A Novel Early Life Stress Model Affects Brain Development and Behavior in Mice
Abstract
1. Introduction
2. Results
2.1. Novel ELS Model Induces Anxiety- and Depression-like Behavior in Offspring Mice
2.2. Novel ELS Model Induces Deficiency of Social Behavior in the Offspring Mice
2.3. Novel ELS Model Impairs the Cognitive Ability of the Offspring Mice
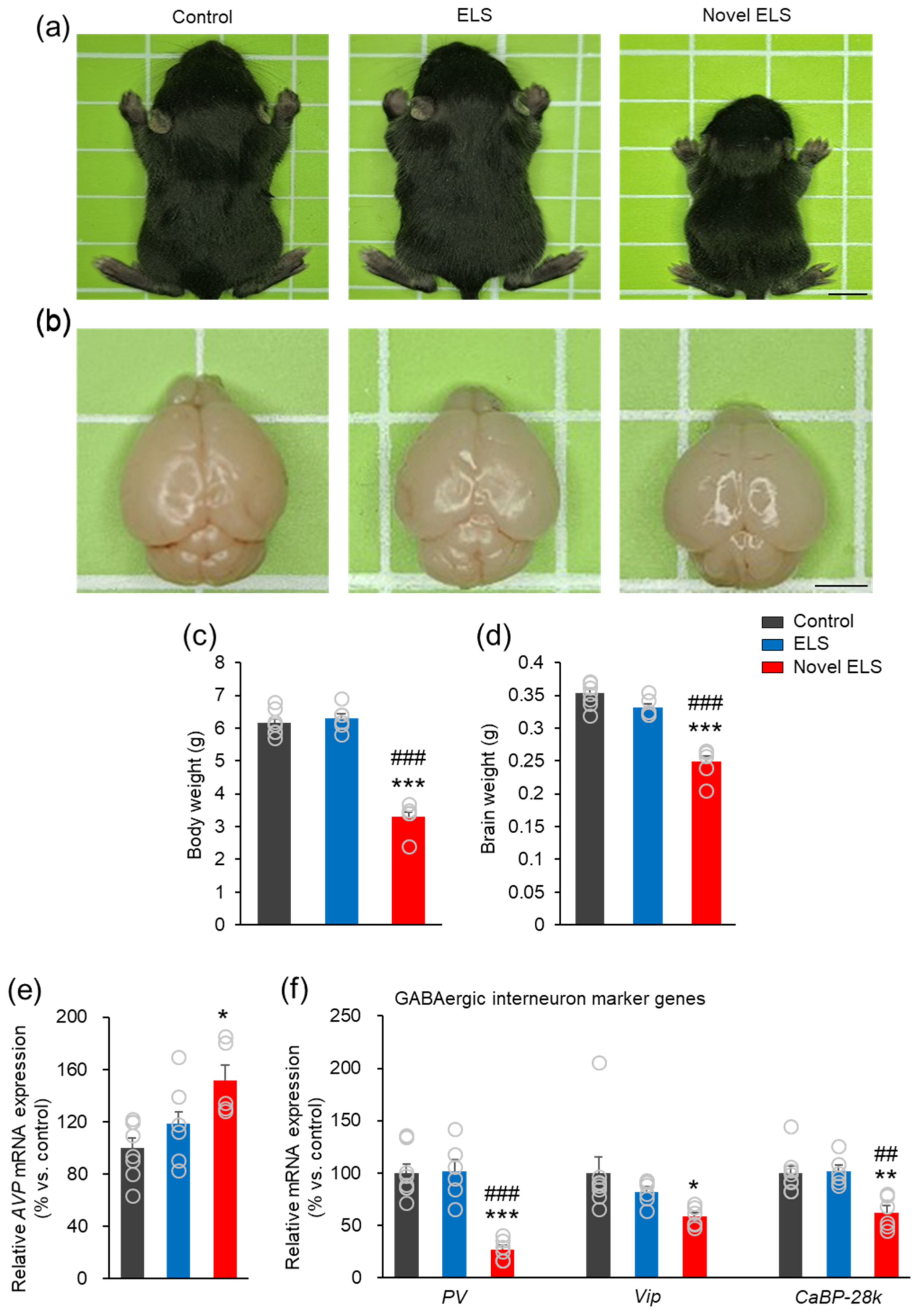
2.4. Novel ELS Affects the Growth of Offspring Mice in the Early Stage and the Expression of Genes Associated with Stress and GABAergic Interneurons
2.5. Novel ELS Model Alters the Number of Cortical GABAergic Interneuron Subpopulations and Microglial Cell Populations in Adult ELS Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Early Life Stress Paradigm
4.3. Behavioral Analysis
4.3.1. Tail Suspension Test
4.3.2. Forced Swimming Test
4.3.3. Elevated Plus-Maze Test
4.3.4. Open-Field Test
4.3.5. Social Interaction Test
4.3.6. Three-Chamber Social Test
4.3.7. Morris Water Maze
4.3.8. Novel Object Recognition
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Immunofluorescence
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hegde, A.; Mitra, R. Environment and early life: Decisive factors for stress-resilience and vulnerability. Int. Rev. Neurobiol. 2020, 150, 155–185. [Google Scholar]
- Apaydin, D.C.; Jaramillo, P.A.; Corradi, L.; Cosco, F.; Rathjen, F.G.; Kammertoens, T.; Filosa, A.; Sawamiphak, S. Early-Life Stress Regulates Cardiac Development through an IL-4-Glucocorticoid Signaling Balance. Cell. Rep. 2020, 33, 108404. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hirai, S.; Hosokawa, M.; Saito, T.; Sakuma, H.; Saido, T.; Hasegawa, M.; Okado, H. Early-life stress induces the development of Alzheimer’s disease pathology via angiopathy. Exp. Neurol. 2021, 337, 113552. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Suchecki, D. Maternal regulation of the infant’s hypothalamic-pituitary-adrenal axis stress response: Seymour ‘Gig’ Levine’s legacy to neuroendocrinology. J. Neuroendocrinol. 2018, 30, e12610. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.L.; Panina, Y.A.; Malinovskaya, N.A.; Salmina, A.B. Early life stress and brain plasticity: From molecular alterations to aberrant memory and behavior. Rev. Neurosci. 2021, 32, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Lesuis, S.L.; Lucassen, P.J.; Krugers, H.J. Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology 2019, 149, 195–203. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Ruigrok, S.R.; Amelianchik, A.; Ivan, D.; van Dam, A.M.; Lucassen, P.J.; Korosi, A. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav. Immun. 2017, 63, 160–175. [Google Scholar] [CrossRef]
- Own, L.S.; Iqbal, R.; Patel, P.D. Maternal separation alters serotonergic and HPA axis gene expression independent of separation duration in mice. Brain Res. 2013, 1515, 29–38. [Google Scholar] [CrossRef]
- Cuarenta, A.; Kigar, S.L.; Henion, I.C.; Chang, L.; Bakshi, V.P.; Auger, A.P. Early life stress during the neonatal period alters social play and Line1 during the juvenile stage of development. Sci. Rep. 2021, 11, 3549. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Manzano-Nieves, G.; Goodwill, H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016, 82, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; Andrade da Costa, B.L.S.; De Stasi, A.M.; Bacci, A.; Imamura Kawasawa, Y.; Vaidya, V.A.; Gaspar, P. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Vetulani, J. Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013, 65, 1451–1461. [Google Scholar] [CrossRef]
- Nephew, B.; Murgatroyd, C. The role of maternal care in shaping CNS function. Neuropeptides 2013, 47, 371–378. [Google Scholar] [CrossRef]
- Miyaso, H.; Nagahori, K.; Takano, K.; Omotehara, T.; Kawata, S.; Li, Z.L.; Kuramasu, M.; Wu, X.; Ogawa, Y.; Itoh, M. Neonatal maternal separation causes decreased numbers of sertoli cell, spermatogenic cells, and sperm in mice. Toxicol. Mech. Methods 2021, 31, 116–125. [Google Scholar] [CrossRef]
- Tsotsokou, G.; Nikolakopoulou, M.; Kouvelas, E.D.; Mitsacos, A. Neonatal maternal separation affects metabotropic glutamate receptor 5 expression and anxiety-related behavior of adult rats. Eur. J. Neurosci. 2021, 54, 4550–4564. [Google Scholar] [CrossRef]
- Jolodar, S.K.; Bigdeli, M.; Moghaddam, A.H. Hypericin Ameliorates Maternal Separation-Induced Cognitive Deficits and Hippocampal Inflammation in Rats. Mini Rev. Med. Chem. 2021, 21, 1144–1149. [Google Scholar] [CrossRef]
- McEwen, B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003, 9, 149–154. [Google Scholar] [CrossRef]
- Daniels, W.M.; Pietersen, C.Y.; Carstens, M.E.; Stein, D.J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis. 2004, 19, 3–14. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Z.; Wang, G.; Xiao, L.; Wang, H.; Sun, L.; Xie, Y. Long-term maternal separation potentiates depressive-like behaviours and neuroinflammation in adult male C57/BL6J mice. Pharmacol. Biochem. Behav. 2020, 196, 172953. [Google Scholar] [CrossRef] [PubMed]
- Haikonen, J.; Englund, J.; Amarilla, S.P.; Kharybina, Z.; Shintyapina, A.; Kegler, K.; Garcia, M.S.; Atanasova, T.; Taira, T.; Hartung, H.; et al. Aberrant cortical projections to amygdala GABAergic neurons contribute to developmental circuit dysfunction following early life stress. iScience 2023, 26, 105724. [Google Scholar] [CrossRef] [PubMed]
- Holland, F.H.; Ganguly, P.; Potter, D.N.; Chartoff, E.H.; Brenhouse, H.C. Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci. Lett. 2014, 566, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Banqueri, M.; Méndez, M.; Gómez-Lázaro, E.; Arias, J.L. Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 2019, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Maita, I.; Phan, M.L.; Gu, E.; Kwok, C.; Dieterich, A.; Gergues, M.M.; Yohn, C.N.; Wang, Y.; Zhou, J.-N.; et al. Early-life stress alters affective behaviors in adult mice through persistent activation of CRH-BDNF signaling in the oval bed nucleus of the stria terminalis. Transl. Psychiatry 2020, 10, 396. [Google Scholar] [CrossRef]
- Brydges, N.M.; Hall, J.; Best, C.; Rule, L.; Watkin, H.; Drake, A.J.; Lewis, C.; Thomas, K.L.; Hall, J. Childhood stress impairs social function through AVP-dependent mechanisms. Transl. Psychiatry 2019, 9, 330. [Google Scholar] [CrossRef]
- Guadagno, A.; Belliveau, C.; Mechawar, N.; Walker, C.D. Effects of Early Life Stress on the Developing Basolateral Amygdala-Prefrontal Cortex Circuit: The Emerging Role of Local Inhibition and Perineuronal Nets. Front. Hum. Neurosci. 2021, 15, 669120. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Ryabushkina, Y.; Kovner, A.; Lepeshko, A.; Bondar, N. Repeated and single maternal separation specifically alter microglial morphology in the prefrontal cortex and neurogenesis in the hippocampus of 15-day-old male mice. Neuroreport 2020, 31, 1256–1264. [Google Scholar] [CrossRef]
- Seidel, K.; Helmeke, C.; Poeggel, G.; Braun, K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev. Neurobiol. 2008, 68, 1137–1152. [Google Scholar] [CrossRef]
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688. [Google Scholar] [CrossRef]
- Tata, D. Maternal separation as a model of early stress: Effects on aspects of emotional behavior and neuroendocrine function. Hell. J. Psychol. 2012, 9, 84–101. [Google Scholar]
- Litvin, Y.; Tovote, P.; Pentkowski, N.S.; Zeyda, T.; King, L.B.; Vasconcellos, A.J.; Dunlap, C.; Spiess, J.; Blanchard, D.C.; Blanchard, R.J. Maternal separation modulates short-term behavioral and physiological indices of the stress response. Horm. Behav. 2010, 58, 241–249. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Magalhaes, T.; Correia, D.; de Carvalho, L.M.; Damasceno, S.; Brunialti Godard, A.L. Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav. 2018, 8, e00841. [Google Scholar] [CrossRef] [PubMed]
- Francis-Oliveira, J.; Shieh, I.C.; Vilar Higa, G.S.; Barbosa, M.A.; De Pasquale, R. Maternal separation induces changes in TREK-1 and 5HT1A expression in brain areas involved in the stress response in a sex-dependent way. Behav. Brain Res. 2021, 396, 112909. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.P.; Gunnar, M.R. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage 2020, 209, 116493. [Google Scholar] [CrossRef]
- Adjimann, T.S.; Argañaraz, C.V.; Soiza-Reilly, M. Serotonin-related rodent models of early-life exposure relevant for neurodevelopmental vulnerability to psychiatric disorders. Transl. Psychiatry 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Atsak, P.; Cardenas, J.; Kosmidis, S.; Leonardo, E.D.; Dranovsky, A. Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Sci. Rep. 2019, 9, 4120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.K.; Delpech, J.-C.; Thompson, G.J.; Wei, L.; Hao, J.; Herman, P.; Hyder, F.; Kaffman, A. Amygdala hyper-connectivity in a mouse model of unpredictable early life stress. Transl. Psychiatry 2018, 8, 49. [Google Scholar] [CrossRef]
- Gómez-González, B.; Escobar, A. Altered functional development of the blood–brain barrier after early life stress in the rat. Brain Res. Bull. 2009, 79, 376–387. [Google Scholar] [CrossRef]
- Roque, A.; Valles Méndez, K.M.; Ruiz, R.; Pineda, E.; Lajud, N. Early life stress induces a transient increase in hippocampal corticotropin-releasing hormone in rat neonates that precedes the effects on hypothalamic neuropeptides. Eur. J. Neurosci. 2022, 55, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.; Sudheimer, K.; Tirouvanziam, R.; Williams, L.M.; O’Hara, R. The long-term impact of early adversity on late-life psychiatric disorders. Curr. Psychiatry Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Ruiz, C.M.; Rismanchi, N.; Sanchez, G.A.; Castillo, E.; Huang, J.; Cross, C.; Baram, T.Z.; Mahler, S.V. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol. Stress 2018, 8, 57–67. [Google Scholar] [CrossRef]
- Goff, B.; Tottenham, N. Early-life adversity and adolescent depression: Mechanisms involving the ventral striatum. CNS Spectr. 2015, 20, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Penza, K.M.; Heim, C.; Nemeroff, C.B. Neurobiological effects of childhood abuse: Implications for the pathophysiology of depression and anxiety. Arch. Women’s Ment. Health 2003, 6, 15–22. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.X.; Wang, Y.; Wang, D.; Song, Y.; Zou, J.X.; Pan, H.Q.; Zhai, X.Z.; Zhang, Y.M.; Zhang, Y.B.; et al. Early life stress induces anxiety-like behavior during adulthood through dysregulation of neuronal plasticity in the basolateral amygdala. Life Sci. 2021, 285, 119959. [Google Scholar] [CrossRef]
- Kambali, M.Y.; Anshu, K.; Kutty, B.M.; Muddashetty, R.S.; Laxmi, T.R. Effect of early maternal separation stress on attention, spatial learning and social interaction behaviour. Exp. Brain Res. 2019, 237, 1993–2010. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Han, L.; Zhu, G. Early maternal separation promotes apoptosis in dentate gyrus and alters neurological behaviors in adolescent rats. Int. J. Clin. Exp. Pathol. 2017, 10, 10812–10820. [Google Scholar]
- Jiang, Z.; Zhu, Z.; Zhao, M.; Wang, W.; Li, H.; Liu, D.; Pan, F. H3K9me2 regulation of BDNF expression in the hippocampus and medial prefrontal cortex is involved in the depressive-like phenotype induced by maternal separation in male rats. Psychopharmacology 2021, 238, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Brenneman, D.E. VIP: Molecular biology and neurobiological function. Mol. Neurobiol. 1989, 3, 201–236. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, A.I.; Toth, K.; Danos, P.; Freund, T.F. Subpopulations of GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in the rat hippocampus. J. Comp. Neurol. 1991, 312, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Kelsom, C.; Lu, W. Development and specification of GABAergic cortical interneurons. Cell Biosci. 2013, 3, 19. [Google Scholar] [CrossRef]
- Schwaller, B.; Meyer, M.; Schiffmann, S. ‘New’ functions for ‘old’ proteins: The role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum 2002, 1, 241–258. [Google Scholar] [CrossRef]
- Schmidt, H. Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 2012, 5, 25. [Google Scholar] [CrossRef]
- Goff, K.M.; Goldberg, E.M. A Role for Vasoactive Intestinal Peptide Interneurons in Neurodevelopmental Disorders. Dev. Neurosci. 2021, 43, 168–180. [Google Scholar] [CrossRef]
- Li, J.T.; Xie, X.M.; Yu, J.Y.; Sun, Y.X.; Liao, X.M.; Wang, X.X.; Su, Y.A.; Liu, Y.J.; Schmidt, M.V.; Wang, X.D.; et al. Suppressed Calbindin Levels in Hippocampal Excitatory Neurons Mediate Stress-Induced Memory Loss. Cell Rep. 2017, 21, 891–900. [Google Scholar] [CrossRef]
- Molinari, S.; Battini, R.; Ferrari, S.; Pozzi, L.; Killcross, A.S.; Robbins, T.W.; Jouvenceau, A.; Billard, J.M.; Dutar, P.; Lamour, Y.; et al. Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proc. Natl. Acad. Sci. USA 1996, 93, 8028–8033. [Google Scholar] [CrossRef]
- Lussier, S.J.; Stevens, H.E. Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev. Neurobiol. 2016, 76, 1078–1091. [Google Scholar] [CrossRef]
- Mossner, J.M.; Batista-Brito, R.; Pant, R.; Cardin, J.A. Developmental loss of MeCP2 from VIP interneurons impairs cortical function and behavior. eLife 2020, 9, e55639. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.J.; Webster, M.J.; Sivagnanasundaram, S.; Duncan, C.; Elashoff, M.; Weickert, C.S. Expression of Interneuron Markers in the Dorsolateral Prefrontal Cortex of the Developing Human and in Schizophrenia. Am. J. Psychiatry 2010, 167, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Bandler, R.C.; Mayer, C.; Fishell, G. Cortical interneuron specification: The juncture of genes, time and geometry. Curr. Opin. Neurobiol. 2017, 42, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Wei, L.; Hao, J.; Yu, X.; Madore, C.; Butovsky, O.; Kaffman, A. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016, 57, 79–93. [Google Scholar] [CrossRef]
- George, E.D.; Bordner, K.A.; Elwafi, H.M.; Simen, A.A. Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neurosci. 2010, 11, 123. [Google Scholar] [CrossRef]
- Arnett, M.G.; Pan, M.S.; Doak, W.; Cyr, P.E.P.; Muglia, L.M.; Muglia, L.J. The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior. Transl. Psychiatry 2015, 5, e542. [Google Scholar] [CrossRef][Green Version]
- Shin, S.; Pribiag, H.; Lilascharoen, V.; Knowland, D.; Wang, X.Y.; Lim, B.K. Drd3 Signaling in the Lateral Septum Mediates Early Life Stress-Induced Social Dysfunction. Neuron 2018, 97, 195–208.e6. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Soldado-Magraner, S.; Alvarez-Sánchez, M.; Bohacek, J.; Vernaz, G.; Shu, H.; Franklin, T.B.; Wolfer, D.; Mansuy, I.M. Early life stress in fathers improves behavioural flexibility in their offspring. Nat. Commun. 2014, 5, 5466. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Jung, E.-M.; Yoo, Y.-M.; Lee, J.-H.; Jeung, E.-B. Perinatal Exposure to Triclosan Results in Abnormal Brain Development and Behavior in Mice. Int. J. Mol. Sci. 2020, 21, 4009. [Google Scholar] [CrossRef]
- Jung, E.M.; Moffat, J.J.; Liu, J.; Dravid, S.M.; Gurumurthy, C.B.; Kim, W.Y. Arid1b haploinsufficiency disrupts cortical interneuron development and mouse behavior. Nat. Neurosci. 2017, 20, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Edfawy, M.; Guedes, J.R.; Pereira, M.I.; Laranjo, M.; Carvalho, M.J.; Gao, X.; Ferreira, P.A.; Caldeira, G.; Franco, L.O.; Wang, D.; et al. Abnormal mGluR-mediated synaptic plasticity and autism-like behaviours in Gprasp2 mutant mice. Nat. Commun. 2019, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Choi, K.C.; Frank, H.Y.; Jeung, E.B. Effects of 17beta-estradiol and xenoestrogens on mouse embryonic stem cells. Toxicol. Vitr. 2010, 24, 1538–1545. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.S.; Choi, S.M.; Lee, S.H.; Moon, H.J.; Jung, E.-M. A Novel Early Life Stress Model Affects Brain Development and Behavior in Mice. Int. J. Mol. Sci. 2023, 24, 4688. https://doi.org/10.3390/ijms24054688
Shin HS, Choi SM, Lee SH, Moon HJ, Jung E-M. A Novel Early Life Stress Model Affects Brain Development and Behavior in Mice. International Journal of Molecular Sciences. 2023; 24(5):4688. https://doi.org/10.3390/ijms24054688
Chicago/Turabian StyleShin, Hyun Seung, Soo Min Choi, Seung Hyun Lee, Ha Jung Moon, and Eui-Man Jung. 2023. "A Novel Early Life Stress Model Affects Brain Development and Behavior in Mice" International Journal of Molecular Sciences 24, no. 5: 4688. https://doi.org/10.3390/ijms24054688
APA StyleShin, H. S., Choi, S. M., Lee, S. H., Moon, H. J., & Jung, E.-M. (2023). A Novel Early Life Stress Model Affects Brain Development and Behavior in Mice. International Journal of Molecular Sciences, 24(5), 4688. https://doi.org/10.3390/ijms24054688






