Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma
Abstract
:1. Introduction
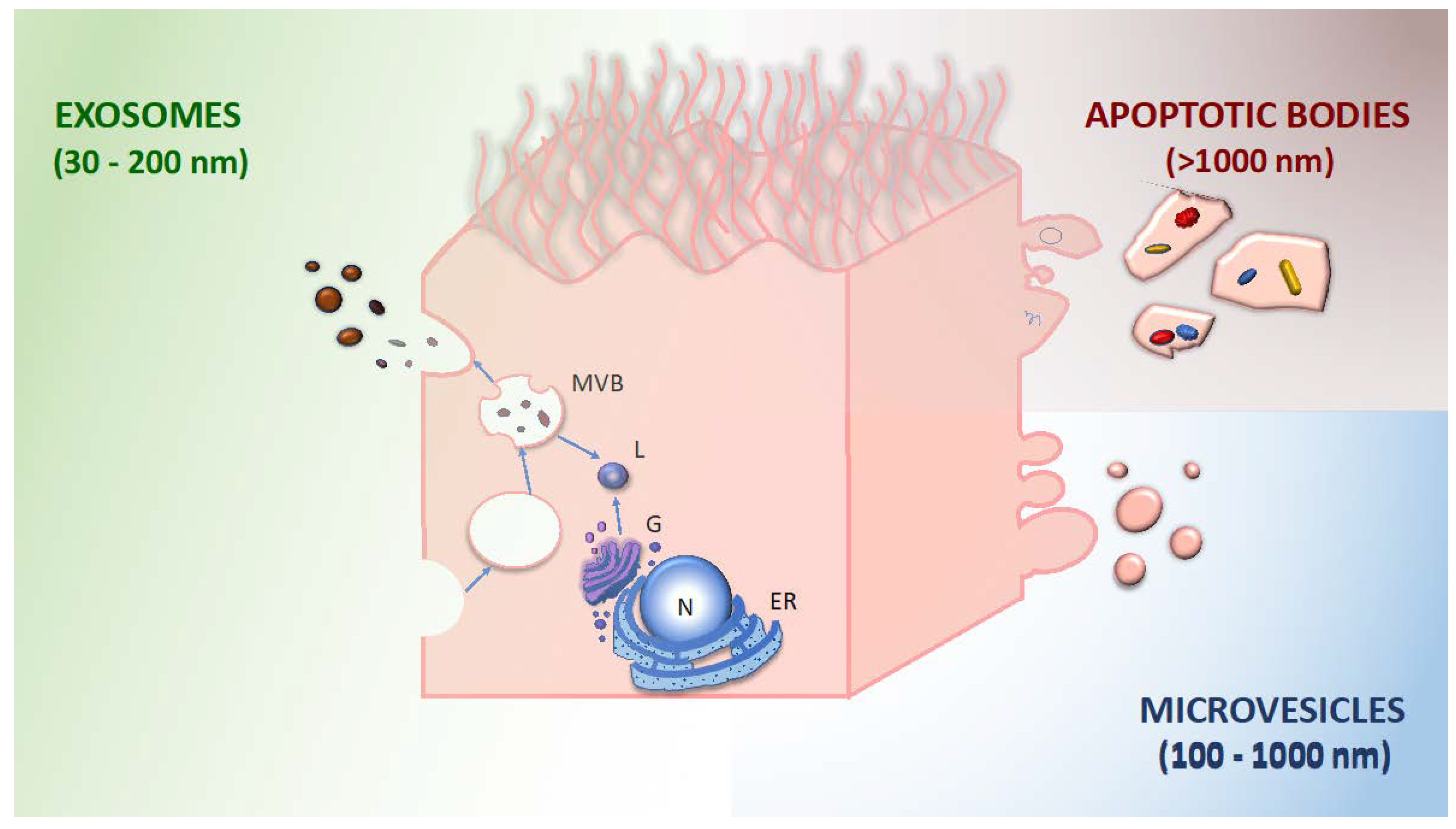
2. Extracellular Vesicles
- exosomes: 30–200 nm diameter; generated by inward budding of the membrane (endocytosis), subsequent formation of multivesicular bodies, and release by exocytosis; and characterized by tetraspanins (CD9, CD63, CD81, and CD82) and other surface markers derived from the multivesicular body.
- microvesicles: 100–1000 nm diameter; released by budding and shedding from the plasma membrane of activated cells. They share the same membrane components with the parent cell.
- apoptotic bodies: 1000–4000 nm diameter; released through blebbing of apoptotic cell membrane by cells undergoing apoptosis; and enriched in phosphatidylserine, annexin V.
3. Mechanistic Hints from Preclinical Studies
3.1. Epithelial Cells
3.2. Immune Cells
3.3. Other Non-Immune Cells
4. Extracellular Vesicles as Asthma Sensors in Humans
5. EV in Our Guests: How Microbiome Introduces Higher Levels of Complexity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.K.W.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S.; International Study of Asthma and Allergies in Childhood. Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: Phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, Update 2022. Available online: https://ginasthma.org (accessed on 31 January 2023).
- Tay, T.R.; Hew, M. Comorbid “treatable traits” in difficult asthma: Current evidence and clinical evaluation. Allergy 2017, 73, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Sforza, M.; Calzetta, L. The impact of comorbidities on severe asthma. Curr. Opin. Pulm. Med. 2020, 26, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A. Allergic rhinitis and asthma: Epidemiology and common pathophysiology. Allergy Asthma Proc. 2014, 35, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2019, 8, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Crimi, C.; Ferri, S.; Campisi, R.; Crimi, N. The Link between Asthma and Bronchiectasis: State of the Art. Respiration 2020, 99, 463–476. [Google Scholar] [CrossRef]
- Turrin, M.; Rizzo, M.; Bonato, M.; Bazzan, E.; Cosio, M.G.; Semenzato, U.; Saetta, M.; Baraldo, S. Differences Between Early- and Late-Onset Asthma: Role of Comorbidities in Symptom Control. J. Allergy Clin. Immunol. Pr. 2022, 10, 3196–3203. [Google Scholar] [CrossRef]
- Djukanović, R.; Roche, W.R.; Wilson, J.W.; Beasley, C.R.W.; Twentyman, O.P.; Howarth, P.H.; Holgate, S.T. Mucosal inflammation in asthma. Am. Rev. Respir. Dis. 1990, 142, 434–457. [Google Scholar] [CrossRef]
- Maison, N.; Omony, J.; Illi, S.; Thiele, D.; Skevaki, C.; Dittrich, A.-M.; Bahmer, T.; Rabe, K.F.; Weckmann, M.; Happle, C.; et al. T2-high asthma phenotypes across lifespan. Eur. Respir. J. 2022, 60, 2102288. [Google Scholar] [CrossRef]
- Sporik, R.; Holgate, S.T.; Platts-Mills, T.A.; Cogswell, J.J. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N. Engl. J. Med. 1990, 323, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Permaul, P.; Hoffman, E.B.; Fu, C.; Sheehan, W.J.; Baxi, S.N.; Gaffin, J.M.; Lane, J.P.; Bailey, A.; King, E.; Chapman, M.; et al. Allergens in urban schools and homes of children with asthma. Pediatr. Allergy Immunol. 2012, 23, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, M.; Zock, J.-P.; Accordini, S.; Heinrich, J.; Jarvis, D.; Künzli, N.; Antó, J.M.; Norbäck, D.; Svanes, C.; Verlato, G. Indoor Working Group of the European Community Respiratory Health Survey II. Risk factors for new-onset cat sensitization among adults: A population-based international cohort study. J. Allergy Clin. Immunol. 2012, 129, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonato, M.; Tiné, M.; Bazzan, E.; Biondini, D.; Saetta, M.; Baraldo, S. Early Airway Pathological Changes in Children: New Insights into the Natural History of Wheezing. J. Clin. Med. 2019, 8, 1180. [Google Scholar] [CrossRef] [Green Version]
- Bonato, M.; Bazzan, E.; Snijders, D.; Tinè, M.; Biondini, D.; Turato, G.; Balestro, E.; Papi, A.; Cosio, M.G.; Barbato, A.; et al. Clinical and Pathologic Factors Predicting Future Asthma in Wheezing Children. A Longitudinal Study. Am. J. Respir. Cell Mol. Biol. 2018, 59, 458–466. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Saglani, S.; Baraldo, S. Remodelling Phenotypes Take Centre Stage in the Prediction of Preschool Wheeze Attacks. Am. J. Respir. Crit. Care Med. 2023, 207, 381–382. [Google Scholar] [CrossRef]
- Olin, J.T.; Wechsler, M.E. Asthma: Pathogenesis and novel drugs for treatment. BMJ 2014, 349, g5517. [Google Scholar] [CrossRef] [Green Version]
- Li-Weber, M.; Krammer, P.H. Regulation of IL4 gene expression by T cells and therapeutic perspectives. Nat. Rev. Immunol. 2003, 3, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, K.; Takeyama, K.; Lee, H.M.; Ueki, I.F.; Lausier, J.A.; Nadel, J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999, 162, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Hershey, G.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E. Harrison: Principles of Internal Medicine XVI; Kasper, D., Braunwald, E., Fauci, A., Hauser, S., Longo, D., Jameson, L., Eds.; Mc Graw Hill: New York, NY, USA, 2005. [Google Scholar]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic mechanisms in asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- Rivas, M.N.; Burton, O.T.; Oettgen, H.C.; Chatila, T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 2016, 138, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.J.; Antignano, F.; Halim, T.Y.; Hirota, J.A.; Blanchet, M.-R.; Zaph, C.; Takei, F.; McNagny, K.M. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J. Allergy Clin. Immunol. 2014, 133, 1142–1148. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Cherrier, D.E.; Serafini, N.; Di Santo, J.P. Innate Lymphoid Cell Development: A T Cell Perspective. Immunity 2018, 48, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef]
- Mindt, B.C.; Fritz, J.H.; Duerr, C.U. Group 2 Innate Lymphoid Cells in Pulmonary Immunity and Tissue Homeostasis. Front. Immunol. 2018, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Li, B.W.S.; Stadhouders, R.; De Bruijn, M.J.W.; Lukkes, M.; Beerens, D.M.J.M.; Brem, M.D.; Kleinjan, A.; Bergen, I.; Vroman, H.; Kool, M.; et al. Group 2 Innate Lymphoid Cells Exhibit a Dynamic Phenotype in Allergic Airway Inflammation. Front. Immunol. 2017, 8, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.W.S.; de Bruijn, M.J.W.; Lukkes, M.; van Nimwegen, M.; Bergen, I.M.; KleinJan, A.; GeurtsvanKessel, C.H.; Andeweg, A.; Rimmelzwaan, G.F.; Hendriks, R.W. T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. Eur. J. Immunol. 2018, 49, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Zhang, Y.; Liu, L.; Cao, L.; Liu, Y.; Dong, L. Type 2 innate lymphoid cells: A novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir. Med. 2015, 109, 1391–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.-N.; Tan, W.-P.; Fan, X.-L.; Guo, Y.-B.; Qin, Z.-L.; Li, C.-L.; Chen, D.; Bin Lin, Z.; Wen, W.; Fu, Q.-L. Increased Group 2 Innate Lymphoid Cells Are Correlated with Eosinophilic Granulocytes in Patients with Allergic Airway Inflammation. Int. Arch. Allergy Immunol. 2018, 176, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hesse, L.; van Ieperen, N.; Habraken, C.; Petersen, A.H.; Korn, S.; Smilda, T.; Goedewaagen, B.; Ruiters, M.H.; van der Graaf, A.C.; Nawijn, M.C. Subcutaneous immunotherapy with purified Der p1 and 2 suppresses type 2 immunity in a murine asthma model. Allergy 2018, 73, 862–874. [Google Scholar] [CrossRef] [Green Version]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef]
- Préfontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein st2 and induces t helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Blom, L.; Poulsen, B.C.; Jensen, B.M.; Hansen, A.; Poulsen, L.K. IL-33 induces il-9 production in human cd4+ t cells and basophils. PLoS ONE 2011, 6, e21695. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ji, N.; Ma, Q.; Zhu, R.; Chen, Z.; Wang, Z.; Qian, Y.; Wu, C.; Hu, F.; Huang, M.; et al. Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis. Front. Immunol. 2020, 11, 1598. [Google Scholar] [CrossRef]
- Hackett, T.-L. Epithelial–mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [Green Version]
- Henderson, M.C.; Azorsa, D.O. The genomic and proteomic content of cancer cell-derived exosomes. Front. Oncol. 2012, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Gordon, C.; Gudi, K.; Krause, A.; Sackrowitz, R.; Harvey, B.-G.; Strulovici-Barel, Y.; Mezey, J.G.; Crystal, R.G. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Koga, H.; Sugiyama, S.; Kugiyama, K.; Fukushima, H.; Watanabe, K.; Sakamoto, T.; Yoshimura, M.; Jinnouchi, H.; Ogawa, H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur. Heart J. 2006, 27, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, R.A.; Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Valle, M.; Aime, G.; Ahn, Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003, 41, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesri, M.; Altieri, D.C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 1998, 161, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Njock, M.-S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edrissi, H.; Schock, S.C.; Hakim, A.M.; Thompson, C.S. Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro. Brain Res. 2015, 1634, 83–93. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Isobe, H.; Lesèche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, I.; Aquila, S. Exosomes in human atherosclerosis: An ultrastructural analysis study. Ultrastruct. Pathol. 2016, 40, 101–106. [Google Scholar] [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.-M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Min, P.-K.; Kim, J.-Y.; Chung, K.-H.; Lee, B.K.; Cho, M.; Lee, D.-L.; Hong, S.-Y.; Choi, E.-Y.; Yoon, Y.-W.; Hong, B.-K.; et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2013, 227, 323–328. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Eikenboom, J.; Tesselaar, M.; Huisman, M.; Nijkeuter, M.; Osanto, S.; Bertina, R. Plasma levels of microparticle-associated tissue factor activity in patients with clinically suspected pulmonary embolism. Thromb. Res. 2010, 126, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Bal, L.; Ederhy, S.; Di Angelantonio, E.; Toti, F.; Zobairi, F.; Dufaitre, G.; Meuleman, C.; Mallat, Z.; Boccara, F.; Tedgui, A.; et al. Factors influencing the level of circulating procoagulant microparticles in acute pulmonary embolism. Arch. Cardiovasc. Dis. 2010, 103, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramberg, C.; Wilsgård, L.; Latysheva, N.; Brækkan, S.K.; Hindberg, K.; Sovershaev, T.; Snir, O.; Hansen, J. Plasma procoagulant phospholipid clotting time and venous thromboembolism risk. Res. Pract. Thromb. Haemost. 2021, 5, e12640. [Google Scholar] [CrossRef] [PubMed]
- Bal, L.; Ederhy, S.; Di Angelantonio, E.; Toti, F.; Zobairi, F.; Dufaitre, G.; Meuleman, C.; Mallat, Z.; Boccara, F.; Tedgui, A.; et al. Circulating procoagulant microparticles in acute pulmonary embolism: A case–control study. Int. J. Cardiol. 2010, 145, 321–322. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, A.; Kaur, J.; Verma, N.; Pandey, A.K.; Das, S.; Bhattacharyya, S.; Guchhait, P. Upregulation of cytokine signalling in platelets increases risk of thrombophilia in severe COVID-19 patients. Blood Cells Mol. Dis. 2022, 94, 102653. [Google Scholar] [CrossRef]
- Buzás, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Palkovits, J.; Novacek, G.; Kollars, M.; Hron, G.; Osterode, W.; Quehenberger, P.; Kyrle, P.A.; Vogelsang, H.; Reinisch, W.; Papay, P.; et al. Tissue factor exposing microparticles in inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Brogan, P.A.; Dillon, M.J. Endothelial microparticles and the diagnosis of the vasculitides. Intern. Med. 2004, 43, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, J.; Lee, H. Emerging role of extracellular vesicles in the respiratory system. Exp. Mol. Med. 2020, 52, 887–895. [Google Scholar] [CrossRef]
- Tinè, M.; Biondini, D.; Damin, M.; Semenzato, U.; Bazzan, E.; Turato, G. Extracellular vesicles in lung cancer: Bystanders or main characters? Biology 2023, 12, 246. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Potaczek, D.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma—More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Radicioni, G.; Abdelwahab, S.; Dang, H.; Carpenter, J.; Chua, M.; Mieczkowski, P.A.; Sheridan, J.T.; Randell, S.H.; Kesimer, M. Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2019, 60, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.-T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019, 26, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2019, 75, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-A.; Sharif, A.S.; Tschumperlin, D.J.; Lau, L.; Limbrey, R.; Howarth, P.; Drazen, J.M. Tissue factor–bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 2012, 130, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Mwase, C.; Phung, T.-K.N.; O’Sullivan, M.J.; Mitchel, J.A.; De Marzio, M.; Kılıç, A.; Weiss, S.T.; Fredberg, J.J.; Park, J.-A. Mechanical Compression of Human Airway Epithelial Cells Induces Release of Extracellular Vesicles Containing Tenascin C. Cells 2022, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, A.; Altraja, A.; Kämpe, M.; Linden, M.; Virtanen, I.; Laitinen, L.A. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am. J. Respir. Crit. Care Med. 1997, 156, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.T.; Schwenzer, A.; Marsh, E.K.; Edwards, M.R.; Sabroe, I.; Midwood, K.S.; Parker, L.C. Airway Epithelial Cells Generate Pro-inflammatory Tenascin-C and Small Extracellular Vesicles in Response to TLR3 Stimuli and Rhinovirus Infection. Front. Immunol. 2019, 10, 1987. [Google Scholar] [CrossRef] [Green Version]
- Chahar, H.S.; Corsello, T.; Kudlicki, A.S.; Komaravelli, N.; Casola, A. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Shin, T.-S.; Kim, J.H.; Kim, Y.-S.; Jeon, S.G.; Zhu, Z.; Gho, Y.S. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy 2010, 65, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Zhang, D.; Zhu, Z.; Cruz, C.S.D.; Jin, Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 2016, 6, 35250. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Zhang, D.; Laskin, D.L.; Jin, Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J. Immunol. 2018, 201, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Ax, E.; Jevnikar, Z.; Cvjetkovic, A.; Malmhäll, C.; Olsson, H.; Rådinger, M.; Lässer, C. T2 and T17 cytokines alter the cargo and function of airway epithelium-derived extracellular vesicles. Respir. Res. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Kesimer, M.; Scull, M.; Brighton, B.; DeMaria, G.; Burns, K.; O'Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef] [Green Version]
- Rose, M.C.; Voynow, J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkoussa, S.; Hulo, S.; Courcot, D.; Billet, S.; Martin, P.J. Extracellular vesicles as actors in the air pollution related cardiopulmonary diseases. Crit. Rev. Toxicol. 2020, 50, 402–423. [Google Scholar] [CrossRef] [PubMed]
- Stassen, F.R.M.; van Eijck, P.H.; Savelkoul, P.H.M.; Wouters, E.F.M.; Rohde, G.G.U.; Briedé, J.J.; Reynaert, N.L.; de Kok, T.M.; Benedikter, B.J. Cell Type- and Exposure-Specific Modulation of CD63/CD81-Positive and Tissue Factor-Positive Extracellular Vesicle Release in response to Respiratory Toxicants. Oxidative Med. Cell. Longev. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, T.; Pergoli, L.; Petrini, S.; Gravendonk, L.; Balia, C.; Scalise, V.; Amoruso, A.; Pedrinelli, R.; Paggiaro, P.; Bollati, V.; et al. Particulate matter induces prothrombotic microparticle shedding by human mononuclear and endothelial cells. Toxicol. Vitr. 2016, 32, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Angelici, L.; Rizzo, G.; Pergoli, L.; Rota, F.; Hoxha, M.; Nordio, F.; Bonzini, M.; Tarantini, L.; Cantone, L.; et al. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J. Appl. Toxicol. 2014, 35, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Wang, N.; Xu, Y.; Hua, L.; Zhou, D.; Zheng, M.; Deng, X. Effects of chronic PM2.5 exposure on pulmonary epithelia: Transcriptome analysis of mRNA-exosomal miRNA interactions. Toxicol. Lett. 2019, 316, 49–59. [Google Scholar] [CrossRef]
- Moon, H.-G.; Kim, S.-H.; Gao, J.; Quan, T.; Qin, Z.; Osorio, J.C.; Rosas, I.O.; Wu, M.; Tesfaigzi, Y.; Jin, Y. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L326–L337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedikter, B.J.; Volgers, C.; van Eijck, P.H.; Wouters, E.F.; Savelkoul, P.H.; Reynaert, N.L.; Haenen, G.R.; Rohde, G.G.; Weseler, A.R.; Stassen, F.R. Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Free. Radic. Biol. Med. 2017, 108, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Walters, M.S.; Shieh, J.-H.; Shen, L.-B.; Gomi, K.; Downey, R.J.; Crystal, R.G.; Moore, M.A.S. Extracellular vesicles from human airway basal cells respond to cigarette smoke extract and affect vascular endothelial cells. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Wouters, E.F.M.; Savelkoul, P.H.M.; Rohde, G.G.U.; Stassen, F.R.M. Extracellular vesicles released in response to respiratory exposures: Implications for chronic disease. J. Toxicol. Environ. Health Part B 2018, 21, 142–160. [Google Scholar] [CrossRef]
- Schindler, V.E.M.; Alhamdan, F.; Preußer, C.; Hintz, L.; Alhamwe, B.A.; Nist, A.; Stiewe, T.; von Strandmann, E.P.; Potaczek, D.P.; Thölken, C.; et al. Side-Directed Release of Differential Extracellular Vesicle-associated microRNA Profiles from Bronchial Epithelial Cells of Healthy and Asthmatic Subjects. Biomedicines 2022, 10, 622. [Google Scholar] [CrossRef]
- Lecce, M.; Molfetta, R.; Milito, N.D.; Santoni, A.; Paolini, R. FcεRI Signaling in the Modulation of Allergic Response: Role of Mast Cell-Derived Exosomes. Int. J. Mol. Sci. 2020, 21, 5464. [Google Scholar] [CrossRef]
- Akuthota, P.; Carmo, L.A.S.; Bonjour, K.; Murphy, R.O.; Silva, T.P.; Gamalier, J.P.; Capron, K.L.; Tigges, J.; Toxavidis, V.; Camacho, V.; et al. Extracellular Microvesicle Production by Human Eosinophils Activated by “Inflammatory” Stimuli. Front. Cell Dev. Biol. 2016, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Cañas, J.A.; Sastre, B.; Mazzeo, C.; Fernández-Nieto, M.; Rodrigo-Muñoz, J.M.; González-Guerra, A.; Izquierdo, M.; Barranco, P.; Quirce, S.; Sastre, J.; et al. Exosomes from eosinophils autoregulate and promote eosinophil functions. J. Leukoc. Biol. 2017, 101, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; Fernández-Nieto, M.; Barranco, P.; Quirce, S.; Sastre, J.; del Pozo, V. Eosinophil-derived exosomes contribute to asthma remodelling by activating structural lung cells. Clin. Exp. Allergy 2018, 48, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Esser, J.; Gehrmann, U.; D'Alexandri, F.L.; Hidalgo-Estévez, A.M.; Wheelock, C.E.; Scheynius, A.; Gabrielsson, S.; Rådmark, O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 2010, 126, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, A.; Roux-Dalvai, F.; Droit, A.; Lavoie, J.-P. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am. J. Respir. Cell Mol. Biol. 2016, 55, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Shefler, I.; Salamon, P.; Levi-Schaffer, F.; Mor, A.; Hershko, A.Y.; Mekori, Y.A. MicroRNA-4443 regulates mast cell activation by T cell–derived microvesicles. J. Allergy Clin. Immunol. 2018, 141, 2132–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallhov, H.; Gutzeit, C.; Hultenby, K.; Valenta, R.; Grönlund, H.; Scheynius, A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy 2015, 70, 1651–1655. [Google Scholar] [CrossRef]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017, 7, 17095. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, C.; Cañas, J.A.; Zafra, M.P.; Marco, A.R.; Fernández-Nieto, M.; Sanz, V.; Mittelbrunn, M.; Izquierdo, M.; Baixauli, F.; Sastre, J.; et al. Exosome secretion by eosinophils: A possible role in asthma pathogenesis. J. Allergy Clin. Immunol. 2015, 135, 1603–1613. [Google Scholar] [CrossRef]
- Xie, H.; He, S.-H. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J. Gastroenterol. 2005, 11, 2851–2857. [Google Scholar] [CrossRef]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.-C.; Peronet, R.; Namane, A.; David, B.; Mécheri, S. Nonspecific B and T cell-stimulatory activity mediated by mast cellsis associated with exosomes. Int. Arch. Allergy Immunol. 2001, 124, 133–136. [Google Scholar] [CrossRef]
- Bourdonnay, E.; Zaslona, Z.; Penke, L.R.K.; Speth, J.; Schneider, D.; Przybranowski, S.; Swanson, J.; Mancuso, P.; Freeman, C.M.; Curtis, J.; et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 2015, 212, 729–742. [Google Scholar] [CrossRef]
- Draijer, C.; Speth, J.M.; Penke, L.R.K.; Zaslona, Z.; Bazzill, J.D.; Lugogo, N.; Huang, Y.J.; Moon, J.J.; Peters-Golden, M. Resident alveolar macrophage-derived vesicular SOCS3 dampens allergic airway inflammation. FASEB J. 2020, 34, 4718–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, X.; Wang, M.; Chen, Z.; Yan, Y.; Gu, W.; Tan, J.; Jiang, W.; Ji, W. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 2018, 86, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Bohle, B.; Johansson, S.M.; Focke-Tejkl, M.; Valenta, R.; Scheynius, A.; Gabrielsson, S. B cell–derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J. Allergy Clin. Immunol. 2007, 120, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Paulie, S.; Gabrielsson, S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur. J. Immunol. 2006, 36, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Vincent-Schneider, H.; Stumptner-Cuvelette, P.; Lankar, D.; Pain, S.; Raposo, G.; Benaroch, P.; Bonnerot, C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 2002, 14, 713–722. [Google Scholar] [CrossRef]
- Shefler, I.; Salamon, P.; Reshef, T.; Mor, A.; Mekori, Y.A. T cell-induced mast cell activation: A role for microparticles released from activated T cells. J. Immunol. 2010, 185, 4206–4212. [Google Scholar] [CrossRef]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Hynes, G.M.; Hinks, T.S. The role of interleukin-17 in asthma: A protective response? ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Mainguy-Seers, S.; Beaudry, F.; Fernandez-Prada, C.; Martin, J.G.; Lavoie, J.-P. Neutrophil Extracellular Vesicles and Airway Smooth Muscle Proliferation in the Natural Model of Severe Asthma in Horses. Cells 2022, 11, 3347. [Google Scholar] [CrossRef] [PubMed]
- Haj-Salem, I.; Plante, S.; Gounni, A.S.; Rouabhia, M.; Chakir, J. Fibroblast-derived exosomes promote epithelial cell proliferation through TGF-β2 signalling pathway in severe asthma. Allergy 2017, 73, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. New therapies for asthma: Is there any progress? Trends Pharmacol. Sci. 2010, 31, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, I.; Cruz, M.-J.; Moreno, R.; Morell, F.; Muñoz, X.; Aran, J.M.; Martínez-González, I.; Cruz, M.-J.; Moreno, R.; Morell, F.; et al. Human mesenchymal stem cells resolve airway inflammation, hyperreactivity, and histopathology in a mouse model of occupational asthma. Stem Cells Dev. 2014, 23, 2352–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.-Y.; Bai, S.-Y.; Li, M.-L.; Zhao, J.-Y.; Sun, J.-M.; Bao, H.-J.; Ren, Y.; Su, X.-M. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomal miR-301a-3p Regulates Airway Smooth Muscle Cells During Asthma by Targeting STAT3. J. Asthma Allergy 2022, 15, 99–110. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Res. Ther. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Abreu, S.C.; Weiss, D.J.; Rocco, P.R.M. Extracellular vesicles derived from mesenchymal stromal cells: A therapeutic option in respiratory diseases? Stem Cell Res. Ther. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Duarte, D.; Taveira-Gomes, T.; Sokhatska, O.; Palmares, C.; Costa, R.; Negrão, R.; Guimarães, J.T.; Delgado, L.; Soares, R.; Moreira, A. Increased circulating platelet microparticles as a potential biomarker in asthma. Allergy 2013, 68, 1073–1075. [Google Scholar] [CrossRef]
- Gao, J.; Xu, X.; Ying, Z.; Jiang, L.; Zhong, M.; Wang, A.; Chen, L.-C.; Lu, B.; Sun, Q. Post-Effect of Air Quality Improvement on Biomarkers for Systemic Inflammation and Microparticles in Asthma Patients after the 2008 Beijing Olympic Games: A Pilot Study. Inflammation 2017, 40, 1214–1224. [Google Scholar] [CrossRef]
- Skeppholm, M.; Mobarrez, F.; Malmqvist, K.; Wallén, H. Platelet-derived microparticles during and after acute coronary syndrome. Thromb. Haemost. 2012, 107, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Bozkaya, G.; Kirbiyik, H.; Alacacioglu, A.; Ates, H.; Sop, G.; Can, G.; Taylan, A.; Piskin, O.; Yildiz, Y.; et al. Evaluation of circulating endothelial and platelet microparticles in men with ankylosing spondylitis. J. Rheumatol. 2012, 39, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Eberhardt, M.; Vera, J.; Cuomo, F.; Blume, K.; Galster, S.; Achenbach, S.; Laffert, B.; Kahlert, H.; Schuler, G.; et al. Plasma-derived extracellular vesicles discriminate type-1 allergy subjects from non-allergic controls. World Allergy Organ. J. 2021, 14, 100583. [Google Scholar] [CrossRef] [PubMed]
- Atashbasteh, M.; Mortaz, E.; Mahdaviani, S.A.; Jamaati, H.; Allameh, A. Expression levels of plasma exosomal miR-124, miR-125b, miR-133b, miR-130a and miR-125b-1-3p in severe asthma patients and normal individuals with emphasis on inflammatory factors. Allergy Asthma Clin. Immunol. 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Zhao, M.; Juanjuan, L.; Weijia, F.; Jing, X.; Qiuhua, H.; Hua, Z.; Fuhe, L.; Hao, P. Expression Levels of MicroRNA-125b in Serum Exosomes of Patients with Asthma of Different Severity and its Diagnostic Significance. Curr. Drug Metab. 2019, 20, 781–784. [Google Scholar] [CrossRef]
- Hir, S.R.; Alizadeh, Z.; Mazinani, M.; Rad, M.M.; Fazlollahi, M.R.; Kazemnejad, A.; Hosseini, A.Z.; Moin, M. Exosomal MicroRNAs as Biomarkers in Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 160–168. [Google Scholar]
- Bahmer, T.; Krauss-Etschmann, S.; Buschmann, D.; Behrends, J.; Watz, H.; Kirsten, A.; Pedersen, F.; Waschki, B.; Fuchs, O.; Pfaffl, M.W.; et al. RNA-seq–based profiling of extracellular vesicles in plasma reveals a potential role of miR-122-5p in asthma. Allergy 2020, 76, 366–371. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.-P.; Geng, X.-R.; Zhao, M.; Ma, S.-B.; Yang, Y.-H.; Deng, Z.-H.; Luo, L.-M.; Pan, X.-Q. Expression Level of MiRNA-126 in Serum Exosomes of Allergic Asthma Patients and Lung Tissues of Asthmatic Mice. Curr. Drug Metab. 2019, 20, 799–803. [Google Scholar] [CrossRef]
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Saavedra-Nieves, P.; Arias, P.; González-Fernández, C.; Mosteiro-Añón, M.; Corbacho-Abelaira, M.D.; Blanco-Aparicio, M.; Méndez-Brea, P.; et al. Serum exosome inflamma-miRs are surrogate biomarkers for asthma phenotype and severity. Allergy 2022, 78, 141–155. [Google Scholar] [CrossRef]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenbäck, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.-I.; Spell, M.L.; Wang, Y.; Chanda, D.; Dager, J.R.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, P.T.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Rådmark, O.; Grönneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 2012, 67, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Levänen, B.; Bhakta, N.R.; Paredes, P.T.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östling, J.; Van Geest, M.; Olsson, H.K.; Dahlen, S.-E.; Viklund, E.; Gustafsson, P.M.; Mirgorodskaya, E.; Olin, A.-C. A novel non-invasive method allowing for discovery of pathologically relevant proteins from small airways. Clin. Proteom. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Comfort, N.; Bloomquist, T.R.; Shephard, A.P.; Petty, C.R.; Cunningham, A.; Hauptman, M.; Phipatanakul, W.; Baccarelli, A. Isolation and characterization of extracellular vesicles in saliva of children with asthma. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Leong, L.E.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2017, 141, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, M.F.; Sanderson, N.D.; Foster, D.; Crook, D.W.; Cane, J.L.; Borg, C.; Connolly, C.; Thulborn, S.; Pavord, I.D.; Klenerman, P.; et al. Identifying Bacterial Airways Infection in Stable Severe Asthma Using Oxford Nanopore Sequencing Technologies. Microbiol. Spectr. 2022, 10, e02279-21. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, J.-P.; Yang, J.; Won, H.-K.; Park, C.S.; Song, W.-J.; Kwon, H.-S.; Kim, T.-B.; Kim, Y.-K.; Park, H.-S.; et al. Metagenome analysis using serum extracellular vesicles identified distinct microbiota in asthmatics. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- An, J.; McDowell, A.; Kim, Y.-K.; Kim, T.-B. Extracellular vesicle-derived microbiome obtained from exhaled breath condensate in patients with asthma. Ann. Allergy Asthma Immunol. 2021, 126, 729–731. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.H.; Lim, D.H. Urine Microbe-Derived Extracellular Vesicles in Children with Asthma. Allergy Asthma Immunol. Res. 2021, 13, 75–87. [Google Scholar] [CrossRef]
- Samra, M.S.; Lim, D.H.; Han, M.Y.; Jee, H.M.; Kim, Y.K.; Kim, J.H. Bacterial Microbiota-derived Extracellular Vesicles in Children With Allergic Airway Diseases: Compositional and Functional Features. Allergy Asthma Immunol. Res. 2021, 13, 56–74. [Google Scholar] [CrossRef] [PubMed]

| Parental Cell | Evidences on EV Release and Function | References |
|---|---|---|
| Immune cells | ||
| Eosinophils | Eosinophil-derived EVs in asthma promote epithelial apoptosis, muscle proliferation, and eosinophil chemotaxis. IFN-γ induced eosinophil EVs are increased in asthma. | [102,103,109] |
| Mast cells | EVs released by FcεRI-engaged Mast Cells contain FcεRI/IgE/antigen complexes and amplify allergic responses by allowing antigen persistency. Mast cell-derived EVs, enriched in MHC class II, ICAM-1, and CD86, induce B and T cell proliferation. | [100,111] |
| Alveolar macrophages | Macrophage-derived EVs inhibited the activation of STATs in epithelial cells through SOCS. Macrophages-derived EVs promote granulocyte migration. | [104,112] |
| B lymphocytes | B-cells release EVs enriched in MHC class II molecule that induce antigen-specific T cell response. | [117] |
| T lymphocytes | T cell-derived EVs favour mast cell activation carrying miR-4443. | [106,120] |
| Neutrophils | EVs released by LPS-activated neutrophils enhance airway smooth muscle proliferation through immune-related proteins, including tenascin-X. | [105,123] |
| Dendritic cells | EVs secreted by dendritic cells (DC) induce antigen-specific CD8+ T-cells and antigen-specific IgG production. EVs released by DC promote CD4+ T cell proliferation and Th2 differentiation through OX40L. | [108,114] |
| Non immune cells | ||
| Epithelial Cells | Pulmonary epithelial cells are the main source of EVs in normal lungs, further amplified by inflammatory stimuli. In Th2 setting epithelial-derived EVs, enriched in specific miRNAs, stimulate airway inflammation and remodeling. EV populations isolated from the apical side differ from the basolateral (in size and miRNA content). | [74,75,78,81,99] |
| Fibroblasts | Fibroblasts-derived EVs from asthmatics expressing lower levels of TGF-β2 increase epithelial cell proliferation. | [124] |
| Mesenchymal Cells | Adipose-derived EVs, which contain miR-301a-3p, limit smooth muscle cells proliferation and migration. Hypoxic umbilical cord mesenchymal cell-derived EVs, enriched in miR-146a-5p, attenuate allergic airway inflammation and remodeling in a chronic asthma model. | [128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinè, M.; Padrin, Y.; Bonato, M.; Semenzato, U.; Bazzan, E.; Conti, M.; Saetta, M.; Turato, G.; Baraldo, S. Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma. Int. J. Mol. Sci. 2023, 24, 4645. https://doi.org/10.3390/ijms24054645
Tinè M, Padrin Y, Bonato M, Semenzato U, Bazzan E, Conti M, Saetta M, Turato G, Baraldo S. Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma. International Journal of Molecular Sciences. 2023; 24(5):4645. https://doi.org/10.3390/ijms24054645
Chicago/Turabian StyleTinè, Mariaenrica, Ylenia Padrin, Matteo Bonato, Umberto Semenzato, Erica Bazzan, Maria Conti, Marina Saetta, Graziella Turato, and Simonetta Baraldo. 2023. "Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma" International Journal of Molecular Sciences 24, no. 5: 4645. https://doi.org/10.3390/ijms24054645
APA StyleTinè, M., Padrin, Y., Bonato, M., Semenzato, U., Bazzan, E., Conti, M., Saetta, M., Turato, G., & Baraldo, S. (2023). Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma. International Journal of Molecular Sciences, 24(5), 4645. https://doi.org/10.3390/ijms24054645






