Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1
Abstract
1. Introduction
2. Results
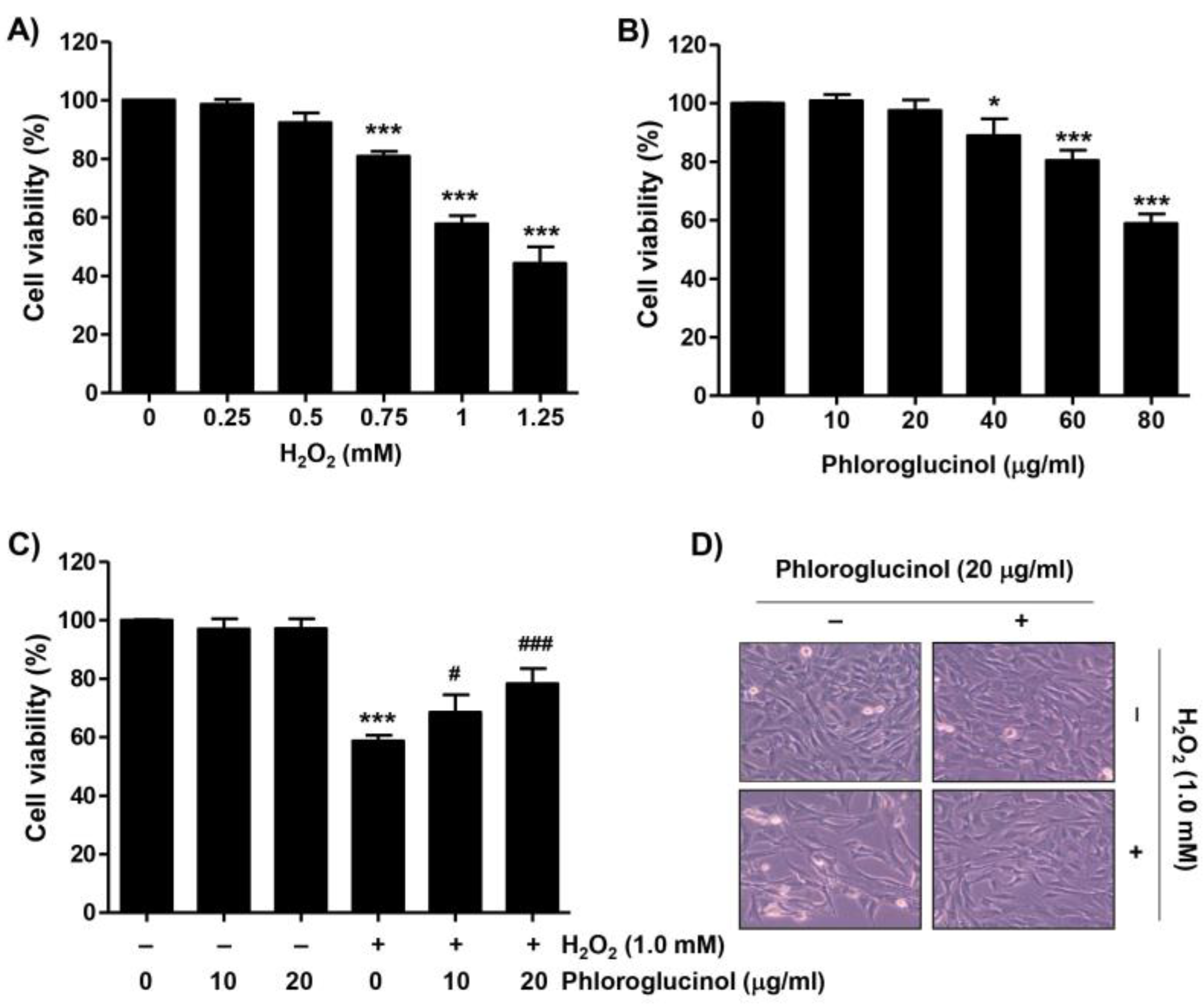
2.1. Phloroglucinol Inhibits the Decrease of Cell Viability Caused by H2O2 in C2C12 Cells
2.2. Phloroglucinol Abolishes H2O2-Induced Apoptosis in C2C12 Cells
2.3. Phloroglucinol Protects H2O2-Induced Mitochondrial Impairment in C2C12 Cells
2.4. Phloroglucinol Reduces H2O2-Induced DNA Damage and ROS Accumulation in C2C12 Cells
2.5. Phloroglucinol Diminishes H2O2-Induced ROS Accumulation and Activates Nrf2/HO-1 Signaling Pathway in C2C12 Cells
2.6. Activation of HO-1 by Phloroglucinol Contributes to Restoration of H2O2-Induced Oxidative Damage and Mitochondrial Dysfunction in C2C12 Cells
2.7. Activation of HO-1 Is Involved in Mitigating H2O2-Induced Cytotoxicity by Phloroglucinol in C2C12 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay and Cell Morphological Change Observation
4.4. Flow Cytometry Analysis
4.5. Apoptosis Analysis via DAPI Staining
4.6. DNA Fragmentation Assay
4.7. Protein Isolation and Immunoblotting
4.8. Comet Assay
4.9. Analysis of Caspase-3 Activity
4.10. Analysis of HO-1 Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Gao, X.; Zheng, L.; Huang, Q.; Zeng, F.; Chen, H.; Farag, M.A.; Zhao, C. Advances in fucoxanthin chemistry and management of neurodegenerative diseases. Phytomedicine 2022, 105, 154352. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Juárez-Portilla, C.; Olivares-Bañuelos, T.; Molina-Jiménez, T.; Sánchez-Salcedo, J.A.; Moral, D.I.D.; Meza-Menchaca, T.; Flores-Muñoz, M.; López-Franco, Ó.; Roldán-Roldán, G.; Ortega, A.; et al. Seaweeds-derived compounds modulating effects on signal transduction pathways: A systematic review. Phytomedicine 2019, 63, 153016. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production—A review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Montero, L.; Del Pilar Sánchez-Camargo, A.; Ibáñez, E.; Gilbert-López, B. Phenolic compounds from edible algae: Bioactivity and health benefits. Curr. Med. Chem. 2018, 25, 4808–4826. [Google Scholar] [CrossRef]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Call the eckols: Present and future potential cancer therapies. Mar. Drugs 2022, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Faezeh Taghizadeh, S.; Panahi, A.; Esmaeilzadeh Kashi, M.; Kretschmer, N.; Asili, J.; Ahmad Emami, S.; Azizi, M.; Shakeri, A. Structural diversity of complex phloroglucinol derivatives from Eucalyptus species. Chem. Biodivers. 2022, 19, e202200025. [Google Scholar] [CrossRef] [PubMed]
- Biessy, A.; Filion, M. Phloroglucinol derivatives in plant-beneficial Pseudomonas spp.: Biosynthesis, regulation, and functions. Metabolites 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Bamunuarachchi, N.I.; Kim, Y.M. Phloroglucinol and its derivatives: Antimicrobial properties toward microbial pathogens. J. Agric. Food Chem. 2022, 70, 4817–4838. [Google Scholar] [CrossRef]
- Clara, B.; Paul, V.; Denis, P.; Stéphanie, M.; Hélène, V.R.; Rémy, B. Efficacy of phloroglucinol for the treatment of pain of gynaecologic or obstetrical origin: A systematic review of literature of randomised controlled trials. Eur. J. Clin. Pharmacol. 2020, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Pouchain, D.; Vanderkam, P.; Perault-Pochat, M.C.; Boussageon, R.; Vaillant-Roussel, H. Efficacy of phloroglucinol for treatment of abdominal pain: A systematic review of literature and meta-analysis of randomised controlled trials versus placebo. Eur. J. Clin. Pharmacol. 2018, 74, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Drysch, M.; Schmidt, S.V.; Becerikli, M.; Reinkemeier, F.; Dittfeld, S.; Wagner, J.M.; Dadras, M.; Sogorski, A.; von Glinski, M.; Lehnhardt, M.; et al. Myostatin deficiency protects C2C12 cells from oxidative stress by inhibiting intrinsic activation of apoptosis. Cells 2021, 10, 1680. [Google Scholar] [CrossRef]
- Cia, D.; Cubizolle, A.; Crauste, C.; Jacquemot, N.; Guillou, L.; Vigor, C.; Angebault, C.; Hamel, C.P.; Vercauteren, J.; Brabet, P. Phloroglucinol protects retinal pigment epithelium and photoreceptor against all-trans-retinal-induced toxicity and inhibits A2E formation. J. Cell. Mol. Med. 2016, 20, 1651–1663. [Google Scholar] [CrossRef]
- So, M.J.; Cho, E.J. Phloroglucinol attenuates free radical-induced oxidative stress. Prev. Nutr. Food Sci. 2014, 19, 129–135. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Kang, K.A.; Lee, N.H.; Hyun, J.W.; Kim, H.S. Phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells. J. Pharmacol. Sci. 2012, 119, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Zhang, R.; Hong, B.H.; Yang, E.J.; Kang, K.A.; Choi, M.; Kim, K.C.; Noh, S.J.; Kim, H.S.; Lee, N.H.; et al. Phloroglucinol attenuates motor functional deficits in an animal model of Parkinson’s disease by enhancing Nrf2 activity. PLoS ONE 2013, 8, e71178. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, H.; Kim, H.S.; Chang, M.J. Phloroglucinol attenuates oligomeric amyloid b peptide1-42-induced astrocytic activation by reducing oxidative stress. J. Pharmacol. Sci. 2021, 145, 308–312. [Google Scholar] [CrossRef]
- Yang, E.J.; Mahmood, U.; Kim, H.; Choi, M.; Choi, Y.; Lee, J.P.; Cho, J.Y.; Hyun, J.W.; Kim, Y.S.; Chang, M.J.; et al. Phloroglucinol ameliorates cognitive impairments by reducing the amyloid β peptide burden and pro-inflammatory cytokines in the hippocampus of 5XFAD mice. Free Radic. Biol. Med. 2018, 126, 221–234. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Kang, K.A.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Hyun, J.W. Phloroglucinol attenuates ultraviolet B-induced 8-oxoguanine formation in human HaCaT keratinocytes through Akt and Erk-mediated Nrf2/Ogg1 signaling pathways. Biomol. Ther. 2021, 29, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.H.; Setiawan, B.; Dewi, F.R.; Noor, Z. Regulation by phloroglucinol of Nrf2/Maf-mediated expression of antioxidant enzymes and inhibition of osteoclastogenesis via the RANKL/RANK signaling pathway: In silico study. Acta. Inform. Med. 2015, 23, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; McArdle, A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J. Physiol. 2011, 589, 2139–2145. [Google Scholar] [CrossRef]
- Jenkins, T.; Gouge, J. Nrf2 in cancer, detoxifying enzymes and cell death programs. Antioxidants 2021, 10, 1030. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Ma, D.; He, Z.C.; Liu, P.; Huang, J.; Fang, Q.; Zhao, J.Y.; Wang, J.S. Heme oxygenase-1 protects bone marrow mesenchymal stem cells from iron overload through decreasing reactive oxygen species and promoting IL-10 generation. Exp. Cell Res. 2018, 362, 28–42. [Google Scholar] [CrossRef]
- Vineetha, V.P.; Soumya, R.S.; Raghu, K.G. Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur. J. Pharmacol. 2015, 754, 162–172. [Google Scholar] [CrossRef]
- Li, J.; Yang, Q.; Han, L.; Pan, C.; Lei, C.; Chen, H.; Lan, X. C2C12 mouse myoblasts damage induced by oxidative stress is alleviated by the antioxidant capacity of the active substance phloretin. Front. Cell. Dev. Biol. 2020, 8, 541260. [Google Scholar] [CrossRef]
- Salucci, S.; Battistelli, M.; Burattini, S.; Squillace, C.; Canonico, B.; Gobbi, P.; Papa, S.; Falcieri, E. C2C12 myoblast sensitivity to different apoptotic chemical triggers. Micron 2010, 41, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M.; Wang, Y.; Always, S.E. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009, 84, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell. Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed. Pharmacother. 2018, 106, 902–909. [Google Scholar] [CrossRef]
- Lalier, L.; Vallette, F.; Manon, S. Bcl-2 Family members and the mitochondrial import machineries: The roads to death. Biomolecules 2022, 12, 162. [Google Scholar] [CrossRef]
- Di Filippo, E.S.; Mancinelli, R.; Pietrangelo, T.; La Rovere, R.M.; Quattrocelli, M.; Sampaolesi, M.; Fulle, S. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem. Biophys. Res. Commun. 2016, 473, 462–470. [Google Scholar] [CrossRef]
- Ji, L.L. Redox signaling in skeletal muscle: Role of aging and exercise. Adv. Physiol. Educ. 2015, 39, 352–359. [Google Scholar] [CrossRef]
- Fulle, S.; Sancilio, S.; Mancinelli, R.; Gatta, V.; Di Pietro, R. Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis. 2013, 4, e955. [Google Scholar] [CrossRef]
- Keum, Y.S. Regulation of Nrf2-mediated phase II detoxification and anti-oxidant genes. Biomol. Ther. 2012, 20, 144–151. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef]
- Yu, L.M.; Zhang, W.H.; Han, X.X.; Li, Y.Y.; Lu, Y.; Pan, J.; Mao, J.Q.; Zhu, L.Y.; Deng, J.J.; Huang, W.; et al. Hypoxia-induced ROS contribute to myoblast pyroptosis during obstructive sleep apnea via the NF-κB/HIF-1α signaling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4596368. [Google Scholar] [CrossRef]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of platycodin D against oxidative stress-induced DNA damage and apoptosis in C2C12 myoblasts. Gen. Physiol. Biophys. 2020, 39, 519–530. [Google Scholar] [CrossRef]
- Park, C.; Ji, S.Y.; Lee, H.; Choi, S.H.; Kwon, C.Y.; Kim, S.Y.; Lee, E.T.; Choo, S.T.; Kim, G.Y.; Choi, Y.H.; et al. Mori Ramulus suppresses hydrogen peroxide-induced oxidative damage in murine myoblast C2C12 cells through activation of AMPK. Int. J. Mol. Sci. 2021, 22, 11729. [Google Scholar] [CrossRef]
- Jeong, M.J.; Lim, D.S.; Kim, S.O.; Park, C.; Leem, S.H.; Lee, H.; Kim, G.Y.; Jeong, S.J.; Choi, Y.H. Protection of oxidative stress-induced DNA damage and apoptosis by rosmarinic acid in murine myoblast C2C12 cells. Biotechnol. Bioprocess Eng. 2022, 27, 171–182. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.H.; Kim, J.H.; Park, S.K.; Jeong, J.W.; Kim, M.Y.; Hong, S.H.; Song, K.S.; Kim, G.Y.; Hyun, J.W.; et al. Urban aerosol particulate matter promotes necrosis and autophagy via reactive oxygen species-mediated cellular disorders that are accompanied by cell cycle arrest in retinal pigment epithelial cells. Antioxidants 2021, 10, 149. [Google Scholar] [CrossRef]
- Sukjamnong, S.; Chen, H.; Saad, S.; Santiyanont, R. Fimbristylis ovata and Artemisia vulgaris extracts inhibited AGE-mediated RAGE expression, ROS generation, and inflammation in THP-1 cells. Toxicol. Res. 2022, 38, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Tacrolimus induces apoptosis in leukemia Jurkat cells through inactivation of the reactive oxygen species-dependent phosphoinositide-3-kinase/Akt signaling pathway. Biotechnol. Bioprocess Eng. 2022, 27, 183–192. [Google Scholar] [CrossRef]
- Choi, Y.H. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2021, 43, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Park, J.P.; Yun, J.W. Carboxylesterase3 (Ces3) Interacts with bone morphogenetic protein 11 and promotes differentiation of osteoblasts via Smad1/5/9 pathway. Biotechnol. Bioprocess Eng. 2022, 27, 1–16. [Google Scholar] [CrossRef]
- Sim, K.H.; Shu, M.S.; Kim, S.; Kim, J.Y.; Choi, B.H.; Lee, Y.J. Cilostazol induces apoptosis and inhibits proliferation of hepatocellular carcinoma cells by activating AMPK. Biotechnol. Bioprocess Eng. 2021, 26, 776–785. [Google Scholar] [CrossRef]
- Jo, H.G.; Park, C.; Lee, H.; Kim, G.Y.; Keum, Y.S.; Hyun, J.W.; Kwon, T.K.; Choi, Y.H.; Hong, S.H. Inhibition of oxidative stress induced-cytotoxicity by coptisine in V79-4 Chinese hamster lung fibroblasts through the induction of Nrf-2 mediated HO-1 expression. Genes Genom. 2021, 43, 17–31. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Cha, H.-J.; Hwangbo, H.; Ji, S.Y.; Kim, D.H.; Kim, M.Y.; Bang, E.; Hong, S.H.; Kim, S.O.; Jeong, S.-J.; et al. Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1. Int. J. Mol. Sci. 2023, 24, 4637. https://doi.org/10.3390/ijms24054637
Park C, Cha H-J, Hwangbo H, Ji SY, Kim DH, Kim MY, Bang E, Hong SH, Kim SO, Jeong S-J, et al. Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1. International Journal of Molecular Sciences. 2023; 24(5):4637. https://doi.org/10.3390/ijms24054637
Chicago/Turabian StylePark, Cheol, Hee-Jae Cha, Hyun Hwangbo, Seon Yeong Ji, Da Hye Kim, Min Yeong Kim, EunJin Bang, Su Hyun Hong, Sung Ok Kim, Soon-Jeong Jeong, and et al. 2023. "Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1" International Journal of Molecular Sciences 24, no. 5: 4637. https://doi.org/10.3390/ijms24054637
APA StylePark, C., Cha, H.-J., Hwangbo, H., Ji, S. Y., Kim, D. H., Kim, M. Y., Bang, E., Hong, S. H., Kim, S. O., Jeong, S.-J., Lee, H., Moon, S.-K., Shim, J.-H., Kim, G.-Y., Cho, S., & Choi, Y. H. (2023). Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1. International Journal of Molecular Sciences, 24(5), 4637. https://doi.org/10.3390/ijms24054637











