Transcriptome and Metabolome Reveal the Molecular Mechanism of Barley Genotypes Underlying the Response to Low Nitrogen and Resupply
Abstract
:1. Introduction
2. Results
2.1. Dry Weight and Biomass under Different Nitrogen Treatments
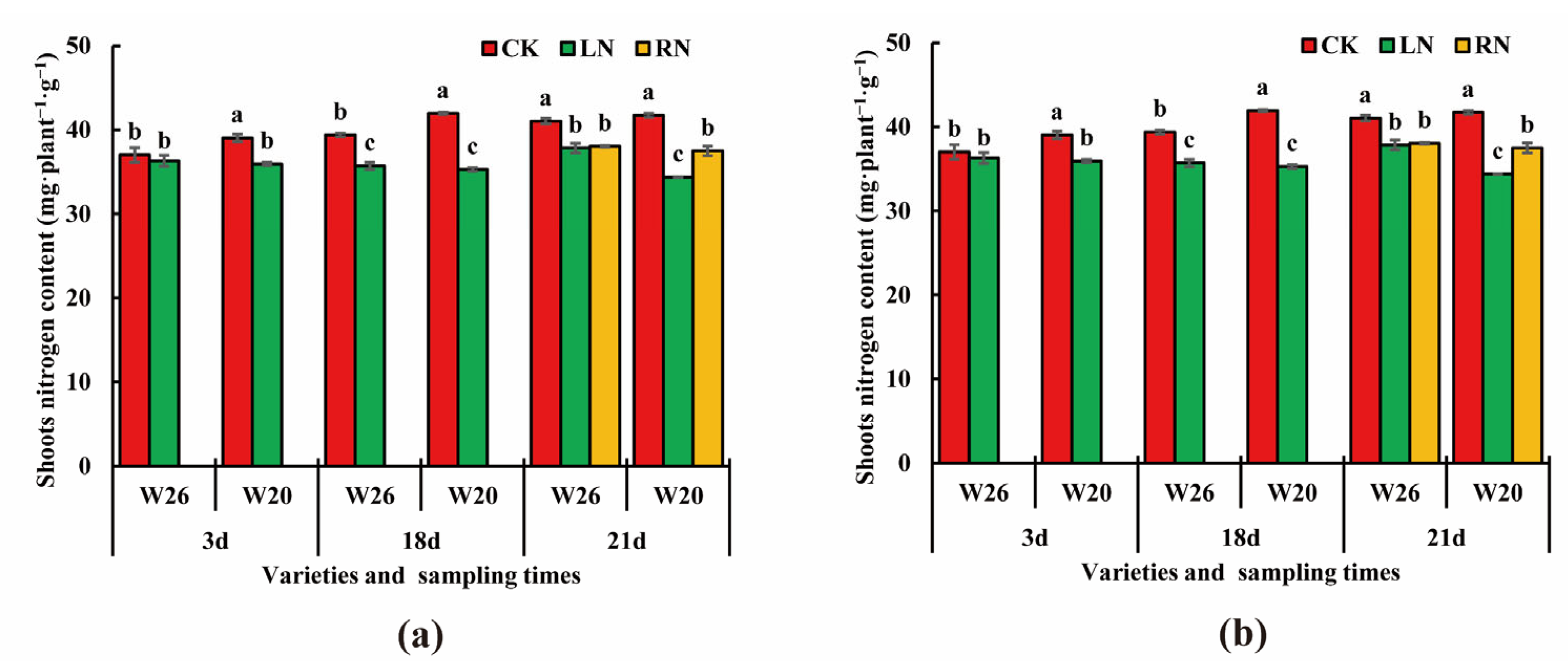
2.2. Nitrogen Content under Different Nitrogen Treatments
2.3. NUE Estimation after 21 Days of Plant Growing
2.4. Transcriptome Profiling under Different Nitrogen Treatments
2.4.1. Results of RNA-Seq
2.4.2. Selection of DEGs under Different Nitrogen Treatments
2.4.3. GO Enrichment Analysis of DEGs
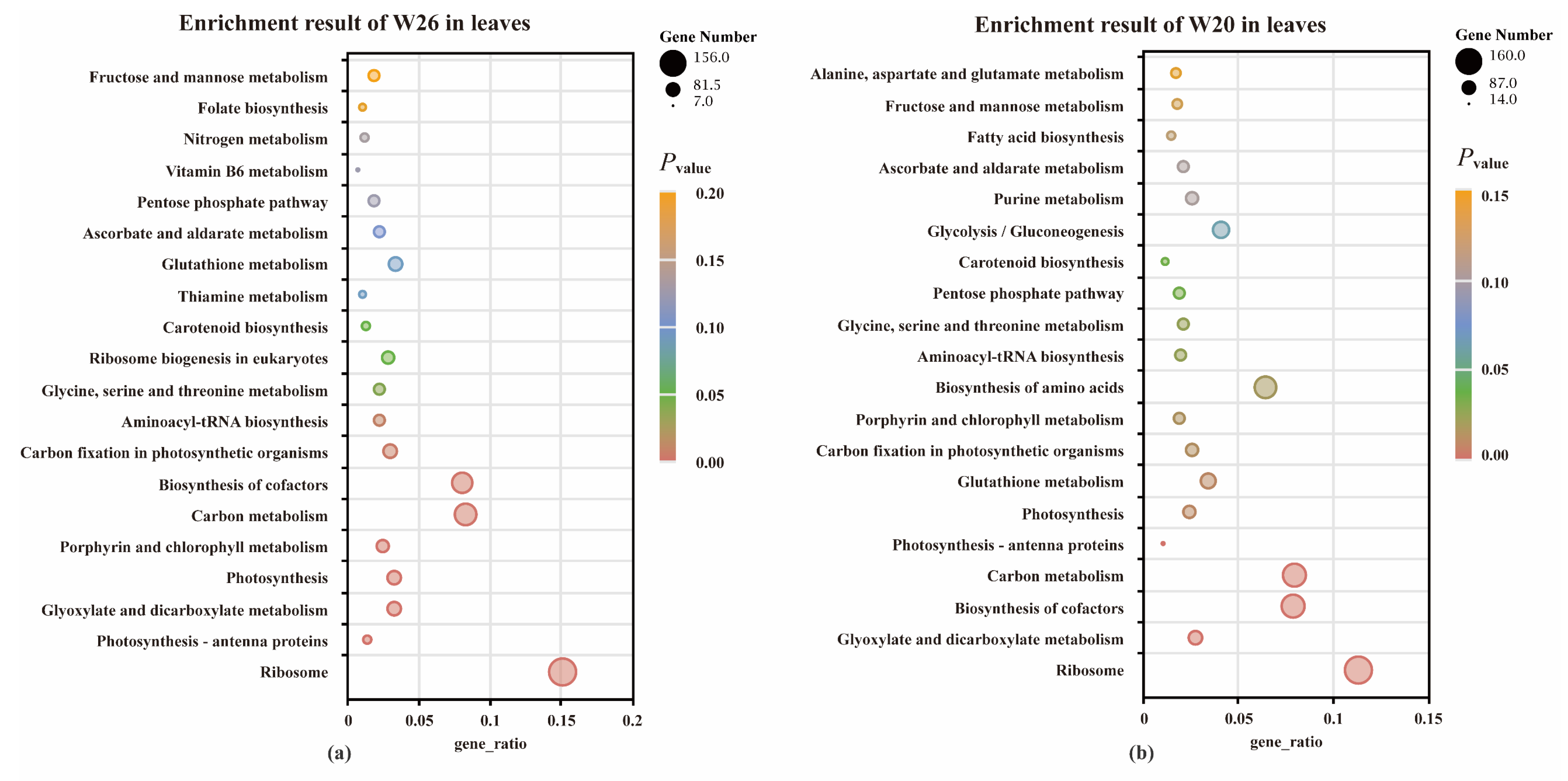
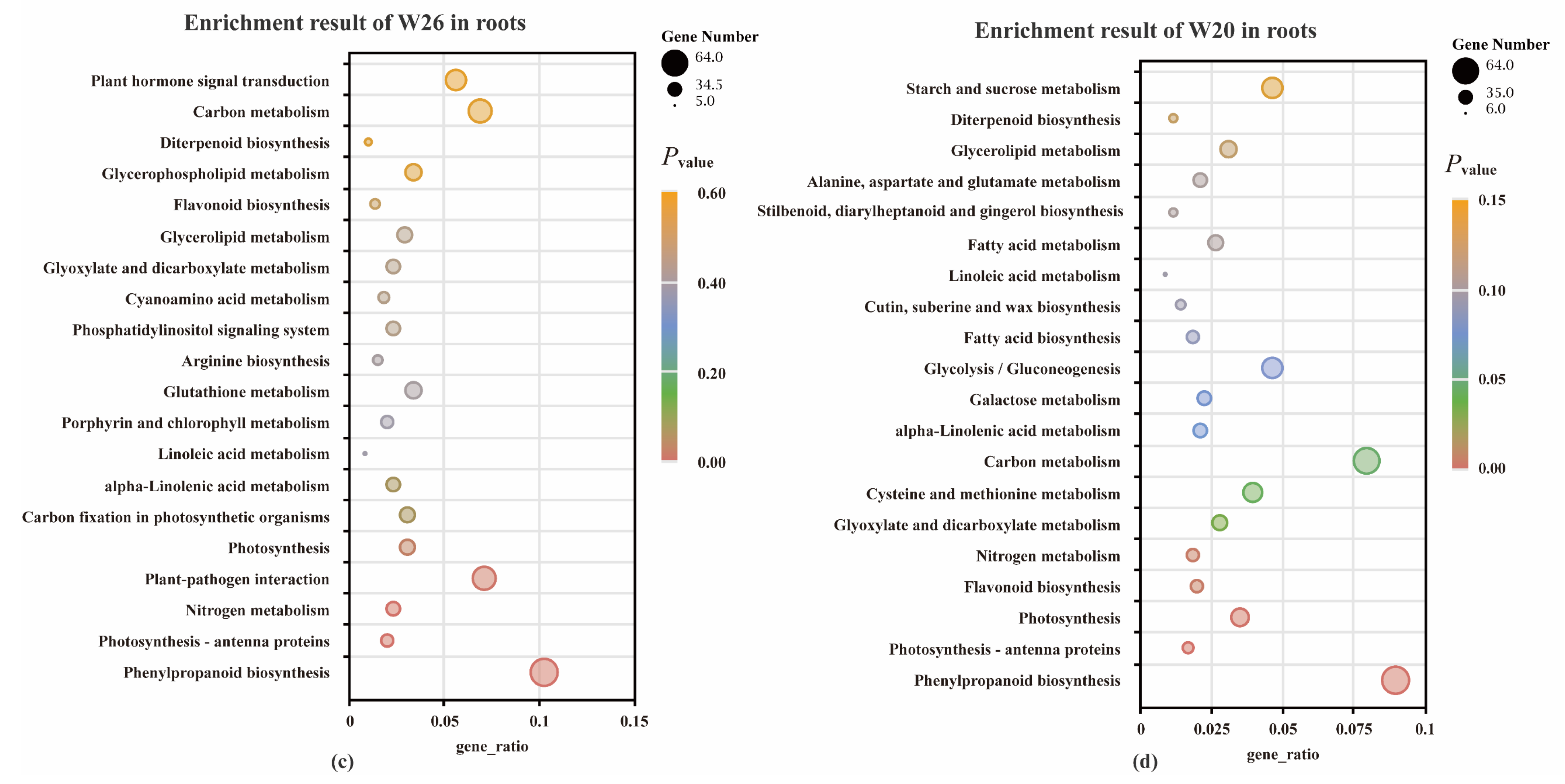
2.4.4. KEGG Enrichment Analysis of DEGs
2.4.5. Validation of DEGs by Quantitative Real-Time PCR (qRT-PCR)
2.5. Metabonomics and Correlation Analysis
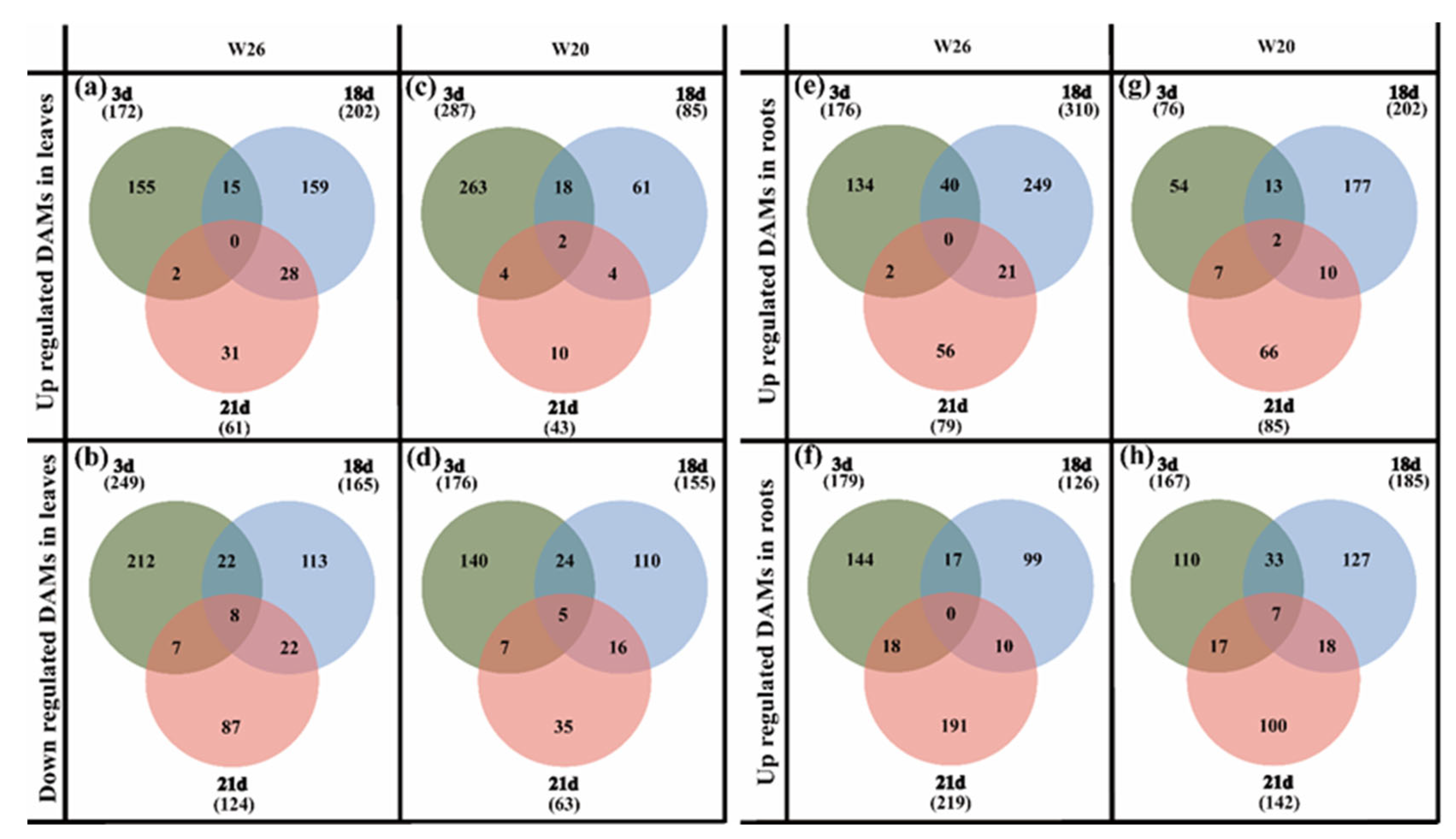
2.5.1. Selection of DAMs under Different Nitrogen Treatments
2.5.2. KEGG Joint Analysis of DAMs and DEGs
2.6. Differential Genes and Metabolites of Nitrogen Metabolism and GSH Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Growing and Sampling
4.2. Extraction of Total RNA and the Construction and Sequencing of RNA-Seq Library
4.3. Sequencing Data Quality Control
4.4. Identification and Analysis of Metabolites
4.5. Selection of DEGs and DAMs
4.6. qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Plett, D.; Heuer, S.; Okamoto, M. Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals: Challenges and future directions. Funct. Plant Biol. 2015, 42, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.J. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Sardans, J. The global nitrogen-phosphorus imbalance. Science 2022, 375, 266–267. [Google Scholar] [CrossRef]
- Zhang, Q. Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [Green Version]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Plett, D.C.; Holtham, L.R.; Okamoto, M.; Garnett, T.P. Nitrate uptake and its regulation in relation to improving nitrogen use efficiency in cereals. Semin. Cell Dev. Biol. 2018, 74, 97–104. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. Found. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Moran-Zuloaga, D.; Dippold, M.; Glaser, B.; Kuzyakov, Y. Organic nitrogen uptake by plants: Reevaluation by position-specific labeling of amino acids. Biogeochemistry 2015, 125, 359–374. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef] [Green Version]
- Scheurwater, I.; Koren, M.; Lambers, H.; Atkin, O.K. The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. J. Exp. Bot. 2002, 53, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Miflin, B.J. Distribution of Nitrate and Nitrite Reductase in Barley. Nature 1967, 214, 1133–1134. [Google Scholar] [CrossRef]
- Temple, S.J.; Vance, C.P.; Stephen Gantt, J. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998, 3, 51–56. [Google Scholar] [CrossRef]
- Prakash, P.; Punekar, N.S.; Bhaumik, P. Structural basis for the catalytic mechanism and α-ketoglutarate cooperativity of glutamate dehydrogenase. J. Biol. Chem. 2018, 293, 6241–6258. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.C.; Flavell, A.J.; George, T.S.; Leat, P.; Mullholland, B.; Ramsay, L.; Revoredo-Giha, C.; Russell, J.; Steffenson, B.J.; Swanston, J.S.; et al. Crops that feed the world 4. Barley: A resilient crop? Strengths and weaknesses in the context of food security. Food Secur. 2011, 3, 141–178. [Google Scholar] [CrossRef]
- Zhou, M.X. Barley Production and Consumption. In Genetics and Improvement of Barley Malt Quality; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–17. [Google Scholar]
- Baik, B.-K.; Ullrich, S.E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008, 48, 233–242. [Google Scholar] [CrossRef]
- Chutimanitsakun, Y.; Cuesta-Marcos, A.; Chao, S.; Corey, A.; Filichkin, T.; Fisk, S.; Kolding, M.; Meints, B.; Ong, Y.-L.; Rey, J.I.; et al. Application of marker-assisted selection and genome-wide association scanning to the development of winter food barley germplasm resources. Plant Breed. 2013, 132, 563–570. [Google Scholar] [CrossRef]
- Keller, B.; Krattinger, S.G. Genomic compartments in barley. Nature 2017, 544, 424–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; He, X.; Wu, D.; Zhu, B.; Cai, S.; Nadira, U.A.; Jabeen, Z.; Zhang, G. Comparative transcriptome profiling of two Tibetan wild barley genotypes in responses to low potassium. PLoS ONE 2014, 9, e100567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, X.; Zeng, J.; Ye, L.; Chen, G.; Han, Z.; Shah, J.M.; Zhang, G. Transcriptome profiling analysis for two Tibetan wild barley genotypes in responses to low nitrogen. BMC Plant Biol. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decouard, B.; Bailly, M.; Rigault, M.; Marmagne, A.; Arkoun, M.; Soulay, F.; Caius, J.; Paysant-Le Roux, C.; Louahlia, S.; Jacquard, C.; et al. Genotypic Variation of Nitrogen Use Efficiency and Amino Acid Metabolism in Barley. Front. Plant Sci. 2021, 12, 807798. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.L.B.; Pedas, P.; Andersen, B.; Svensson, B.; Schjoerring, J.K.; Finnie, C. Responses of barley root and shoot proteomes to long-term nitrogen deficiency, short-term nitrogen starvation and ammonium. Plant Cell Environ. 2011, 34, 2024–2037. [Google Scholar] [CrossRef]
- Karunarathne, S.D.; Han, Y.; Zhang, X.-Q.; Zhou, G.; Hill, C.B.; Chen, K.; Angessa, T.; Li, C. Genome-Wide Association Study and Identification of Candidate Genes for Nitrogen Use Efficiency in Barley (Hordeum vulgare L.). Front. Plant Sci. 2020, 11, 571912. [Google Scholar] [CrossRef]
- Guo, H.; Pu, X.; Jia, H.; Zhou, Y.; Ye, G.; Yang, Y.; Na, T.; Wang, J. Transcriptome analysis reveals multiple effects of nitrogen accumulation and metabolism in the roots, shoots, and leaves of potato (Solanum tuberosum L.). BMC Plant Biol. 2022, 22, 282. [Google Scholar] [CrossRef]
- Yao, Y.; An, L.; Yao, X.; Bai, Y.; Li, X.; Li, X. Transcriptome analysis of Elymus breviaristatus ‘Tongde’ under different nitrogen treatments. Agron. J. 2022, 114, 756–770. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Bai, W.; Yang, X.; Wu, H.; Lei, K.; Huang, R.; Zhang, S.; Huang, Q.; Lin, Q. Comparative Transcriptome Analysis Reveals Different Low-Nitrogen-Responsive Genes in Pepper Cultivars. Horticulturae 2021, 7, 110. [Google Scholar] [CrossRef]
- Ding, Y.; Gardiner, D.M.; Powell, J.J.; Colgrave, M.L.; Park, R.F.; Kazan, K. Adaptive defence and sensing responses of host plant roots to fungal pathogen attack revealed by transcriptome and metabolome analyses. Plant Cell Environ. 2021, 44, 3756–3774. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum strain T22 Primes and Enhances Defense Responses Against Aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef] [Green Version]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize Source Leaf Adaptation to Nitrogen Deficiency Affects Not Only Nitrogen and Carbon Metabolism but Also Control of Phosphate Homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [Green Version]
- Menz, J.; Li, Z.; Schulze, W.X.; Ludewig, U. Early nitrogen-deprivation responses in Arabidopsis roots reveal distinct differences on transcriptome and (phospho-) proteome levels between nitrate and ammonium nutrition. Plant J. 2016, 88, 717–734. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16, 341. [Google Scholar] [CrossRef] [Green Version]
- Ristova, D.; Carré, C.; Pervent, M.; Medici, A.; Kim, G.J.; Scalia, D.; Ruffel, S.; Birnbaum, K.D.; Lacombe, B.; Busch, W.; et al. Combinatorial interaction network of transcriptomic and phenotypic responses to nitrogen and hormones in the Arabidopsis thaliana root. Sci. Signal. 2016, 9, rs13. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Liu, Y.; Shi, L. Integration of the metabolome and transcriptome reveals the resistance mechanism to low nitrogen in wild soybean seedling roots. Environ. Exp. Bot. 2020, 175, 104043. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Wang, Y.; He, T.; Gao, R.; Xu, H.; Guo, G.; Li, Y.; Zhou, L.; Lu, R.; et al. Expression Analysis of Nitrogen Metabolism-Related Genes Reveals Differences in Adaptation to Low-Nitrogen Stress between Two Different Barley Cultivars at Seedling Stage. Int. J. Genom. 2018, 2018, 8152860. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Q.; Jiang, P.; Zhang, W.; Huang, J.; Liu, C.; Halford, N.G.; Lu, R. Novel low-nitrogen stress-responsive long non-coding RNAs (lncRNA) in barley landrace B968 (Liuzhutouzidamai) at seedling stage. BMC Plant Biol. 2020, 20, 142. [Google Scholar] [CrossRef]
- Liaquat, F.; Munis, M.F.H.; Arif, S.; Haroon, U.; Shi, J.; Saqib, S.; Zaman, W.; Che, S.; Liu, Q. PacBio Single-Molecule Long-Read Sequencing Reveals Genes Tolerating Manganese Stress in Schima superba Saplings. Front. Genet. 2021, 12, 635043. [Google Scholar] [CrossRef]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and Transcriptome Analysis of Wheat Seedlings Response to Low Nitrogen Stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavoosi, G.; Balotf, S.; Eshghi, H.; Hasani, H. Analysis of nitrate reductase mRNA expression and nitrate reductase activity in response to nitrogen supply. Mol. Biol. Res. Commun. 2014, 3, 75–84. [Google Scholar] [PubMed]
- Broadley, M.R.; Bowen, H.C.; Cotterill, H.L.; Hammond, J.P.; Meacham, M.C.; Mead, A.; White, P.J. Phylogenetic variation in the shoot mineral concentration of angiosperms. J. Exp. Bot. 2004, 55, 321–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, C.; Lu, R.; Guo, G.; Chen, Z.; He, T.; Gao, R.; Li, Y.; Huang, J. The difference in responses to nitrogen deprivation and re-supply at seedling stage between two barley genotypes differing nitrogen use efficiency. Plant Growth Regul. 2015, 79, 119–126. [Google Scholar] [CrossRef]
- Sun, J.; Bankston, J.; Payandeh, J.; Hinds, T.; Zagotta, W.; Zheng, N. Crystal Structure of a Plant Dual-Affinity Nitrate Transporter. Nature 2014, 507, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.L.; Newstead, S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 2014, 507, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Muños, S.; Cazettes, C.; Fizames, C.; Gaymard, F.; Tillard, P.; Lepetit, M.; Lejay, L.; Gojon, A. Transcript Profiling in the chl1-5 Mutant of Arabidopsis Reveals a Role of the Nitrate Transporter NRT1.1 in the Regulation of Another Nitrate Transporter, NRT2.1[W]. Plant Cell 2004, 16, 2433–2447. [Google Scholar] [CrossRef] [Green Version]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubeš, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zažímalová, E.; et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef] [Green Version]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2016, 63, 220–229. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Wang, J.; Xu, Q. Enhancing glutamine production by optimising the GS-GOGAT pathway in Corynebacterium glutamicum and NH4+-limited fermentation. Authorea, 2021; preprint. [Google Scholar] [CrossRef]
- Kambhampati, S.; Ajewole, E.; Marsolais, F. Advances in Asparagine Metabolism. In Progress in Botany; Cánovas, F.M., Lüttge, U., Matyssek, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 79, pp. 49–74. [Google Scholar]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, G.; Yang, H.; Lu, D. Foliar Brassinolide Sprays Ameliorate Post-Silking Heat Stress on the Accumulation and Remobilization of Biomass and Nitrogen in Fresh Waxy Maize. Agronomy 2022, 12, 1363. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Mehdi, K.; Penninckx, M.J. An important role for glutathione and γ-glutamyltranspeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae. Microbiology 1997, 143, 1885–1889. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Liu, H.; Chen, G.; Zhao, Q.; Guo, H.; Minocha, R.; Long, S.; Tang, Y.; Saad, E.M.; DeLaTorreRoche, R.; et al. Dual roles of glutathione in silver nanoparticle detoxification and enhancement of nitrogen assimilation in soybean (Glycine max (L.) Merrill). Environ. Sci. Nano 2020, 7, 1954–1966. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abiotic Stress, Generation of Reactive Oxygen Species, and Their Consequences. In Reactive Oxygen Species in Plants; Wiley: Hoboken, NJ, USA, 2017; pp. 23–50. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Effect of Salinity Stress on Growth and Physiology of Medicinal Plants. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 177–188. [Google Scholar]
- Schmitt, F.-J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta BBA-Bioenerg. 2014, 1837, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Ihsan, M.Z.; Daur, I.; Alghabari, F.; Alzamanan, S.; Rizwan, S.; Ahmad, M.; Waqas, M.; Shafqat, W. Heat stress and plant development: Role of sulphur metabolites and management strategies. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 332–342. [Google Scholar] [CrossRef]
- Meyer, A.J.; Hell, R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth. Res. 2005, 86, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-glutathione-α-tocopherol Triad in Abiotic Stress Response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Destro, T.; Turetta, L.; Varotto, S.; Caporale, G.; Ferretti, M. Localization of gamma-glutamyl transferase activity and protein in Zea mays organs and tissues. J. Plant Physiol. 2007, 164, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Heischmann, S.; Quinn, K.; Cruickshank-Quinn, C.; Liang, L.-P.; Reisdorph, R.; Reisdorph, N.; Patel, M. Exploratory Metabolomics Profiling in the Kainic Acid Rat Model Reveals Depletion of 25-Hydroxyvitamin D3 during Epileptogenesis. Sci. Rep. 2016, 6, 31424. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Haspel, J.A.; Chettimada, S.; Shaik, R.S.; Chu, J.-H.; Raby, B.A.; Cernadas, M.; Carey, V.; Process, V.; Hunninghake, G.M.; Ifedigbo, E.; et al. Circadian rhythm reprogramming during lung inflammation. Nat. Commun. 2014, 5, 4753. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zaman, W.; Lu, J.; Niu, Q.; Zhang, X.; Ayaz, A.; Saqib, S.; Yang, B.; Zhang, J.; Zhao, H.; et al. Natural lupeol level variation among castor accessions and the upregulation of lupeol synthesis in response to light. Ind. Crop. Prod. 2023, 192, 116090. [Google Scholar] [CrossRef]
- Ren, P.; Meng, Y.; Li, B.; Ma, X.; Si, E.; Lai, Y.; Wang, J.; Yao, L.; Yang, K.; Shang, X.; et al. Molecular Mechanisms of Acclimatization to Phosphorus Starvation and Recovery Underlying Full-Length Transcriptome Profiling in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 500. [Google Scholar] [CrossRef]



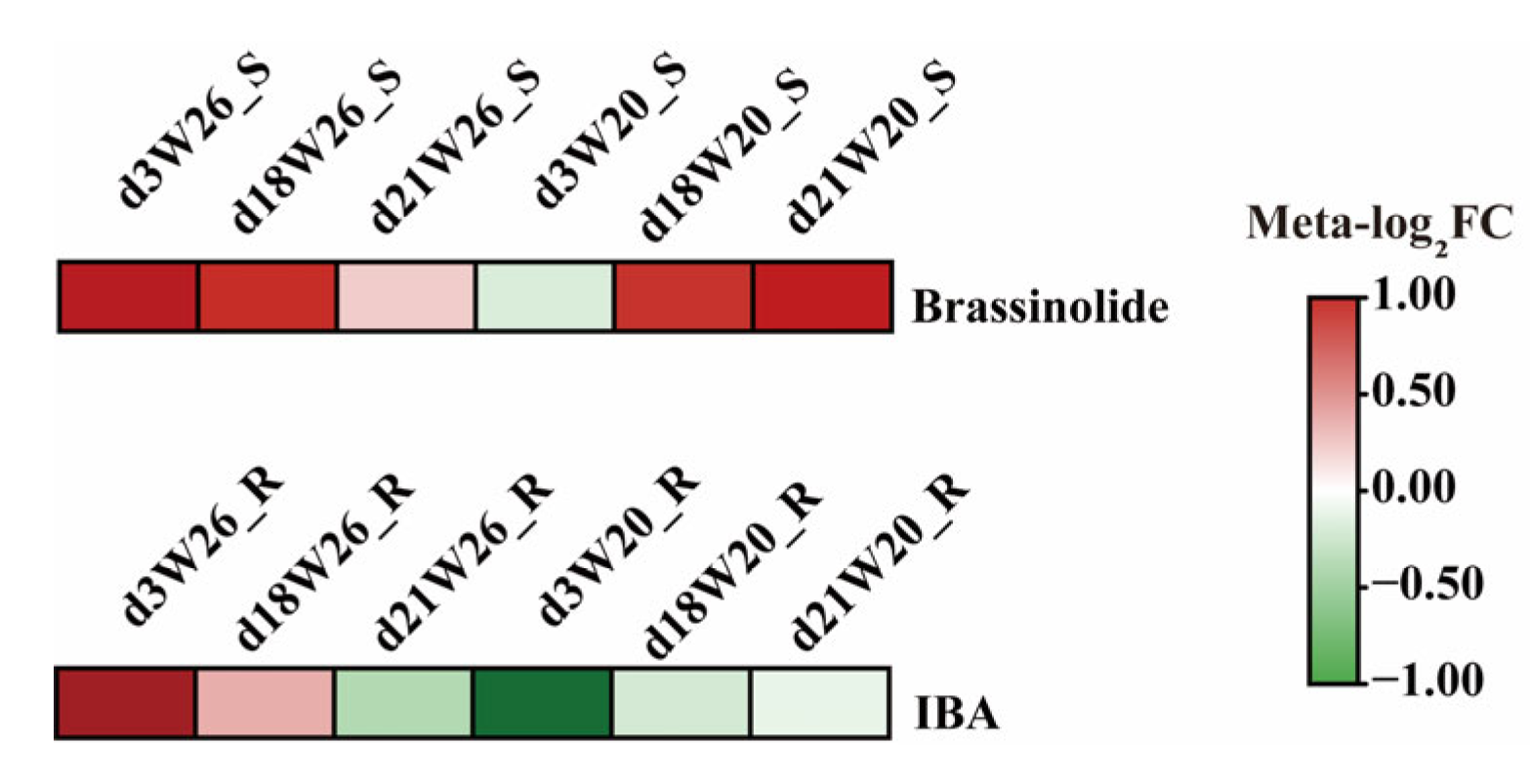

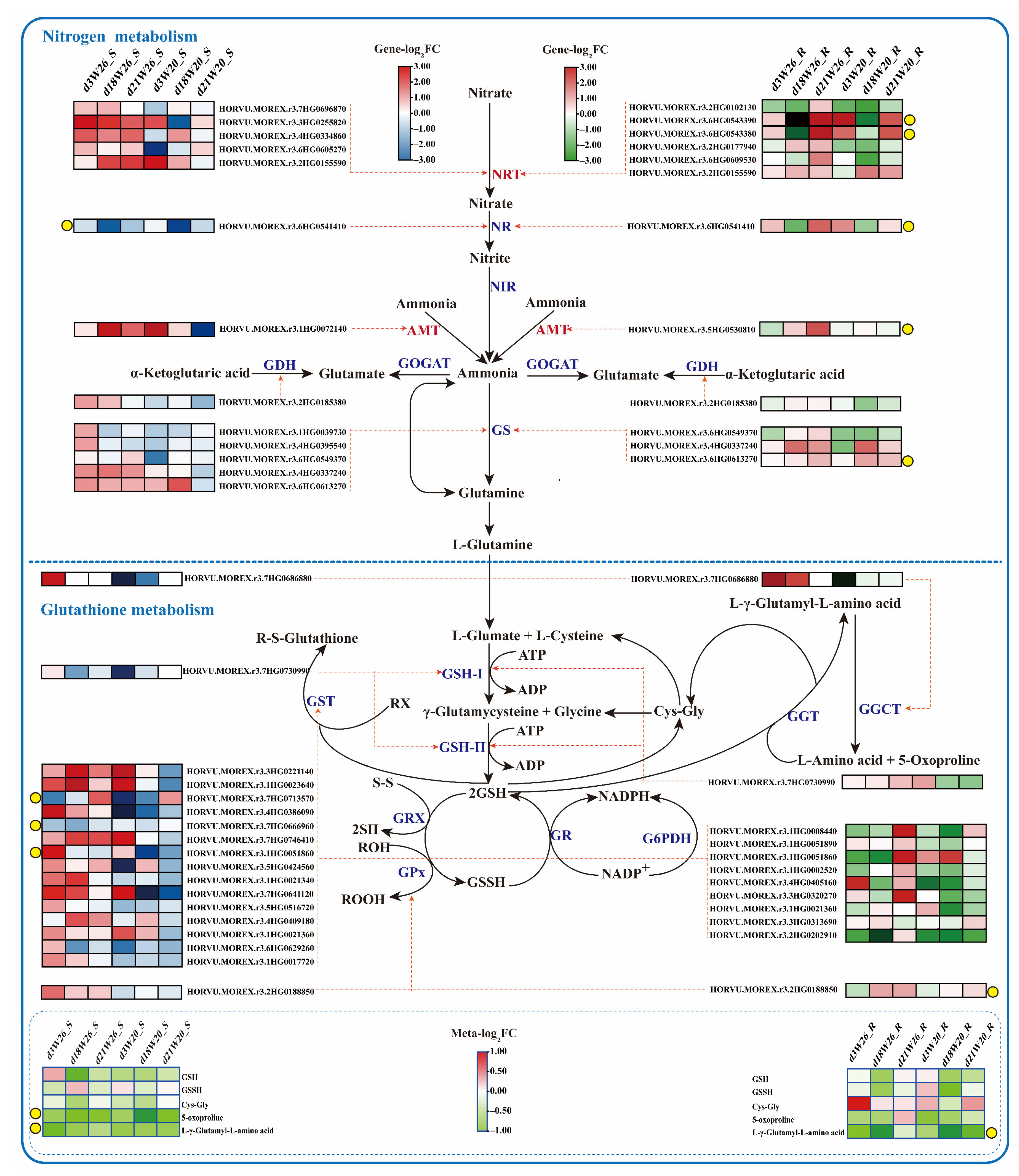
| Genotype | Treatment | NUE (%) |
|---|---|---|
| W26 | CK | 19.50 c |
| LN | 87.52 a | |
| W20 | CK | 19.58 c |
| LN | 61.74 b |
| Macronutrients | Micronutrients | ||
|---|---|---|---|
| Name | Concentration (mM) | Name | Concentration (μM) |
| Ca(NO3)2·4H2O | 0.53 | KI | 5 |
| KNO3 | 0.67 | H3BO3 | 100 |
| NH4NO3 | 0.13 | MnSO4 | 150 |
| KH2PO4 | 0.13 | ZnSO4·7H2O | 30 |
| MgSO4·7H2O | 0.27 | Na2MoO4·2H2O | 1 |
| CuSO4·5H2O | 0.1 | ||
| CoCl2·6H2O | 0.1 | ||
| Na2EDTA·2H2O | 20 | ||
| FeSO4·7H2O | 20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wang, J.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Li, C.; Shang, X.; Meng, Y.; et al. Transcriptome and Metabolome Reveal the Molecular Mechanism of Barley Genotypes Underlying the Response to Low Nitrogen and Resupply. Int. J. Mol. Sci. 2023, 24, 4706. https://doi.org/10.3390/ijms24054706
Wang G, Wang J, Yao L, Li B, Ma X, Si E, Yang K, Li C, Shang X, Meng Y, et al. Transcriptome and Metabolome Reveal the Molecular Mechanism of Barley Genotypes Underlying the Response to Low Nitrogen and Resupply. International Journal of Molecular Sciences. 2023; 24(5):4706. https://doi.org/10.3390/ijms24054706
Chicago/Turabian StyleWang, Gang, Juncheng Wang, Lirong Yao, Baochun Li, Xiaole Ma, Erjing Si, Ke Yang, Chengdao Li, Xunwu Shang, Yaxiong Meng, and et al. 2023. "Transcriptome and Metabolome Reveal the Molecular Mechanism of Barley Genotypes Underlying the Response to Low Nitrogen and Resupply" International Journal of Molecular Sciences 24, no. 5: 4706. https://doi.org/10.3390/ijms24054706






