Sex Differences in Inflammation and Muscle Wasting in Aging and Disease
Abstract
1. Introduction
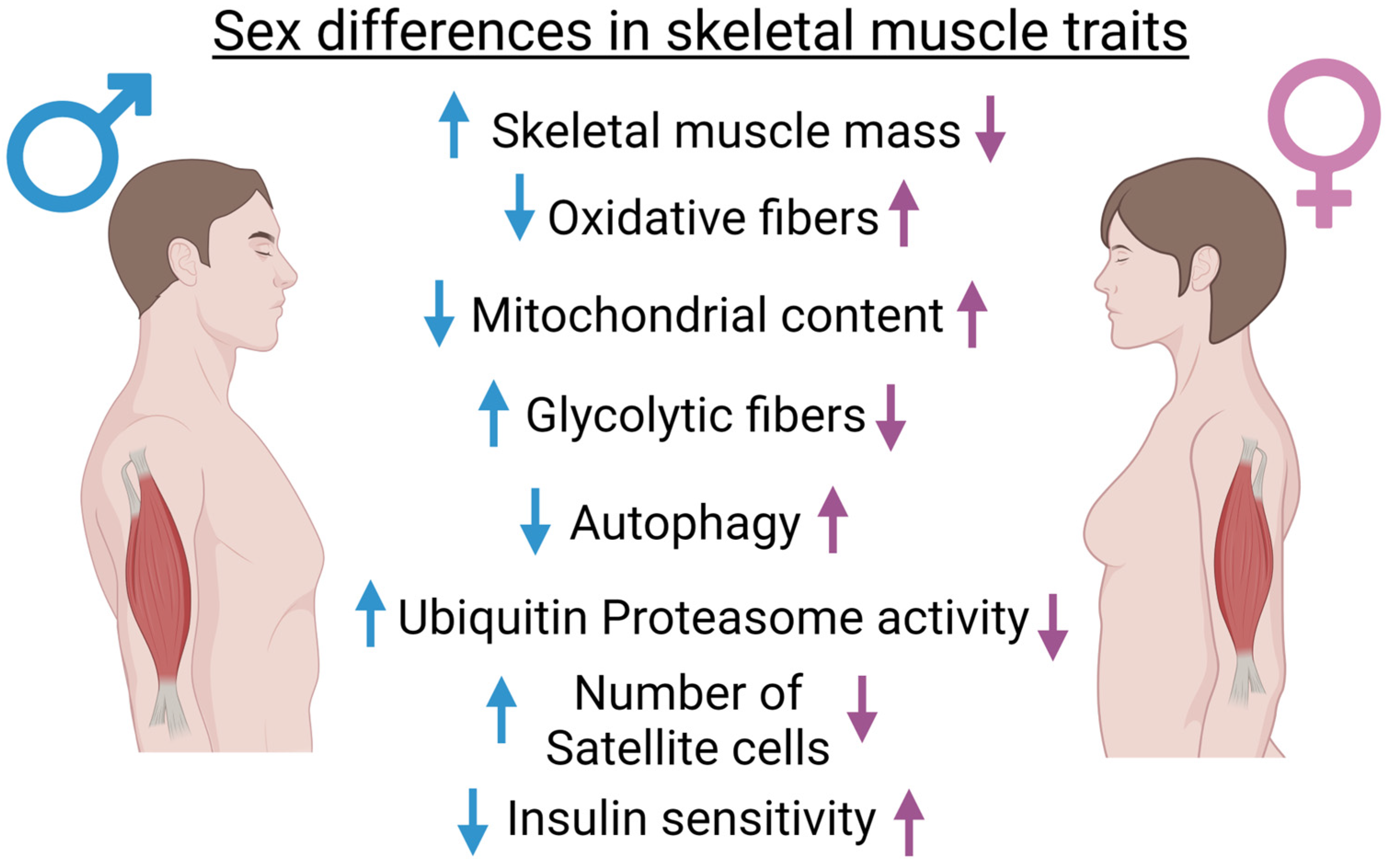
2. Sex Differences in Muscle Homeostasis and Metabolism
3. Sex Differences in Muscle Atrophy Associated with Disuse and Denervation
3.1. Major Differences in Clinical and Pre-Clinical Phenotypes
3.2. Molecular Mechanisms and Sex Differences in Disuse Muscle Atrophy
4. Sex Differences in Aging-Associated Sarcopenia
4.1. Major Differences in Clinical and Pre-Clinical Phenotypes
4.2. Possible Mechanisms Accounting Sex Differences in Aging-Associated Sarcopenia
5. Sex Differences in Cancer Cachexia
5.1. Major Differences in Clinical and Pre-Clinical Phenotypes
5.2. Sex Differences in the Mechanisms Leading to Cachexia
6. Sex Differences in Immune Responses and Inflammation
6.1. Major Differences in the Inflammatory Response
6.2. Inflammation throughout Different Conditions of Muscle Atrophy
7. Conclusions
7.1. Inflammation-Driven Muscle Atrophy: Are Cytokines the Culprit?
7.2. Which Direction Shall We Go?
7.3. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, X.; Berletch, J.B.; Nguyen, D.K.; Disteche, C.M. X chromosome regulation: Diverse patterns in development, tissues and disease. Nat. Rev. Genet. 2014, 15, 367–378. [Google Scholar] [CrossRef]
- Juchniewicz, P.; Piotrowska, E.; Kloska, A.; Podlacha, M.; Mantej, J.; Węgrzyn, G.; Tukaj, S.; Jakóbkiewicz-Banecka, J. Dosage Compensation in Females with X-Linked Metabolic Disorders. Int. J. Mol. Sci. 2021, 22, 4514. [Google Scholar] [CrossRef] [PubMed]
- Migeon, B.R. Why females are mosaics, x-chromosome inactivation, and sex differences in disease. Gend. Med. 2007, 4, 97–105. [Google Scholar] [CrossRef]
- Duman, R.S. Sex-specific disease-associated modules for depression. Nat. Med. 2017, 23, 1015–1017. [Google Scholar] [CrossRef]
- Shakeshaft, A.; Panjwani, N.; Collingwood, A.; Crudgington, H.; Hall, A.; Andrade, D.M.; Beier, C.P.; Fong, C.Y.; Gardella, E.; Gesche, J.; et al. Sex-specific disease modifiers in juvenile myoclonic epilepsy. Sci. Rep. 2022, 12, 2785. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Dell’istituto Super. Di Sanita 2016, 52, 205–212. [Google Scholar] [CrossRef]
- Foltz, S.; Wu, F.; Ghazal, N.; Kwong, J.Q.; Hartzell, H.C.; Choo, H.J. Sex differences in the involvement of skeletal and cardiac muscles in myopathic Ano5−/− mice. Am. J. Physiol. Physiol. 2022, 322, C283–C295. [Google Scholar] [CrossRef] [PubMed]
- Winham, S.J.; de Andrade, M.; Miller, V.M. Genetics of cardiovascular disease: Importance of sex and ethnicity. Atherosclerosis 2015, 241, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.-C.; Efron, P.A.; Ozrazgat-Baslanti, T.; Zhang, J.; Cuschieri, J.; Maier, R.V.; Minei, J.P.; Baker, H.V.; Moore, F.A.; Moldawer, L.L.; et al. Sex-based differences in the genomic response, innate immunity, organ dysfunction, and clinical outcomes after severe blunt traumatic injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2016, 81, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Wyld, M.L.; De La Mata, N.L.; Viecelli, A.; Swaminathan, R.; O’Sullivan, K.M.; O’Lone, E.; Rowlandson, M.; Francis, A.; Wyburn, K.; Webster, A.C. Sex-Based Differences in Risk Factors and Complications of Chronic Kidney Disease. Semin. Nephrol. 2022, 42, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Vinnik, T.; Kreinin, A.; Abildinova, G.; Batpenova, G.; Kirby, M.; Pinhasov, A. Biological Sex and IgE Sensitization Influence Severity of Depression and Cortisol Levels in Atopic Dermatitis. Dermatology 2020, 236, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Muscat, J.; Gaudet, M.M.; Olshan, A.F.; Curado, M.P.; Maso, L.D.; Wünsch-Filho, V.; Sturgis, E.M.; Szeszenia-Dabrowska, N.; Castellsagué, X.; et al. An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case–control studies. Cancer Causes Control. 2011, 22, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, M.A.; Brooks, H.L. Sex-Specific Mechanisms in Inflammation and Hypertension. Curr. Hypertens. Rep. 2019, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Giesen, N.; Chatterjee, M.; Scheid, C.; Poos, A.M.; Besemer, B.; Miah, K.; Benner, A.; Becker, N.; Moehler, T.; Metzler, I.; et al. A phase II clinical trial of combined BRAF/MEK inhibition for BRAFV600E-mutated multiple myeloma. Blood 2023. [Google Scholar] [CrossRef]
- Wolff, C.A.; Lawrence, M.M.; Porter, H.; Zhang, Q.; Reid, J.J.; Laurin, J.L.; Musci, R.V.; Linden, M.A.; Peelor, F.F.; Wren, J.D.; et al. Sex differences in changes of protein synthesis with rapamycin treatment are minimized when metformin is added to rapamycin. Geroscience 2020, 43, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-activated STAT3–IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nature 2018, 20, 917–927. [Google Scholar] [CrossRef]
- Riera, C.S.; Lozanoska-Ochser, B.; Testa, S.; Fornetti, E.; Bouché, M.; Madaro, L. Muscle Diversity, Heterogeneity, and Gradients: Learning from Sarcoglycanopathies. Int. J. Mol. Sci. 2021, 22, 2502. [Google Scholar] [CrossRef]
- Ji, H.; Kwan, A.C.; Chen, M.T.; Ouyang, D.; Ebinger, J.E.; Bell, S.P.; Niiranen, T.J.; Bello, N.A.; Cheng, S. Sex Differences in Myocardial and Vascular Aging. Circ. Res. 2022, 130, 566–577. [Google Scholar] [CrossRef]
- Kozdag, G.; Ertas, G.; Emre, E.; Akay, Y.; Celikyurt, U.; Sahin, T.; Gorur, G.; Karauzum, K.; Yilmaz, I.; Ural, D.; et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex. Hear. Inst. J. 2013, 40, 521–528. [Google Scholar]
- Liu, M.; Zhang, Z.; Zhou, C.; Ye, Z.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Qin, X. Predicted fat mass and lean mass in relation to all-cause and cause-specific mortality. J. Cachex-Sarcopenia Muscle 2022, 13, 1064–1075. [Google Scholar] [CrossRef]
- Nunes, E.A.; Stokes, T.; McKendry, J.; Currier, B.S.; Phillips, S.M. Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: Mechanisms, prevention, and recovery strategies. Am. J. Physiol. Physiol. 2022, 322, C1068–C1084. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, N.; Nanda, P.; Devi, S.; Mohapatra, S. Sarcopenia: An Age-Related Multifactorial Disorder. Curr. Aging Sci. 2022. [Google Scholar] [CrossRef]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef]
- Berardi, E.; Madaro, L.; Lozanoska-Ochser, B.; Adamo, S.; Thorrez, L.; Bouche, M.; Coletti, D. A pound of flesh: What cachexia is and what it is not. Diagnostics 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2003, 18, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bouchè, M.; Lozanoska-Ochser, B.; Proietti, D.; Madaro, L. Do neurogenic and cancer-induced muscle atrophy follow common or divergent paths? Eur. J. Transl. Myol. 2018, 28, 7931. [Google Scholar] [CrossRef] [PubMed]
- Pigna, E.; Sanna, K.; Coletti, D.; Li, Z.; Parlakian, A.; Adamo, S.; Moresi, V. Increasing autophagy does not affect neurogenic muscle atrophy. Eur. J. Transl. Myol. 2018, 28, 7687. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. BioMed Cent. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.; Ono-Moore, K.D.; Chintapalli, S.V.; Rutkowsky, J.M.; Tolentino, T.; Lloyd, K.C.K.; Olfert, I.M.; Adams, S.H. Sex differences in skeletal muscle revealed through fiber type, capillarity, and transcriptomics profiling in mice. Physiol. Rep. 2021, 9, e15031. [Google Scholar] [CrossRef] [PubMed]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.M.; Bedrin, N.G.; Subramanian, M.; Berking, J.; Ades, P.A.; Toth, M.J.; Miller, M.S. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: Relationship to single-fiber function. J. Appl. Physiol. 2014, 116, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachex-Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Reiß, J.; Schulzke, J.; Valentini, L.; Pirlich, M. Effect of sexual dimorphism on muscle strength in cachexia. J. Cachex-Sarcopenia Muscle 2012, 3, 111–116. [Google Scholar] [CrossRef]
- De Jonghe, B. Paresis Acquired in the Intensive Care UnitA Prospective Multicenter Study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.C.; Fu, M.H.; Isfort, R.J.; Varbanov, A.R.; Qu, X.A.; Tarnopolsky, M.A. Sex Differences in Global mRNA Content of Human Skeletal Muscle. PLoS ONE 2009, 4, e6335. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Alcolea, M.P.; Valle, A.; Oliver, J.O.; Roca, P.; García-Palmer, F.J. Skeletal Muscle of Female Rats Exhibit Higher Mitochondrial Mass and Oxidative-Phosphorylative Capacities Compared to Males. Cell. Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Madsen, K.; Meinild-Lundby, A.-K.; Edin, F.; Lundby, C. Sexual dimorphism of substrate utilization: Differences in skeletal muscle mitochondrial volume density and function. Exp. Physiol. 2018, 103, 851–859. [Google Scholar] [CrossRef]
- Miotto, P.M.; McGlory, C.; Holloway, T.M.; Phillips, S.M.; Holloway, G.P. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R909–R915. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Lim, S.; Haynie, W.A.; Brown, J.L.; Deaver, J.W.; Da Silva, F.M.; Jansen, L.T.; Lee, D.E.; Wiggs, M.P.; Washington, T.A.; et al. Female mice may have exacerbated catabolic signalling response compared to male mice during development and progression of disuse atrophy. J. Cachex-Sarcopenia Muscle 2021, 12, 717–730. [Google Scholar] [CrossRef]
- Thompson, J.R.; Swanson, S.A.; Casale, G.P.; Johanning, J.M.; Papoutsi, E.; Koutakis, P.; Miserlis, D.; Zhu, Z.; Pipinos, I.I. Gastrocnemius mitochondrial respiration: Are there any differences between men and women? J. Surg. Res. 2013, 185, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Tunstal, R.J.; Mehan, K.A.; Cameron-Smith, D.; McKenna, M.J.; Spriet, L.L.; Hargreaves, M.; Snow, R.J. Human skeletal muscle creatine transporter mRNA and protein expression in healthy, young males and females. Mol. Cell. Biochem. 2003, 244, 151–157. [Google Scholar] [CrossRef]
- Beaudry, K.M.; Devries, M.C. Sex-based differences in hepatic and skeletal muscle triglyceride storage and metabolism. Appl. Physiol. Nutr. Metab. 2019, 44, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Mittendorfer, B. Sexual dimorphism in skeletal muscle protein turnover. J. Appl. Physiol. 2016, 120, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.S.; Choi, J.-W.; Choi, D.K.; Mukherjee, R.; Liu, H.; Yun, J.W. Gender Dimorphism in Skeletal Muscle Proteome Between Lean and Diet-induced Obese Rats. Cell. Physiol. Biochem. 2011, 28, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Church, D.D.; Kim, I.; Park, S.; Wolfe, R.R.; Ferrando, A.A. Comparison of basal whole-body protein kinetics and muscle protein synthesis between young and older adults. Physiol. Rep. 2020, 8, e14633. [Google Scholar] [CrossRef]
- Hansen, M.; Kjaer, M. Influence of Sex and Estrogen on Musculotendinous Protein Turnover at Rest and After Exercise. Exerc. Sport Sci. Rev. 2014, 42, 183–192. [Google Scholar] [CrossRef]
- West, D.W.D.; Burd, N.; Churchward-Venne, T.A.; Camera, D.; Mitchell, C.; Baker, S.K.; Hawley, J.; Coffey, V.G.; Phillips, S. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J. Appl. Physiol. 2012, 112, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.A.; Barrett, E.J.; Genco, M.L.; Wei, L.; Spraggins, T.A.; Fryburg, D.A. Tissue Composition Affects Measures of Postabsorptive Human Skeletal Muscle Metabolism: Comparison across Genders 1. J. Clin. Endocrinol. Metab. 1999, 84, 1007–1010. [Google Scholar] [CrossRef]
- Horstman, A.M.H.; Kouw, I.W.K.; van Dijk, J.-W.; Hamer, H.M.; Groen, B.B.L.; van Kranenburg, J.; Gorissen, S.H.M.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Whey Protein Ingestion Is Greater in Middle-Aged Women Compared With Men. J. Clin. Endocrinol. Metab. 2018, 104, 994–1004. [Google Scholar] [CrossRef]
- Coletti, D.; Teodori, L.; Lin, Z.; Beranudin, J.F.; Adamo, S. Restoration versus reconstruction: Cellular mechanisms of skin, nerve and muscle regeneration compared. Regen. Med. Res. 2013, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Beltrà, M.; Pin, F.; Costamagna, D.; Duelen, R.; Renzini, A.; Ballarò, R.; Garcia-Castillo, L.; Iannuzzi, A.; Moresi, V.; Coletti, D.; et al. PGC-1α in the myofibers regulates the balance between myogenic and adipogenic progenitors affecting muscle regeneration. iScience 2022, 25, 105480. [Google Scholar] [CrossRef]
- Toschi, A.; Severi, A.; Coletti, D.; Catizone, A.; Musarò, A.; Molinaro, M.; Nervi, C.; Adamo, S.; Scicchitano, B.M. Skeletal Muscle Regeneration in Mice Is Stimulated by Local Overexpression of V1a-Vasopressin Receptor. Mol. Endocrinol. 2011, 25, 1661–1673. [Google Scholar] [CrossRef][Green Version]
- Benedetti, A.; Fiore, P.F.; Madaro, L.; Lozanoska-Ochser, B.; Bouché, M. Targeting PKCθ Promotes Satellite Cell Self-Renewal. Int. J. Mol. Sci. 2020, 21, 2419. [Google Scholar] [CrossRef]
- Fiore, P.F.; Benedetti, A.; Sandonà, M.; Madaro, L.; De Bardi, M.; Saccone, V.; Puri, P.L.; Gargioli, C.; Lozanoska-Ochser, B.; Bouché, M. Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury. Int. J. Mol. Sci. 2020, 21, 932. [Google Scholar] [CrossRef] [PubMed]
- Zhenlin, L.; Parlakian, A.; Coletti, D.; Alonso-Martin, S.; Hourdé, C.; Joanne, P.; Gao-Li, J.; Blanc, J.; Ferry, A.; Paulin, D.; et al. Synemin acts as a regulator of signalling molecules in skeletal muscle hypertrophy. J. Cell Sci. 2014, 127, 4589–4601. [Google Scholar] [CrossRef]
- Musarò, A.; Giacinti, C.; Pelosi, L.; Dobrowolny, G.; Barberi, L.; Nardis, C.; Coletti, D.; Scicchitano, B.M.; Adamo, S.; Molinaro, M. Stem cell-mediated muscle regeneration and repair in aging and neuromuscular diseases. Eur. J. Histochem. 2007, 51. [Google Scholar]
- Neal, A.; Boldrin, L.; Morgan, J.E. The Satellite Cell in Male and Female, Developing and Adult Mouse Muscle: Distinct Stem Cells for Growth and Regeneration. PLoS ONE 2012, 7, e37950. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.; Toivonen, J.M.; Calvo, A.C.; Miana-Mena, F.J.; Zaragoza, P.; Muñoz, M.J.; Montarras, D.; Osta, R. Sex, fiber-type, and age dependent in vitro proliferation of mouse muscle satellite cells. J. Cell. Biochem. 2011, 112, 2825–2836. [Google Scholar] [CrossRef]
- Lee, D.-M.; Bajracharya, P.; Lee, E.J.; Kim, J.-E.; Lee, H.-J.; Chun, T.; Kim, J.; Cho, K.H.; Chang, J.; Hong, S.; et al. Effects of gender-specific adult bovine serum on myogenic satellite cell proliferation, differentiation and lipid accumulation. Vitr. Cell. Dev. Biol.-Anim. 2011, 47, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Horwath, O.; Moberg, M.; Larsen, F.J.; Philp, A.; Apró, W.; Ekblom, B. Influence of sex and fiber type on the satellite cell pool in human skeletal muscle. Scand. J. Med. Sci. Sport. 2020, 31, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Bahri, O.A.; Naldaiz-Gastesi, N.; Kennedy, D.C.; Wheatley, A.M.; Izeta, A.; McCullagh, K.J.A. The panniculus carnosus muscle: A novel model of striated muscle regeneration that exhibits sex differences in the mdx mouse. Sci. Rep. 2019, 9, 15964. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Tawil, R.; Thornton, C.A. Sex-Related Differences in Gene Expression in Human Skeletal Muscle. PLoS ONE 2008, 3, e1385. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Giza, C.C.; Serpa, R.O.; Greco, T.; Robert, H.; Folkerts, M.; Prins, M.L. Sex Differences in Neurophysiological Changes Following Voluntary Exercise in Adolescent Rats. Front. Neurol. 2021, 12, 685822. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Brobst, D.; Chan, W.S.; Tse, M.C.L.; Herlea-Pana, O.; Ahuja, P.; Bi, X.; Zaw, A.M.; Kwong, Z.S.W.; Jia, W.-H.; et al. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci. Signal. 2019, 12, eaau1468. [Google Scholar] [CrossRef]
- Jia, W.-H.; Wang, N.-Q.; Yin, L.; Chen, X.; Hou, B.-Y.; Qiang, G.-F.; Chan, C.B.; Yang, X.-Y.; Du, G.-H. Effect of skeletal muscle phenotype and gender on fasting-induced myokine expression in mice. Biochem. Biophys. Res. Commun. 2019, 514, 407–414. [Google Scholar] [CrossRef]
- Molinero, A.; Fernandez-Perez, A.; Mogas, A.; Giralt, M.; Comes, G.; Fernandez-Gayol, O.; Vallejo, M.; Hidalgo, J. Role of muscle IL-6 in gender-specific metabolism in mice. PLoS ONE 2017, 12, e0173675. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.A.; Bs, J.A.F.; Phillips, C.; Brown, M. Hindlimb skeletal muscle function in myostatin-deficient mice. Muscle Nerve 2010, 43, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Osepchook, C.C.; Jeanplong, F.; Falconer, S.J.; Matthews, K.G.; Conaglen, J.V.; Gerrard, D.F.; Smith, H.K.; Wilkins, R.J.; Bass, J.J.; et al. The decrease in mature myostatin protein in male skeletal muscle is developmentally regulated by growth hormone. J. Physiol. 2009, 587, 669–677. [Google Scholar] [CrossRef]
- Paul, R.G.; Hennebry, A.S.; Elston, M.S.; Conaglen, J.V.; McMahon, C.D. Regulation of murine skeletal muscle growth by STAT5B is age- and sex-specific. Skelet. Muscle 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Jia, W.-H.; Wang, N.-Q.; Yin, L.; Chen, X.; Hou, B.-Y.; Wang, J.-H.; Qiang, G.-F.; Chan, C.B.; Yang, X.-Y.; Du, G.-H. Effects of fasting on the expression pattern of FGFs in different skeletal muscle fibre types and sexes in mice. Biol. Sex Differ. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bredewold, R.; Veenema, A.H. Sex differences in the regulation of social and anxiety-related behaviors: Insights from vasopressin and oxytocin brain systems. Curr. Opin. Neurobiol. 2018, 49, 132–140. [Google Scholar] [CrossRef]
- Adamo, S.; Pigna, E.; Lugarà, R.; Moresi, V.; Coletti, D.; Bouché, M. Skeletal Muscle: A Significant Novel Neurohypophyseal Hormone-Secreting Organ. Front. Physiol. 2019, 9, 1885. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Rossi, E.; Scicchitano, B.M.; Coletti, D.; Moresi, V.; Adamo, S. Neurohypophyseal hormones: Novel actors of striated muscle development and homeostasis. Eur. J. Transl. Myol. 2014, 24, 3790. [Google Scholar] [CrossRef][Green Version]
- Naro, F.; De Arcangelis, V.; Coletti, D.; Molinaro, M.; Zani, B.; Vassanelli, S.; Reggiani, C.; Teti, A.; Adamo, S. Increase in cytosolic Ca2+induced by elevation of extracellular Ca2+in skeletal myogenic cells. Am. J. Physiol. Physiol. 2003, 284, C969–C976. [Google Scholar] [CrossRef]
- Alvisi, M.; De Arcangelis, V.; Ciccone, L.; Palombi, V.; Alessandrini, M.; Nemoz, G.; Molinaro, M.; Adamo, S.; Naro, F. V1a vasopressin receptor expression is modulated during myogenic differentiation. Differentiation 2008, 76, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.; Diel, P. How Sex Hormones Promote Skeletal Muscle Regeneration. Sport Med. 2013, 43, 1089–1100. [Google Scholar] [CrossRef]
- Alexander, S.E.; Pollock, A.C.; Lamon, S. The effect of sex hormones on skeletal muscle adaptation in females. Eur. J. Sport Sci. 2021, 22, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- McMillin, S.L.; Minchew, E.C.; Lowe, D.A.; Spangenburg, E.E. Skeletal muscle wasting: The estrogen side of sexual dimorphism. Am. J. Physiol. Physiol. 2022, 322, C24–C37. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Fuxjager, M.J.; Miles, M.C.; Schlinger, B.A. Evolution of the androgen-induced male phenotype. J. Comp. Physiol. A 2017, 204, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Sex Differences in Exercise Metabolism and the Role of 17-Beta Estradiol. Med. Sci. Sports Exerc. 2008, 40, 648–654. [Google Scholar] [CrossRef]
- Lindegaard, B.; Abildgaard, J.; Heywood, S.E.; Pedersen, B.K.; Febbraio, M.A. Female sex hormones are necessary for the metabolic effects mediated by loss of Interleukin 18 signaling. Mol. Metab. 2018, 12, 89–97. [Google Scholar] [CrossRef]
- Brooks, N.E.; Myburgh, K.H. Skeletal muscle wasting with disuse atrophy is multi-dimensional: The response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 2014, 5, 99. [Google Scholar] [CrossRef]
- Lee, P.H.U.; Chung, M.; Ren, Z.; Mair, D.B.; Kim, D.-H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol. Physiol. 2022, 322, C567–C580. [Google Scholar] [CrossRef]
- Lipes, J.; Mardini, L.; Jayaraman, D. Sex and Mortality of Hospitalized Adults After Admission to an Intensive Care Unit. Am. J. Crit. Care 2013, 22, 314–319. [Google Scholar] [CrossRef]
- Yasuda, N.; Glover, E.I.; Phillips, S.; Isfort, R.J.; Tarnopolsky, M.A. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J. Appl. Physiol. 2005, 99, 1085–1092. [Google Scholar] [CrossRef]
- Miles, M.P.; Heil, D.P.; Larson, K.R.; Conant, S.B.; Schneider, S.M. Prior Resistance Training and Sex Influence Muscle Responses to Arm Suspension. Med. Sci. Sport Exerc. 2005, 37, 1983–1989. [Google Scholar] [CrossRef]
- Shaffer, N.C.; Huang, Y.; Abraham, D.S.; Cheng, Y.; Lu, W.; Gruber-Baldini, A.L.; Hochberg, M.C.; Guralnik, J.; Magaziner, J.; Orwig, D. Comparing Longitudinal Sarcopenia Trends by Definitions across Men and Women after Hip Fracture. J. Am. Geriatr. Soc. 2020, 68, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.-G.; Kwon, T.-Y.; Lee, K.-B. Rectus femoris muscle atrophy and recovery caused by preoperative pretibial traction in femoral shaft fractures-comparison between traction period. Orthop. Traumatol. Surg. Res. 2017, 103, 691–695. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; Hoffman, R.L.; Russ, D.W. Restoration of Voluntary Muscle Strength after 3 Weeks of Cast Immobilization is Suppressed in Women Compared with Men. Arch. Phys. Med. Rehabil. 2009, 90, 178–180. [Google Scholar] [CrossRef]
- Stroud, J.E.; Gale, M.S.; Zwart, S.R.; Heer, M.; Smith, S.M.; Montina, T.; Metz, G.A.S. Longitudinal metabolomic profiles reveal sex-specific adjustments to long-duration spaceflight and return to Earth. Cell. Mol. Life Sci. 2022, 79, 1–18. [Google Scholar] [CrossRef]
- Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Hackett, S.B.; Sharma, H.; Cruickshank, N.C.; Slauterbeck, J.R.; Savage, P.D.; Ades, P.A.; Maughan, D.W.; et al. Chronic disuse and skeletal muscle structure in older adults: Sex-specific differences and relationships to contractile function. Am. J. Physiol. Physiol. 2015, 308, C932–C943. [Google Scholar] [CrossRef]
- Callahan, D.M.; Miller, M.S.; Sweeny, A.P.; Tourville, T.W.; Slauterbeck, J.R.; Savage, P.D.; Maugan, D.W.; Ades, P.A.; Beynnon, B.D.; Toth, M.J. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J. Physiol. 2014, 592, 4555–4573. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef]
- Carraro, U.; Coletti, D.; Kern, H. The Ejtm Specials “The Long-Term Denervated Muscle”. Eur. J. Transl. Myol. 2014, 24. [Google Scholar] [CrossRef]
- Oga, S.; Goto, K.; Sakamoto, J.; Honda, Y.; Sasaki, R.; Ms, K.I.; Kataoka, H.; Nakano, J.; Origuchi, T.; Okita, M. Mechanisms underlying immobilization-induced muscle pain in rats. Muscle Nerve 2020, 61, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, P.A.; Kneppers, A.E.M.; Schols, A.M.W.J.; Kelders, M.C.J.M.; De Theije, C.C.; Verdijk, L.B.; van Loon, L.J.C.; Langen, R.C.J.; Gosker, H.R. Skeletal muscle unloading results in increased mitophagy and decreased mitochondrial biogenesis regulation. Muscle Nerve 2019, 60, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.A.; Thomopoulos, S.; Abu-Amer, Y.; Shen, K.C. Tenotomy-induced muscle atrophy is sex-specific and independent of NFκB. eLife 2022, 11, e82016. [Google Scholar] [CrossRef]
- Deguise, M.-O.; De Repentigny, Y.; Tierney, A.; Beauvais, A.; Michaud, J.; Chehade, L.; Thabet, M.; Paul, B.; Reilly, A.; Gagnon, S.; et al. Motor transmission defects with sex differences in a new mouse model of mild spinal muscular atrophy. Ebiomedicine 2020, 55, 102750. [Google Scholar] [CrossRef]
- Renzini, A.; Pigna, E.; Rocchi, M.; Cedola, A.; Gigli, G.; Moresi, V.; Coletti, D. Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice. Int. J. Mol. Sci. 2022, 24, 98. [Google Scholar] [CrossRef]
- Furukawa, T.; Nakao, K.; Sugita, H.; Tsukagoshi, H. Kugelberg-Welander Disease: With Particular Reference to Sex-Influenced Manifestations. Arch. Neurol. 1968, 19, 156–162. [Google Scholar] [CrossRef]
- Tobias, M.; Marin, M.; Kelley, D. The roles of sex, innervation, and androgen in laryngeal muscle of Xenopus laevis. J. Neurosci. 1993, 13, 324–333. [Google Scholar] [CrossRef]
- Kume-Kick, J.; Strand, F.L. Sex Hormones Affect Muscle Contractility and Motor Functional Recovery Following Peroneal Nerve Crush. Exp. Neurol. 1994, 128, 115–123. [Google Scholar] [CrossRef]
- Musacchia, X.J.; Steffen, J.M.; Fell, R.D. Disuse atrophy of skeletal muscle: Animal models. Exerc. Sport Sci. Rev. 1988, 16, 61–88. [Google Scholar] [CrossRef]
- Coletti, D.; Aulino, P.; Pigna, E.; Barteri, F.; Moresi, V.; Annibali, D.; Adamo, S.; Berardi, E. Spontaneous Physical Activity Downregulates Pax7 in Cancer Cachexia. Stem Cells Int. 2015, 2016, 1–9. [Google Scholar] [CrossRef]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-κB–mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Natsume, T.; Tsuzuki, T.; Chang, S.-W.; Kakigi, R.; Sugiura, T.; Naito, H. Sex differences in forkhead box O3a signaling response to hindlimb unloading in rat soleus muscle. J. Physiol. Sci. 2018, 69, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Trevino, M.B.; Zhang, X.; Standley, R.A.; Wang, M.; Han, X.; dos Reis, F.C.G.; Periasamy, M.; Yu, G.; Kelly, D.P.; Goodpaster, B.H.; et al. Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy. Am. J. Physiol. Metab. 2019, 317, E899–E910. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Lim, S.; Haynie, W.S.; Jansen, L.T.; Westervelt, L.C.; Amos, M.G.; Washington, T.A.; Greene, N.P. Altering aspects of mitochondrial quality to improve musculoskeletal outcomes in disuse atrophy. J. Appl. Physiol. 2020, 129, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Lim, S.; Haynie, W.S.; Brown, J.L.; Lee, D.E.; Dunlap, K.R.; Jansen, L.T.; Washington, T.A.; Wiggs, M.P.; Greene, N.P. Mitochondrial aberrations during the progression of disuse atrophy differentially affect male and female mice. J. Cachex-Sarcopenia Muscle 2021, 12, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; DeRuisseau, K.C. Mechanisms of disuse muscle atrophy: Role of oxidative stress. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R337–R344. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Roubenoff, R.; Hughes, V.A. Sarcopenia: Current Concepts. J. Gerontol. Ser. A 2000, 55, M716–M724. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Renzini, A.; Cavioli, G.; Seelaender, M.; Coletti, D.; Gigli, G.; Cedola, A. Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy. Metabolites 2022, 12, 1149. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Park, S. Sex Differences of Sarcopenia in an Elderly Asian Population: The Prevalence and Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 11980. [Google Scholar] [CrossRef]
- Gallagher, D.; Ruts, E.; Visser, M.; Heshka, S.; Baumgartner, R.N.; Wang, J.; Pierson, R.N.; Pi-Sunyer, F.X.; Heymsfield, S.B. Weight stability masks sarcopenia in elderly men and women. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E366–E375. [Google Scholar] [CrossRef]
- Kasai, T.; Ishiguro, N.; Matsui, Y.; Harada, A.; Takemura, M.; Yuki, A.; Kato, Y.; Otsuka, R.; Ando, F.; Shimokata, H. Sex- and age-related differences in mid-thigh composition and muscle quality determined by computed tomography in middle-aged and elderly Japanese. Geriatr. Gerontol. Int. 2014, 15, 700–706. [Google Scholar] [CrossRef]
- Haynes, E.M.K.; Neubauer, N.; Cornett, K.; O’Connor, B.P.; Jones, G.R.; Jakobi, J.M. Age and sex-related decline of muscle strength across the adult lifespan: A scoping review of aggregated data. Appl. Physiol. Nutr. Metab. 2020, 45, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Matsui, Y.; Tomida, M.; Suzuki, Y.; Nishita, Y.; Tange, C.; Shimokata, H.; Imagama, S.; Otsuka, R.; Arai, H. Differences in the mass and quality of the quadriceps with age and sex and their relationships with knee extension strength. J. Cachex-Sarcopenia Muscle 2021, 12, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Bedrin, N.G.; Callahan, D.M.; Previs, M.J.; Jennings, M.E., II; Ades, P.A.; Maughan, D.W.; Palmer, B.M.; Toth, M.J. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J. Appl. Physiol. 2013, 115, 1004–1014. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Yu, F.; Höök, P.; Ramamurthy, B.; Marx, J.O.; Pircher, P. Effects of aging on regulation of muscle contraction at the motor unit, muscle cell, and molecular levels. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S28–S43. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.; Won, C.W. Sex differences in impact of sarcopenia on falls in community-dwelling Korean older adults. BMC Geriatr. 2021, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Qualls, C.R.; Cesari, M.; Rolland, Y.; Vlietstra, L.; Vellas, B. Relationship of Incident Falls with Balance Deficits and Body Composition in Male and Female Community-Dwelling Elders. J. Nutr. Health Aging 2018, 23, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L.; Féart, C.; Mann, G.; Viña, J.; Chatterji, S.; Chodzko-Zajko, W.; Harmand, M.G.-C.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J. Gerontol. Ser. A 2012, 68, 62–67. [Google Scholar] [CrossRef]
- Gordon, E.; Peel, N.; Samanta, M.; Theou, O.; Howlett, S.; Hubbard, R. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2016, 89, 30–40. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Voshaar, R.C.O. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Mayerl, H.; Stolz, E.; Freidl, W. Frailty and depression: Reciprocal influences or common causes? Soc. Sci. Med. 2020, 263, 113273. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell. Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paturi, S.; Gutta, A.K.; Katta, A.; Kakarla, S.K.; Arvapalli, R.K.; Gadde, M.K.; Nalabotu, S.K.; Rice, K.M.; Wu, M.; Blough, E. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech. Ageing Dev. 2010, 131, 202–209. [Google Scholar] [CrossRef]
- Szulc, P.; Schoppet, M.; Goettsch, C.; Rauner, M.; Dschietzig, T.B.; Chapurlat, R.; Hofbauer, L.C. Endocrine and Clinical Correlates of Myostatin Serum Concentration in Men—the STRAMBO Study. J. Clin. Endocrinol. Metab. 2012, 97, 3700–3708. [Google Scholar] [CrossRef]
- Peng, L.-N.; Lee, W.-J.; Liu, L.-K.; Lin, M.-H.; Chen, L.-K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachex-Sarcopenia Muscle 2018, 9, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ratkevicius, A.; Joyson, A.; Selmer, I.; Dhanani, T.; Grierson, C.; Tommasi, A.M.; Devries, A.; Rauchhaus, P.; Crowther, D.; Alesci, S.; et al. Serum Concentrations of Myostatin and Myostatin-Interacting Proteins Do Not Differ Between Young and Sarcopenic Elderly Men. J. Gerontol. Ser. A 2011, 66, 620–626. [Google Scholar] [CrossRef]
- Fife, E.; Kostka, J.; Kroc, Ł.; Guligowska, A.; Pigłowska, M.; Sołtysik, B.; Kaufman-Szymczyk, A.; Fabianowska-Majewska, K.; Kostka, T. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr. 2018, 18, 200. [Google Scholar] [CrossRef]
- Park, Y.-M.; Jankowski, C.M.; Ozemek, C.; Hildreth, K.L.; Kohrt, W.M.; Moreau, K.L. Appendicular lean mass is lower in late compared with early perimenopausal women: Potential role of FSH. J. Appl. Physiol. 2020, 128, 1373–1380. [Google Scholar] [CrossRef]
- La Colla, A.; Pronsato, L.; Milanesi, L.; Vasconsuelo, A. 17β-Estradiol and testosterone in sarcopenia: Role of satellite cells. Ageing Res. Rev. 2015, 24, 166–177. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lu, L.; Sun, Q.; Ye, X.; Sun, L.; Liu, X.; Zong, G.; Jin, Q.; Li, H.; Lin, X. Poor Vitamin D Status Is Prospectively Associated with Greater Muscle Mass Loss in Middle-Aged and Elderly Chinese Individuals. J. Acad. Nutr. Diet. 2014, 114, 1544–1551.e2. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Yun, S.; Oh, K.; Kim, K. Relation of serum 25-hydroxyvitamin D status with skeletal muscle mass by sex and age group among Korean adults. Br. J. Nutr. 2015, 114, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Aktas, A.; Lorton, C.M.; Griffin, O.; Higgins, K.; Roulston, F.; Stewart, G.; Corkery, N.; Barnes, E.; Walsh, D. Application of the 2011 international consensus cancer cachexia classification in routine oncology dietetic practice: An observational study. Nutr. Clin. Pract. 2022. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachex-Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: A meta-analysis. J. Cachex-Sarcopenia Muscle 2021, 12, 1122–1135. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, Estrogen Receptors Effects on Cardiac and Skeletal Muscle Mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Cardinale, D.A.; Larsen, F.J.; Schiffer, T.A.; Morales-Alamo, D.; Ekblom, B.; Calbet, J.A.L.; Holmberg, H.-C.; Boushel, R. Superior Intrinsic Mitochondrial Respiration in Women Than in Men. Front. Physiol. 2018, 9, 1133. [Google Scholar] [CrossRef]
- Baracos, V.E.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body composition in patients with non−small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010, 91, S1133–S1137. [Google Scholar] [CrossRef]
- Wallengren, O.; Iresjö, B.-M.; Lundholm, K.; Bosaeus, I. Loss of muscle mass in the end of life in patients with advanced cancer. Support. Care Cancer 2014, 23, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Anoveros-Barrera, A.; Bhullar, A.S.; Stretch, C.; Esfandiari, N.; Dunichand-Hoedl, A.R.; Martins, K.J.; Bigam, D.; Khadaroo, R.G.; McMullen, T.; Bathe, O.F.; et al. Clinical and biological characterization of skeletal muscle tissue biopsies of surgical cancer patients. J. Cachex-Sarcopenia Muscle 2019, 10, 1356–1377. [Google Scholar] [CrossRef]
- Burkart, M.; Schieber, M.; Basu, S.; Shah, P.; Venugopal, P.; Borgia, J.A.; Gordon, L.; Karmali, R. Evaluation of the impact of cachexia on clinical outcomes in aggressive lymphoma. Br. J. Haematol. 2019, 186, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Greenman, A.C.; Albrecht, D.M.; Halberg, R.B.; Diffee, G.M. Sex differences in skeletal muscle alterations in a model of colorectal cancer. Physiol. Rep. 2020, 8, e14391. [Google Scholar] [CrossRef]
- de Castro, G.S.; Simoes, E.; Lima, J.D.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef]
- Aversa, Z.; Pin, F.; Lucia, S.; Penna, F.; Verzaro, R.; Fazi, M.; Colasante, G.; Tirone, A.; Fanelli, F.R.; Ramaccini, C.; et al. Autophagy is induced in the skeletal muscle of cachectic cancer patients. Sci. Rep. 2016, 6, 30340. [Google Scholar] [CrossRef]
- Camargo, R.G.; Ribeiro, H.Q.T.; Geraldo, M.V.; Matos-Neto, E.; Neves, R.X.; Carnevali, L.C., Jr.; Donatto, F.F.; Alcântara, P.S.M.; Ottoch, J.P.; Seelaender, M. Cancer Cachexia and MicroRNAs. Mediat. Inflamm. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Lautaoja, J.H.; Lalowski, M.; Nissinen, T.A.; Hentilä, J.J.; Shi, Y.; Ritvos, O.; Cheng, S.; Hulmi, J.J. Muscle and serum metabolomes are dysregulated in colon-26 tumor-bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am. J. Physiol. Metab. 2019, 316, E852–E865. [Google Scholar] [CrossRef]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: Role of Activin. J. Cachex-Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Herpich, M.C.; Franz, M.K.; Ost, M.; Otten, L.; Coleman, V.; Klaus, S.; Müller-Werdan, U.; Norman, K. Associations Between Serum GDF15 Concentrations, Muscle Mass, and Strength Show Sex-Specific Differences in Older Hospital Patients. Rejuvenation Res. 2021, 24, 14–19. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.; Frandsen, U.; Prokhorova, T.; Kamper, R.S.; Haddock, B.; Aagaard, P.; Suetta, C. Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: The Copenhagen Sarcopenia Study. J. Cachex-Sarcopenia Muscle 2021, 12, 1418–1427. [Google Scholar] [CrossRef]
- Crunkhorn, S. Blocking GDF15 signalling reverses cachexia. Nat. Rev. Drug Discov. 2020, 19, 588. [Google Scholar] [CrossRef]
- Garcia, J.M.; Garcia-Touza, M.; Hijazi, R.A.; Taffet, G.; Epner, D.; Mann, D.; Smith, R.G.; Cunningham, G.R.; Marcelli, M. Active Ghrelin Levels and Active to Total Ghrelin Ratio in Cancer-Induced Cachexia. J. Clin. Endocrinol. Metab. 2005, 90, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Hetzler, K.L.; Hardee, J.P.; Puppa, M.J.; Narsale, A.A.; Sato, S.; Davis, J.M.; Carson, J.A. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 816–825. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef]
- Bonafè, M.; Olivieri, F.; Cavallone, L.; Giovagnetti, S.; Mayegiani, F.; Cardelli, M.; Pieri, C.; Marra, M.; Antonicelli, R.; Lisa, R.; et al. A gender--dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur. J. Immunol. 2001, 31, 2357–2361. [Google Scholar] [CrossRef]
- Hetzler, K.L.; Hardee, J.P.; LaVoie, H.A.; Murphy, E.A.; Carson, J.A. Ovarian function’s role during cancer cachexia progression in the female mouse. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E447–E459. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex Disparities in Cancer Incidence by Period and Age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex Disparities in Cancer Mortality and Survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef]
- Wilkinson, N.M.; Chen, H.-C.; Lechner, M.G.; Su, M.A. Sex Differences in Immunity. Annu. Rev. Immunol. 2022, 40, 75–94. [Google Scholar] [CrossRef]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.J.; Carson, B.D.; Subramanian, S.; Afentoulis, M.; Vandenbark, A.A.; Ziegler, S.F.; Offner, H. Cutting Edge: Estrogen Drives Expansion of the CD4+CD25+ Regulatory T Cell Compartment. J. Immunol. 2004, 173, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Teilmann, S.C.; Clement, C.A.; Thorup, J.; Byskov, A.G.; Christensen, S.T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J. Endocrinol. 2006, 191, 525–535. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Bupp, M.R.G.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Jenny, N.S. Inflammation in aging: Cause, effect, or both? Discov. Med. 2012, 13, 451–460. [Google Scholar] [PubMed]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells 2021, 10, 3402. [Google Scholar] [CrossRef]
- Mutin-Carnino, M.; Carnino, A.; Roffino, S.; Chopard, A. Effect of Muscle Unloading, Reloading and Exercise on Inflammation during a Head-down Bed Rest. Int. J. Sport. Med. 2013, 35, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Jurdana, M.; Jenko-Pražnikar, Z.; Mohorko, N.; Petelin, A.; Jakus, T.; Šimunič, B.; Pišot, R. Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age 2015, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Strollo, F.; Vernikos, J. Aging-like metabolic and adrenal changes in microgravity: State of the art in preparation for Mars. Neurosci. Biobehav. Rev. 2021, 126, 236–242. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- de Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin. Nutr. 2020, 40, 2443–2455. [Google Scholar] [CrossRef]
- Shang, M.; Cappellesso, F.; Amorim, R.; Serneels, J.; Virga, F.; Eelen, G.; Carobbio, S.; Rincon, M.Y.; Maechler, P.; De Bock, K.; et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 2020, 587, 626–631. [Google Scholar] [CrossRef]
- Rizzo, G.; Di Maggio, R.; Benedetti, A.; Morroni, J.; Bouche, M.; Lozanoska-Ochser, B. Splenic Ly6Chi monocytes are critical players in dystrophic muscle injury and repair. J. Clin. Investig. 2020, 5, e130807. [Google Scholar] [CrossRef]
- Lozanoska-Ochser, B.; Benedetti, A.; Rizzo, G.; Marrocco, V.; Di Maggio, R.; Fiore, P.; Bouche, M. Targeting early PKCθ-dependent T-cell infiltration of dystrophic muscle reduces disease severity in a mouse model of muscular dystrophy. J. Pathol. 2017, 244, 323–333. [Google Scholar] [CrossRef]
- Berardi, E.; Aulino, P.; Murfuni, I.; Toschi, A.; Padula, F.; Scicchitano, B.M.; Coletti, D.; Adamo, S. Skeletal muscle is enriched in hematopoietic stem cells and not inflammatory cells in cachectic mice. Neurol. Res. 2008, 30, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Coletti, D.; Moresi, V.; Adamo, S.; Molinaro, M.; Sassoon, D. Tumor necrosis factor-α gene transfer induces cachexia and inhibits muscle regeneration. Genes 2005, 43, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Garcia-Alvarez, G.; Pristerà, A.; Rizzuto, E.; Albertini, M.C.; Rocchi, M.; Marazzi, G.; Sassoon, D.; Adamo, S.; Coletti, D. Modulation of Caspase Activity Regulates Skeletal Muscle Regeneration and Function in Response to Vasopressin and Tumor Necrosis Factor. PLoS ONE 2009, 4, e5570. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sifuentes, Y.; Maney, D.L. Reporting and misreporting of sex differences in the biological sciences. eLife 2021, 10, e70817. [Google Scholar] [CrossRef]
- Torregrosa, C.; Chorin, F.; Beltran, E.E.M.; Neuzillet, C.; Cardot-Ruffino, V. Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives. Cancers 2022, 14, 5402. [Google Scholar] [CrossRef]
- Grande, A.J.; Silva, V.; Neto, L.S.; Basmage, J.P.T.; Peccin, M.S.; Maddocks, M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2021, 2021, CD010804. [Google Scholar] [CrossRef]
- Besson, T.; Macchi, R.; Rossi, J.; Morio, C.Y.M.; Kunimasa, Y.; Nicol, C.; Vercruyssen, F.; Millet, G.Y. Sex Differences in Endurance Running. Sport. Med. 2022, 52, 1235–1257. [Google Scholar] [CrossRef]
- Roberts, B.M.; Nuckols, G.; Krieger, J.W. Sex Differences in Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2020, 34, 1448–1460. [Google Scholar] [CrossRef]
- Kornstein, S. Exploring the Biological Contributions to Human Health: Does Sex Matter? J. Women’s Health Gender-Based Med. 2001, 10, 433–439. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef]
- Winkelman, C. Inactivity and Inflammation in the Critically Ill Patient. Crit. Care Clin. 2007, 23, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Terry, V.; Thomas, D.M. Precision medicine: Affording the successes of science. Npj Precis. Oncol. 2023, 7, 3. [Google Scholar] [CrossRef]

| Muscle Conditions | Sex-Related Differences in Muscle Traits | Reference(s) |
|---|---|---|
| Physiological | Mass | [30] |
| Energy metabolism | [32,33,34] | |
| Mitochondrial content | [42] | |
| Protein turnover | [47,50] | |
| Insulin sensitivity | [66,88] | |
| Muscle regeneration | [61,62,65] | |
| Disuse | Muscle weight | [38,93] |
| Muscle force | [92,98] | |
| Myofilament cross bridge kinetics | [99] | |
| Recovery | [96] | |
| Aging | Muscle weight | [126,127] |
| Muscle force | [128,129] | |
| Myofilament cross bridge kinetics | [130] | |
| Frailty | [136,137,138] | |
| Cancer Cachexia | Muscle weight | [162,163] |
| Muscle force | [37,162,163] | |
| Overall survival | [158,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Peruta, C.; Lozanoska-Ochser, B.; Renzini, A.; Moresi, V.; Sanchez Riera, C.; Bouché, M.; Coletti, D. Sex Differences in Inflammation and Muscle Wasting in Aging and Disease. Int. J. Mol. Sci. 2023, 24, 4651. https://doi.org/10.3390/ijms24054651
Della Peruta C, Lozanoska-Ochser B, Renzini A, Moresi V, Sanchez Riera C, Bouché M, Coletti D. Sex Differences in Inflammation and Muscle Wasting in Aging and Disease. International Journal of Molecular Sciences. 2023; 24(5):4651. https://doi.org/10.3390/ijms24054651
Chicago/Turabian StyleDella Peruta, Chiara, Biliana Lozanoska-Ochser, Alessandra Renzini, Viviana Moresi, Carles Sanchez Riera, Marina Bouché, and Dario Coletti. 2023. "Sex Differences in Inflammation and Muscle Wasting in Aging and Disease" International Journal of Molecular Sciences 24, no. 5: 4651. https://doi.org/10.3390/ijms24054651
APA StyleDella Peruta, C., Lozanoska-Ochser, B., Renzini, A., Moresi, V., Sanchez Riera, C., Bouché, M., & Coletti, D. (2023). Sex Differences in Inflammation and Muscle Wasting in Aging and Disease. International Journal of Molecular Sciences, 24(5), 4651. https://doi.org/10.3390/ijms24054651










