink, S.J.; Müller, M.; Knecht, V. Interbilayer repulsion forces between tension-free lipid bilayers from simulation. Soft Matter 2013, 9, 10705–10718. [Google Scholar] [CrossRef] [Green Version]
- Kucerka, N.; Tristram-Nagle, S.; Nagle, J. Structure of Fully Hydrated Fluid Phase Lipid Bilayers with Monounsaturated Chains. J. Membr. Biol. 2006, 208, 193–202. [Google Scholar] [CrossRef]
- Risselada, H.J.; Marrink, S.J. The molecular face of lipid rafts in model membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 17367–17372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, W.F.D.; MacCallum, J.L.; Hinner, M.J.; Marrink, S.J.; Tieleman, D.P. Molecular View of Cholesterol Flip-Flop and Chemical Potential in Different Membrane Environments. J. Am. Chem. Soc. 2009, 131, 12714–12720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlovská, J.; Uhríková, D.; Kučerka, N.; Teixeira, J.; Devínsky, F.; Lacko, I.; Balgavý, P. Influence of N-dodecyl-N,N-dimethylamine N-oxide on the activity of sarcoplasmic reticulum Ca2þ-transporting ATPase reconstituted into diacylphosphatidylcholine vesicles: Effects of bilayer physical parameters. Biophys. Chem. 2006, 119, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kucerka, N.; Gallová, J.; Uhríková, D.; Balgavý, P.; Bulacu, M.; Marrink, S.; Katsaras, J. Areas of Monounsaturated Diacylphosphatidylcholines. Biophys. J. 2009, 97, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Arnarez, C.; Uusitalo, J.J.; Masman, M.F.; Ingólfsson, H.I.; de Jong, D.H.; Melo, M.N.; Periole, X.; de Vries, A.H.; Marrink, S.J. Dry Martini, a Coarse-Grained Force Field for Lipid Membrane Simulations with Implicit Solvent. J. Chem. Theory Comput. 2015, 11, 260–275. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Deserno, M. Systematic implicit solvent coarse-graining of bilayer membranes: Lipid and phase transferability of the force field. New J. Phys. 2010, 12, 095004. [Google Scholar] [CrossRef]
- Lindblom, G.; Orädd, G. Lipid lateral diffusion and membrane heterogeneity. Biochim. Biophys. Acta 2009, 1788, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Fahey, P.F.; Koppel, D.E.; Barak, L.S.; Wolf, D.E.; Elson, E.L.; Webb, W.W. Lateral Diffusion in Planar Lipid Bilayers. Science 1977, 195, 305–306. [Google Scholar] [CrossRef]
- Lindblom, G.; Orädd, G. NMR Studies of translational diffusion in lyotropic liquid crystals and lipid membranes. Prog. Nucl. Magn. Reson. Spectrosc. 1994, 26, 483–515. [Google Scholar] [CrossRef]
- Apajalahti, T.; Niemelä, P.; Govindan, P.N.; Miettinen, M.S.; Salonen, E.; Marrink, S.-J.; Vattulainen, I. Concerted diffusion of lipids in raft-like membranes. Faraday Discuss. 2010, 144, 411–430. [Google Scholar] [CrossRef]
- Available online: https://www.3ds.com/fileadmin/PRODUCTS-SERVICES/BIOVIA/PDF/BIOVIA-Material-Studio-mesocite.pdf (accessed on 19 January 2023).
- Materials Studio Materials Modeling & Simulation Application|Dassault Systèmes BIOVIA. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio/ (accessed on 19 January 2023).
- Marrink, S.J.; de Vries, A.H.; Mark, A.E. Coarse Grained Model for Semiquantitative Lipid Simulations. J. Phys. Chem. B 2004, 108, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. Constant Temperature Molecular Dynamics Methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef] [Green Version]
- Scholarly Community Encyclopedia. Available online: https://encyclopedia.pub/entry/33716 (accessed on 19 January 2023).
- Djurre, H.; de Jong, G.S.; Bennett, W.F.; Arnarez, C.; Wassenaar, T.A.; Lars, V.S.; Periole, X.; Tieleman, D.P.; Marrink, S.J. Improved Parameters for the Martini Coarse-Grained Protein Force Field. J. Chem. Theory Comput. 2013, 9, 687–697. [Google Scholar] [CrossRef]
- Sun, H.; Pengyu, R.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Alessandri, R.; Uusitalo, J.J.; de Vries, A.H.; Havenith, R.W.A.; Marrink, S.J. Bulk Heterojunction Morphologies with Atomistic Resolution from Coarse-Grain Solvent Evaporation Simulations. J. Am. Chem. Soc. 2017, 139, 3697–3705. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Liu, J.; Alessandri, R.; Qiu, X.; Koopmans, M.; Havenith, R.W.A.; Marrink, S.J.; Chiechi, R.C.; Koster, L.J.A.; Hummelen, J.C. Enhancing doping efficiency by improving host-dopant miscibility for fullerene-based n-type thermoelectrics. J. Mater. Chem. A 2017, 5, 21234–21241. [Google Scholar] [CrossRef] [Green Version]
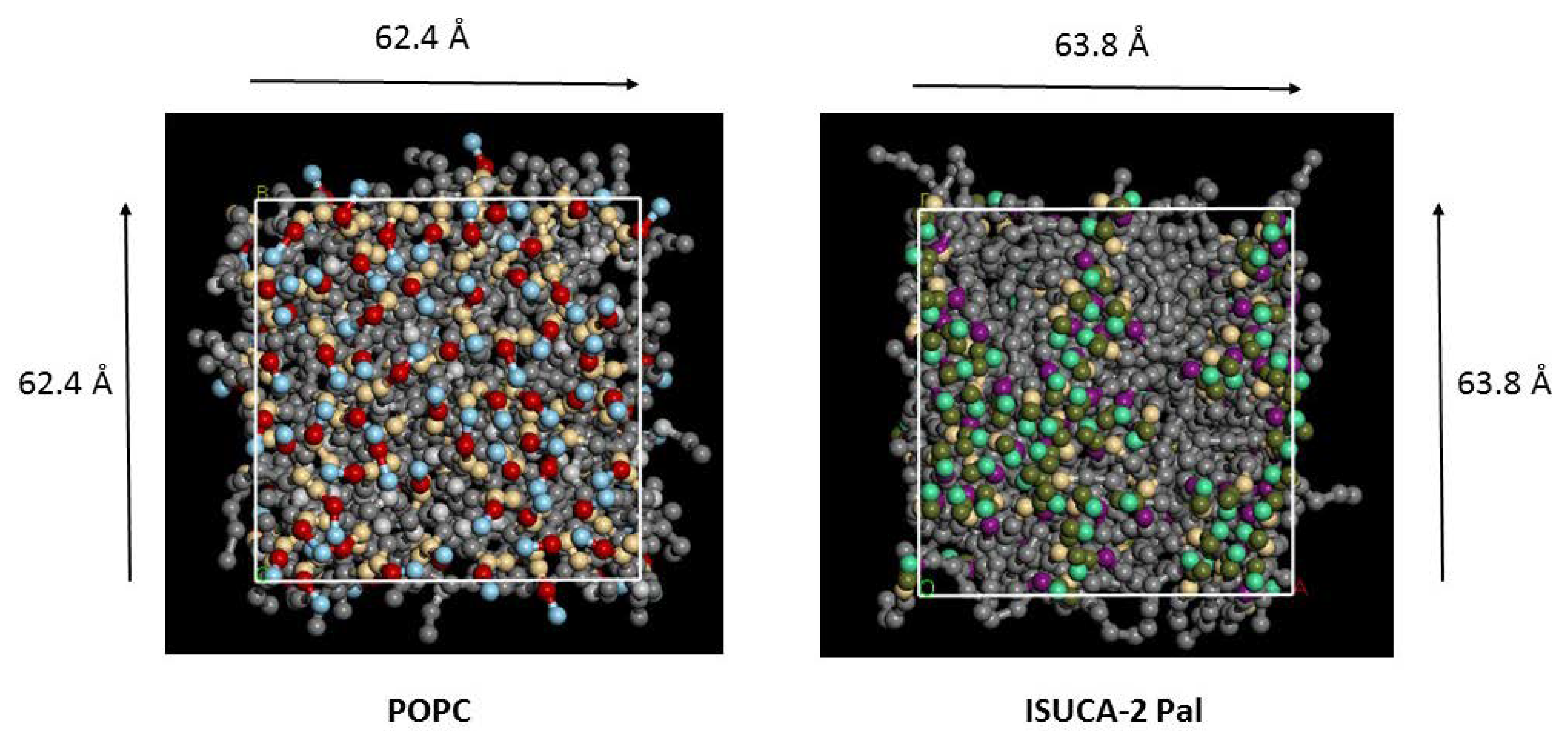
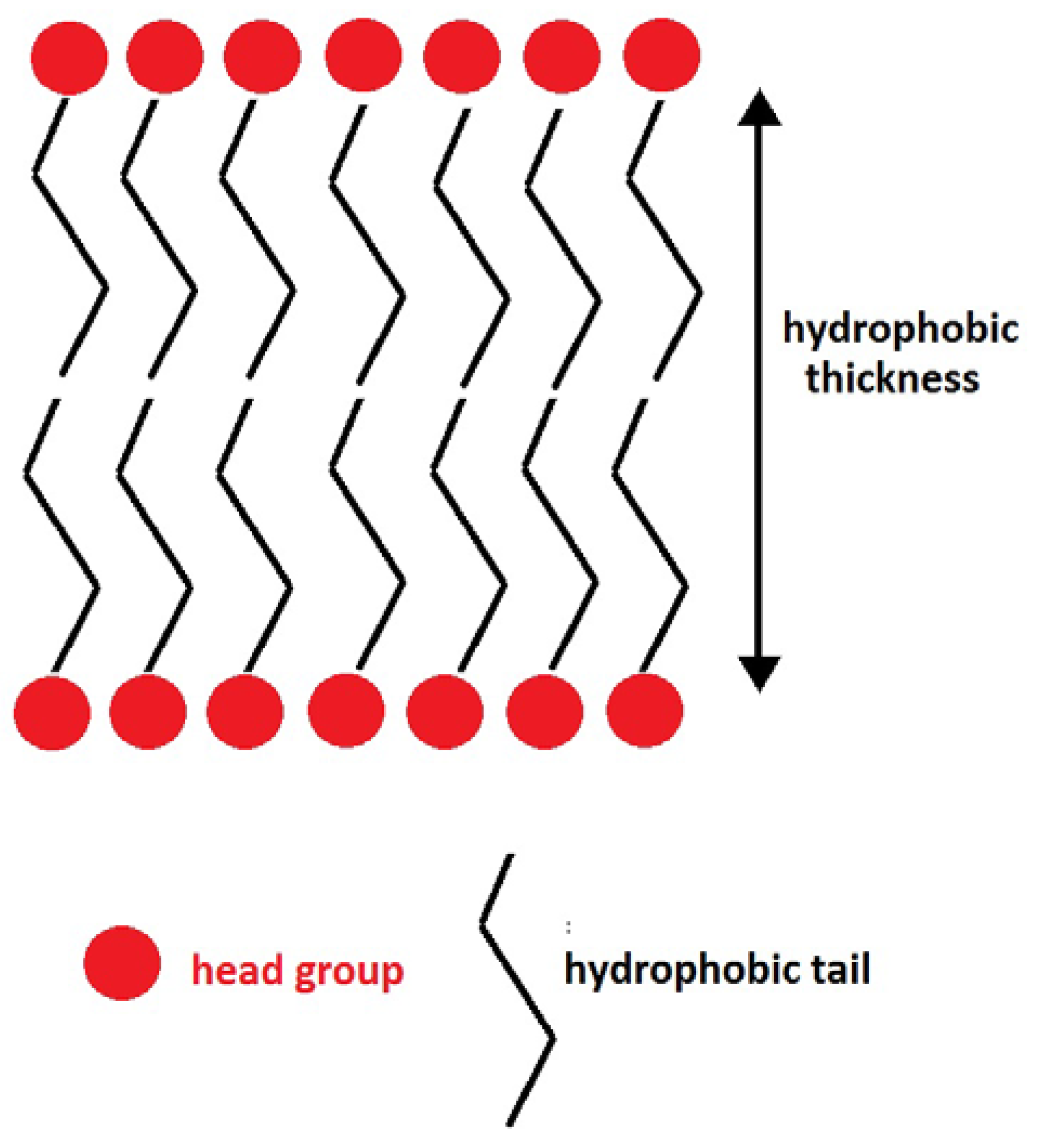
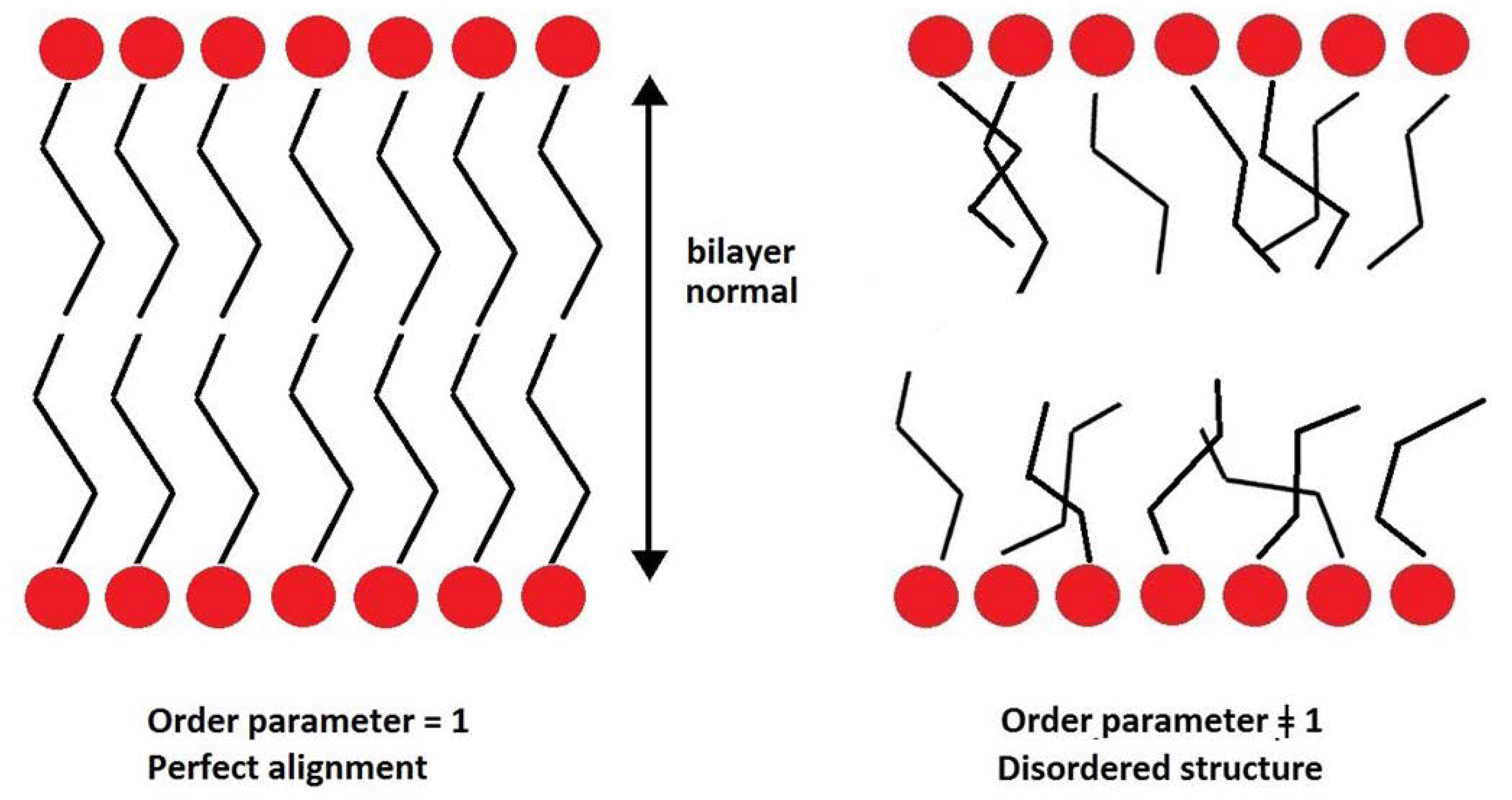
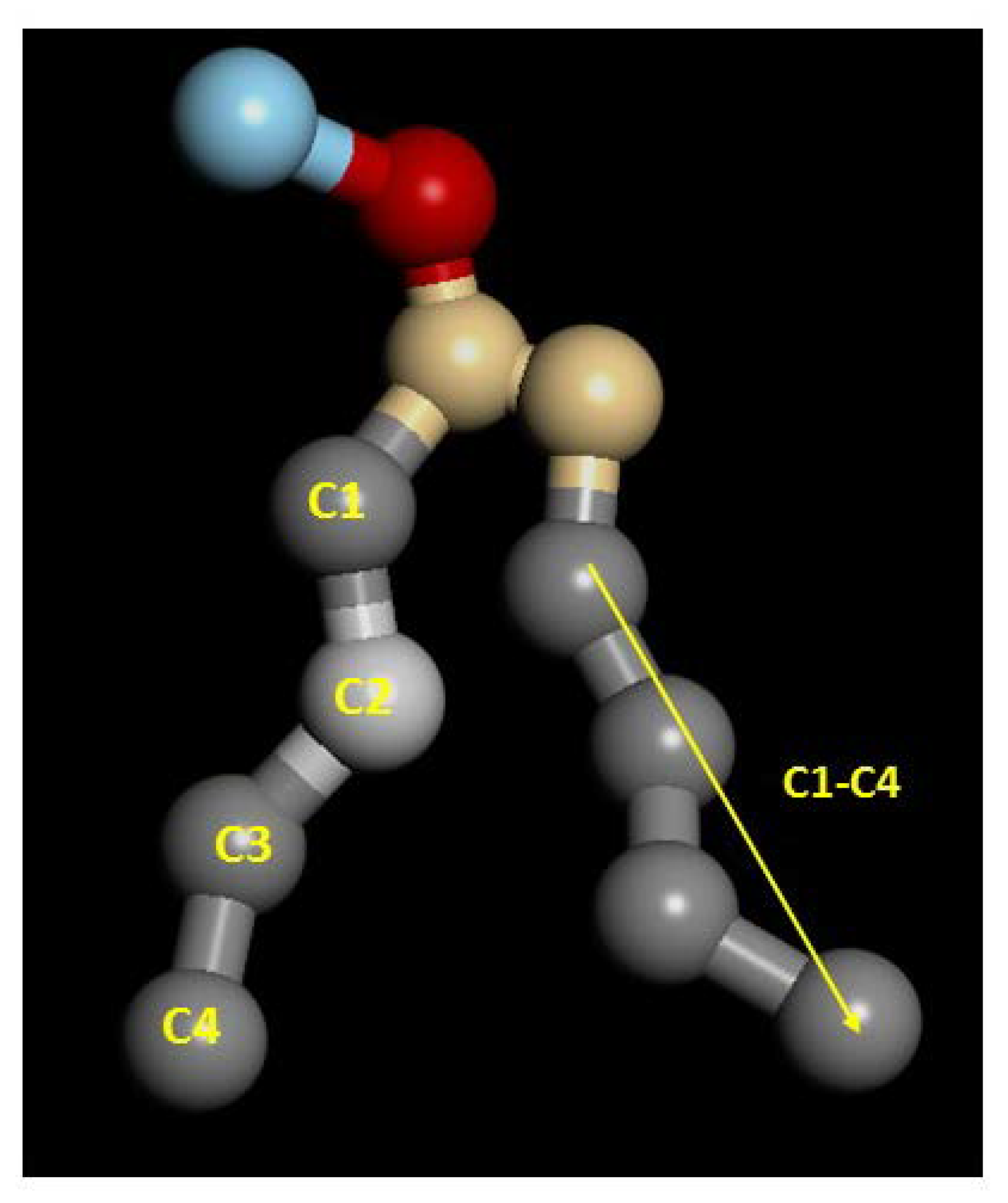
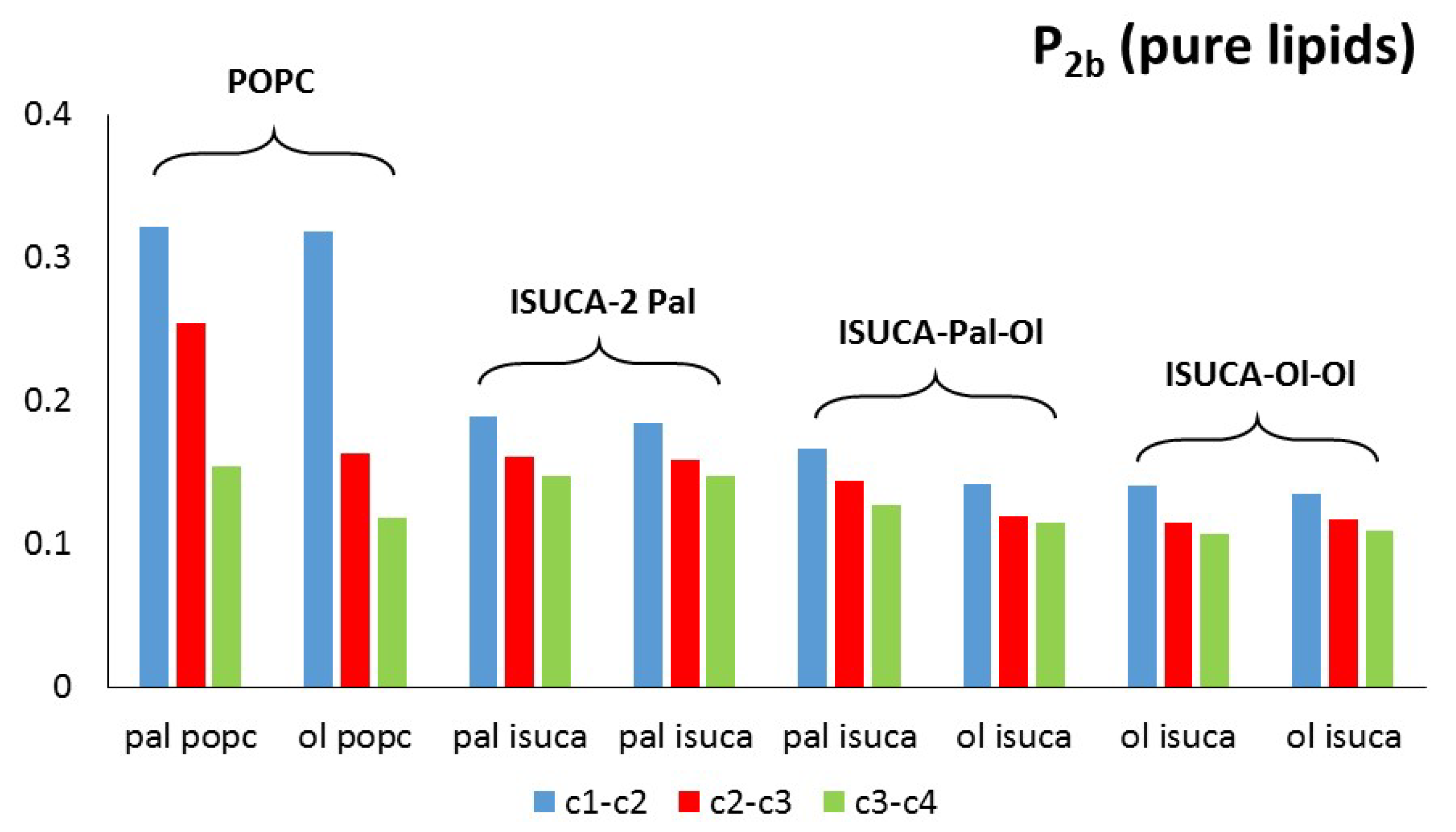

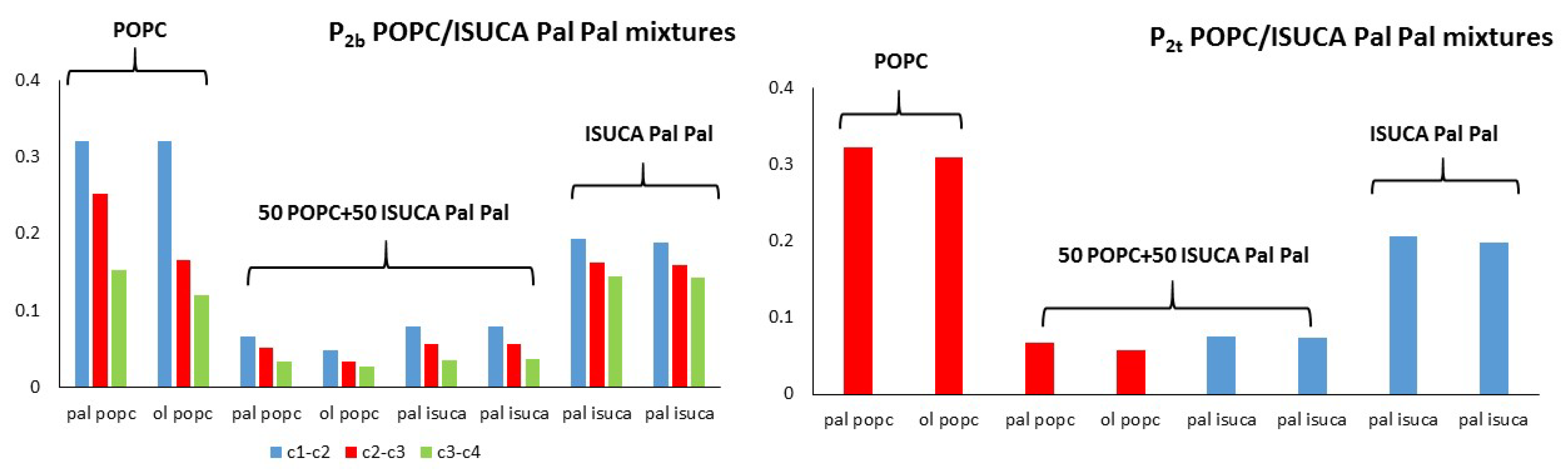
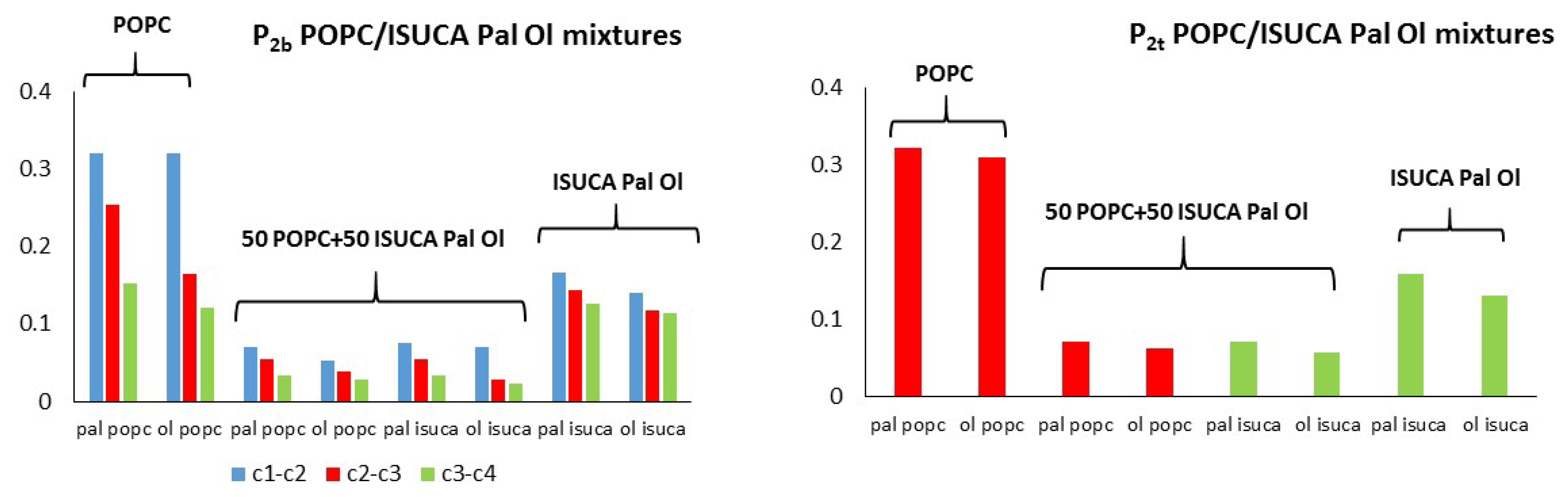
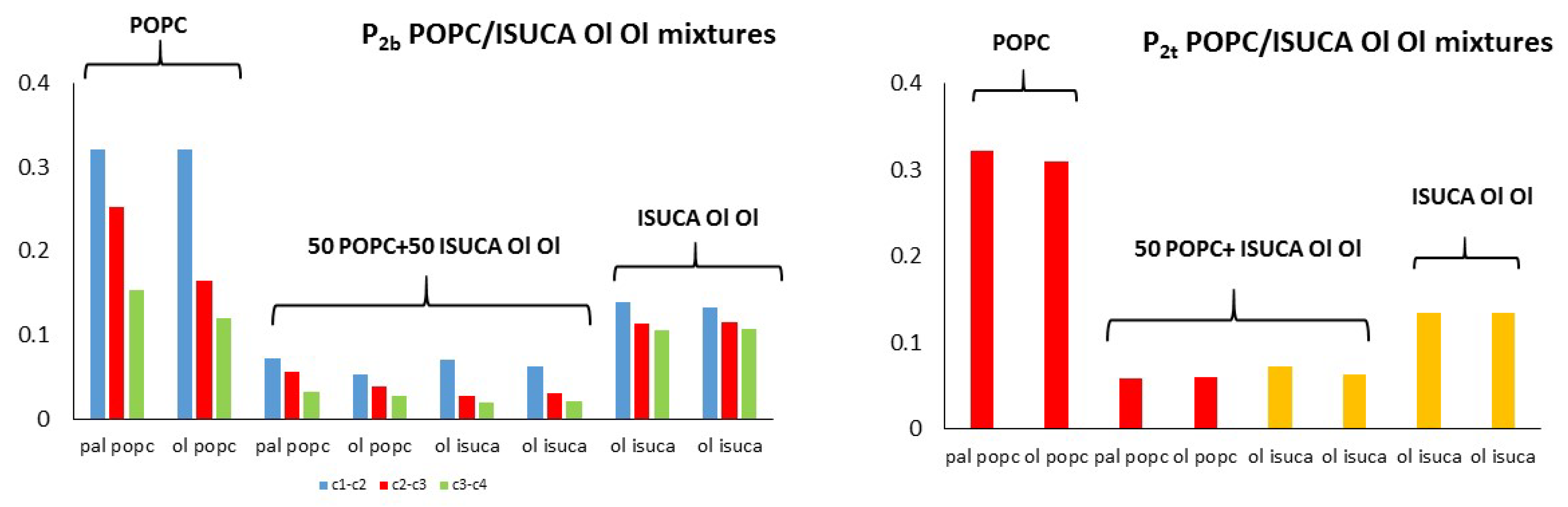
| Model | APL (Å2) | Hydrophobic Thickness (Å) |
|---|---|---|
| POPC | 67.0 ± 0.1 (exp: 62–68) 1 | 14.8 ± 1.2 |
| ISUCA-2 Pal | 70.3 ± 0.2 | 15.7 ± 0.4 |
| ISUCA-2 Ol | 73.1 ± 0.1 | 14.4 ± 0.7 |
| ISUCA-Pal Ol | 71.3 ± 0.1 | 14.5 ± 0.5 |
| 50:50 POPC/ISUCA-2 Pal | 70.3 ± 0.1 | 14.8 ± 0.6 |
| 50:50 POPC/ISUCA-2 Ol | 70.4 ± 0.1 | 11.7 ± 0.4 |
| 50:50 POPC/ISUCA-Pal-Ol | 70.4 ± 0.2 | 12.9 ± 0.8 |
| 90:10 POPC/ISUCA ISUCA-Pal-Ol | 70.4 ± 0.1 | 13.1 ± 0.5 |
| 90:10 POPC/ISUCA+-Pal-Ol (protonated ISUCA) | 70.8 ± 0.1 | 12.5 ± 0.5 |
| Headgroup | Volume (Å3) | |
| POPC | 170 | |
| ISUCA | 158 |
| Model | D (×07 cm2·s−1) |
|---|---|
| POPC | 2.2 (exp.: 1.9) |
| ISUCA-2 Pal | 3.3 |
| ISUCA-2 Ol | 5.7 |
| ISUCA-Pal Ol | 5.6 |
| 50:50 POPC/ISUCA-2 Pal | 3.0 |
| 50:50 POPC/ISUCA-2 Ol | 3.4 |
| 50:50 POPC/ISUCA-Pal-Ol | 3.4 |
| 90:10 POPC/ISUCA ISUCA-Pal-Ol | 2.4 |
| 90:10 POPC/ISUCA+-Pal-Ol | 2.6 |
| (protonated ISUCA) |
| Pure Lipids | Mixtures |
|---|---|
| POPC | 50:50 POPC/ISUCA-2 Pal |
| ISUCA-2 Pal | 50:50 POPC/ISUCA-2 Ol |
| ISUCA-2 Ol | 50:50 POPC/ISUCA-Pal-Ol |
| ISUCA-Pal Ol | 90:10 POPC/ISUCA-Pal-Ol |
| 90:10 POPC/ISUCA+-Pal-Ol (protonated ISUCA) |
| Model | No. of POPC | No. of ISUCA | Model Dimensions (Å3) |
|---|---|---|---|
| POPC | 116 | ||
| ISUCA-2 Pal | 0 | 116 | 63.8 × 63.9 × 90.0 |
| ISUCA-2 Ol | 0 | 116 | 65.1 × 65.1 × 87.9 |
| ISUCA.-Pal Ol | 0 | 116 | 64.3 × 64.3 × 89.5 |
| 50:50 POPC/ISUCA-2 Pal | 58 | 58 | 64.3 × 63.4 × 91.9 |
| 50:50 POPC/ISUCA-2 Ol | 58 | 58 | 64.3 × 63.4 × 91.9 |
| 50:50 POPC/ISUCA-Pal-Ol | 58 | 58 | 64.3 × 63.4 × 91.9 |
| 90:10 POPC/ISUCA ISUCA-Pal-Ol | 104 | 12 | 64.3 × 63.5 × 92.0 |
| 90:10 POPC/ISUCA+-Pal-Ol (protonated ISUCA) 1 | 104 | 12 | 64.4 × 63.5 × 91.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lado-Touriño, I.; Cerpa-Naranjo, A. Coarse-Grained Molecular Dynamics of pH-Sensitive Lipids. Int. J. Mol. Sci. 2023, 24, 4632. https://doi.org/10.3390/ijms24054632
Lado-Touriño I, Cerpa-Naranjo A. Coarse-Grained Molecular Dynamics of pH-Sensitive Lipids. International Journal of Molecular Sciences. 2023; 24(5):4632. https://doi.org/10.3390/ijms24054632
Chicago/Turabian StyleLado-Touriño, Isabel, and Arisbel Cerpa-Naranjo. 2023. "Coarse-Grained Molecular Dynamics of pH-Sensitive Lipids" International Journal of Molecular Sciences 24, no. 5: 4632. https://doi.org/10.3390/ijms24054632
APA StyleLado-Touriño, I., & Cerpa-Naranjo, A. (2023). Coarse-Grained Molecular Dynamics of pH-Sensitive Lipids. International Journal of Molecular Sciences, 24(5), 4632. https://doi.org/10.3390/ijms24054632








