Ergotamine Stimulates Human 5-HT4-Serotonin Receptors and Human H2-Histamine Receptors in the Heart
Abstract
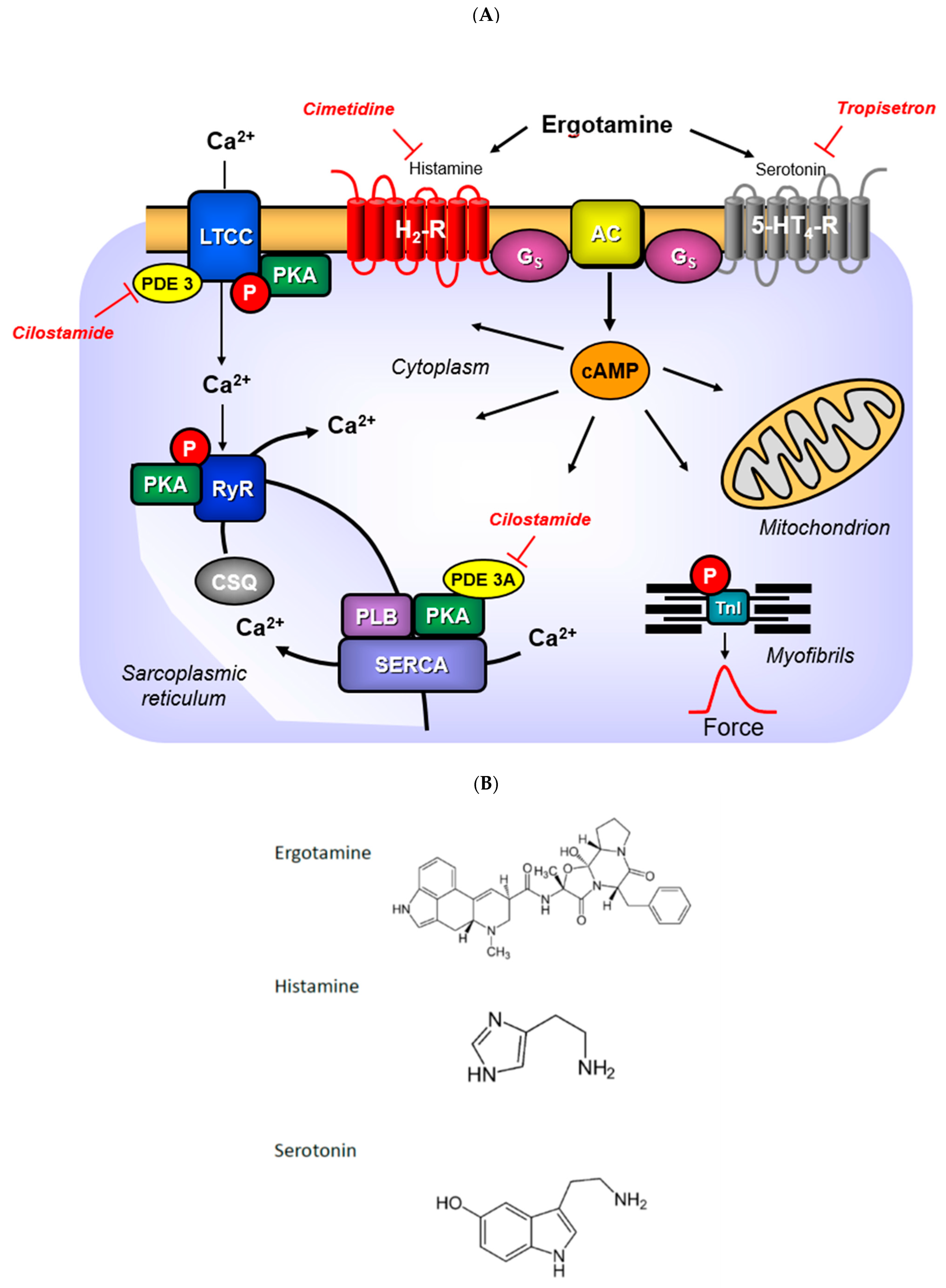
:1. Introduction
2. Results
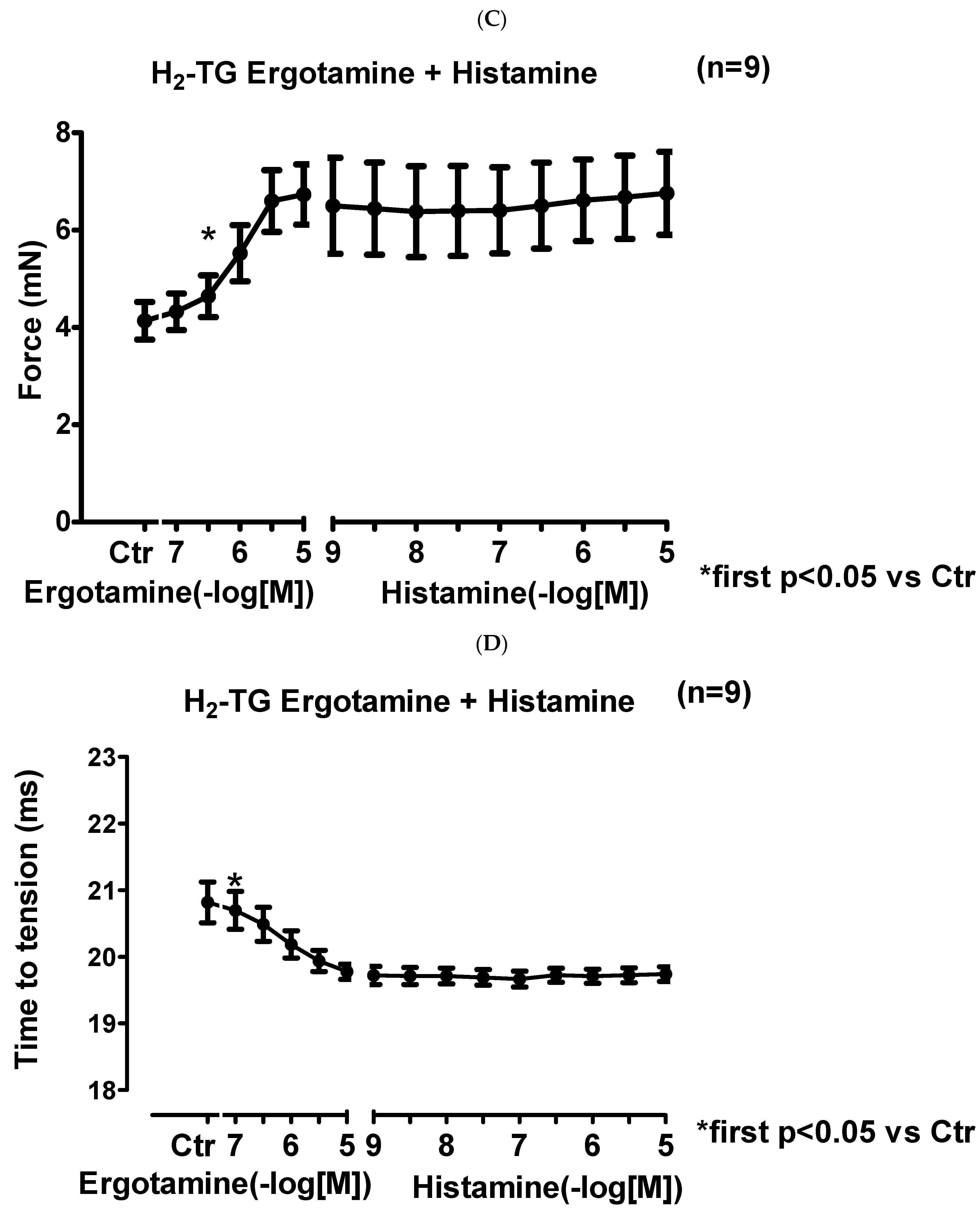
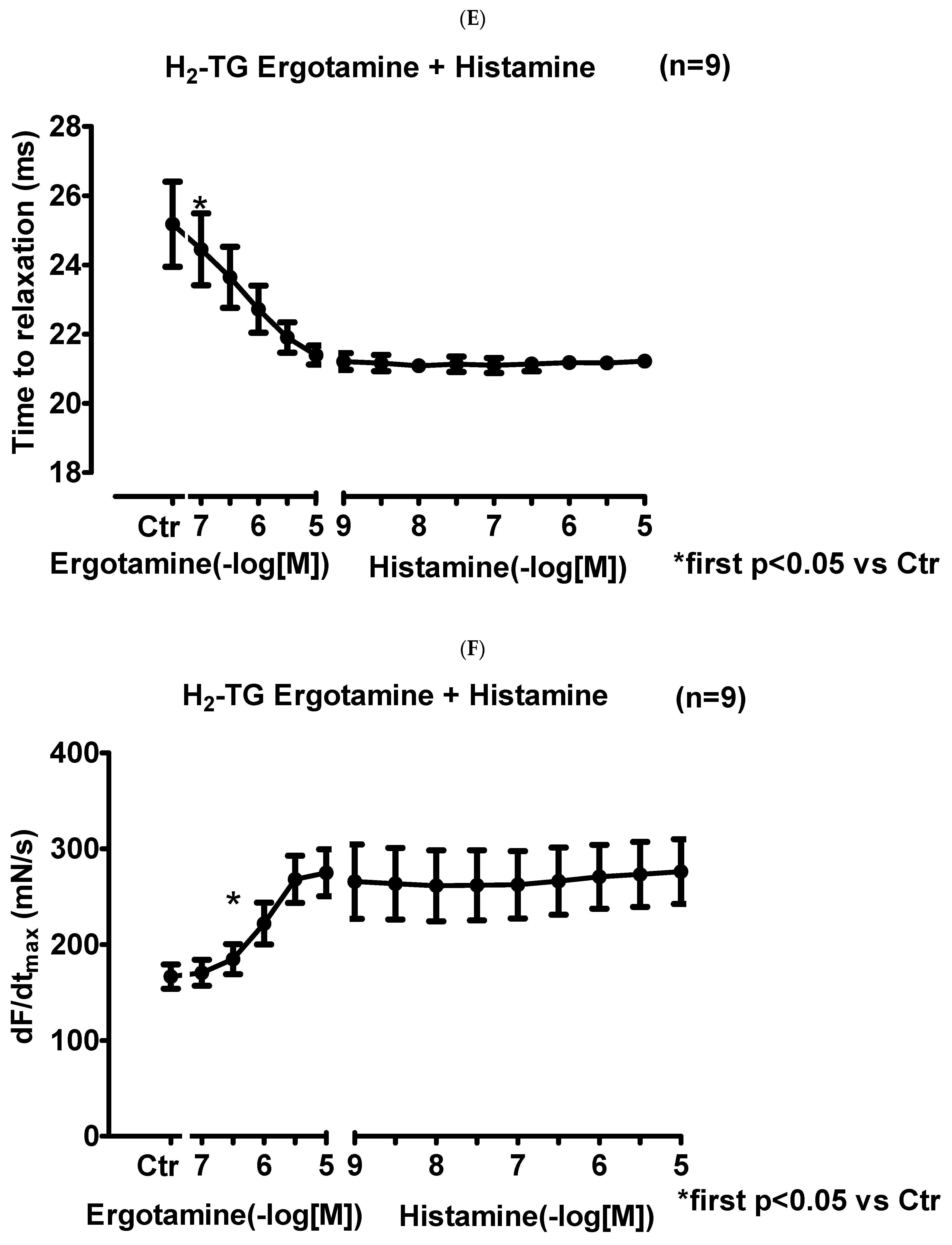
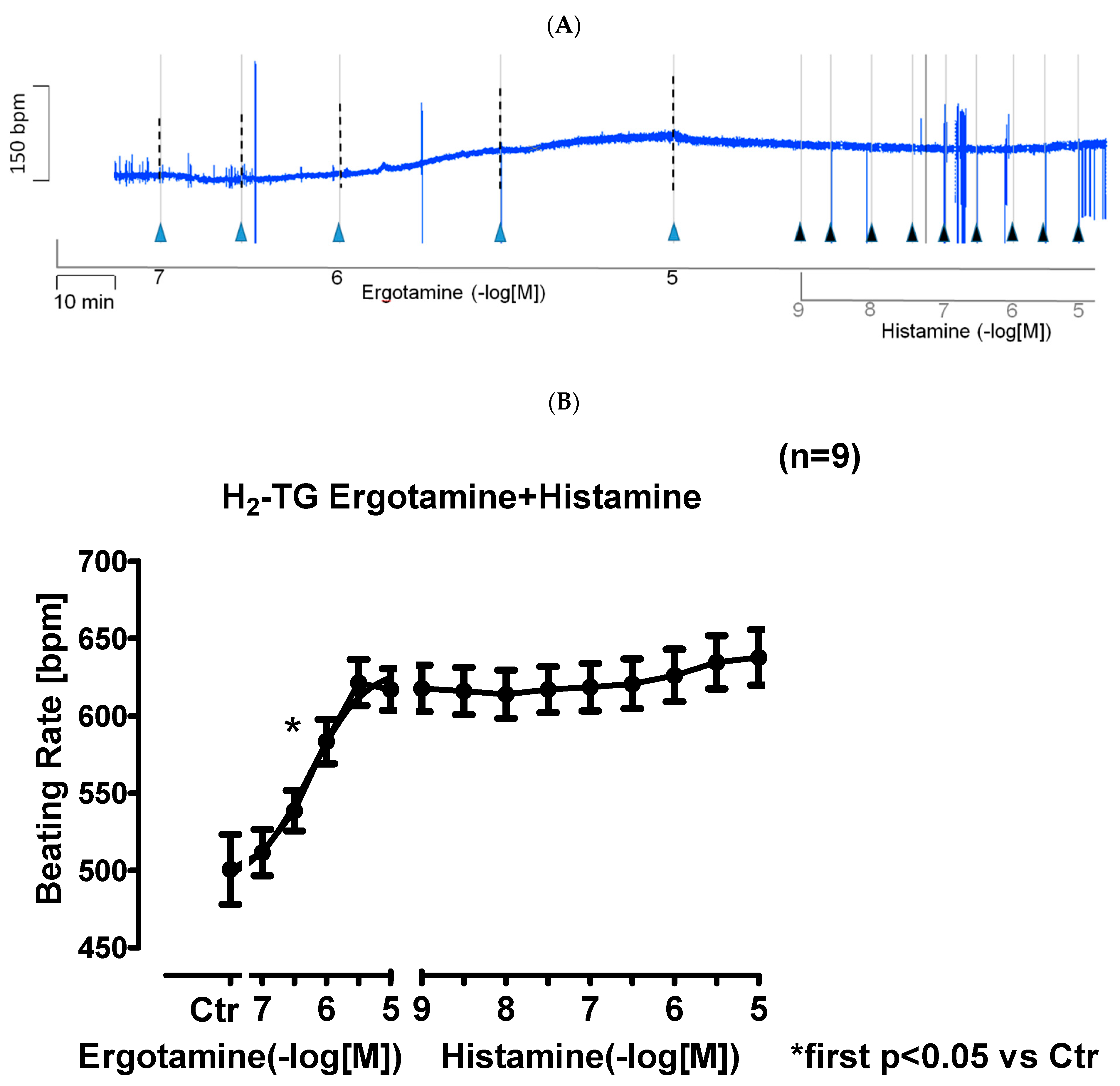
2.1. H2-TG Atrium
2.1.1. Left Atrium
2.1.2. Right Atrium
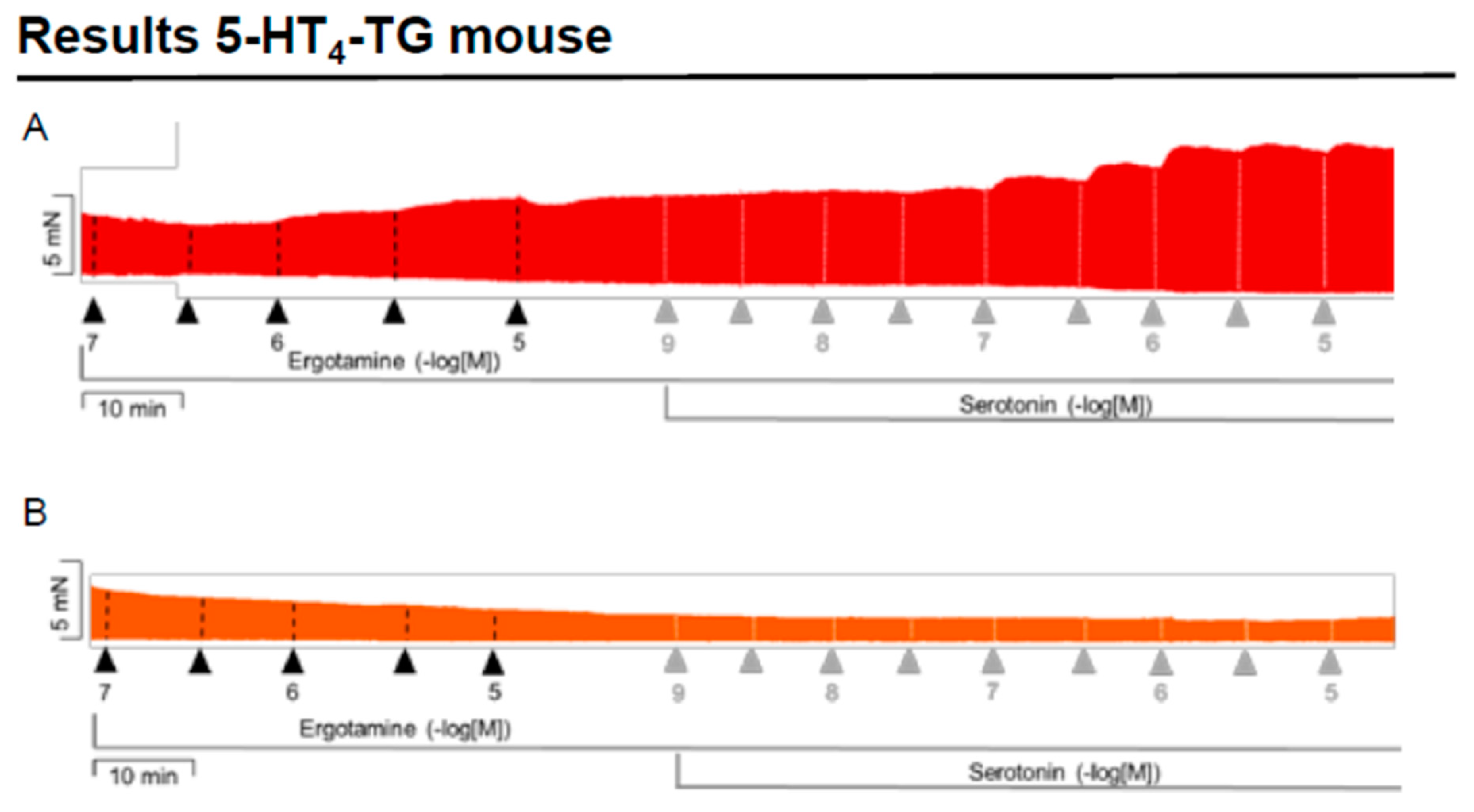
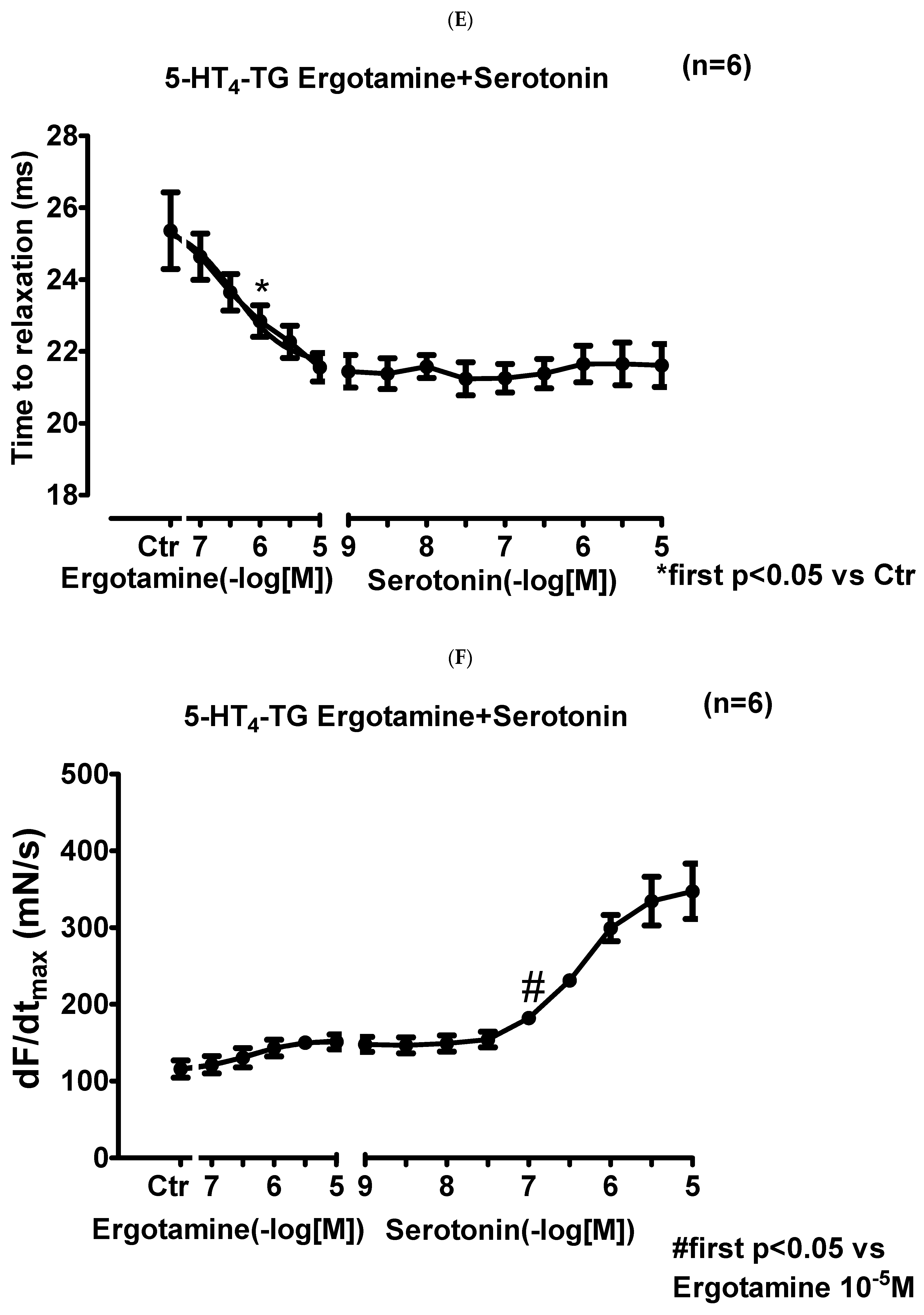
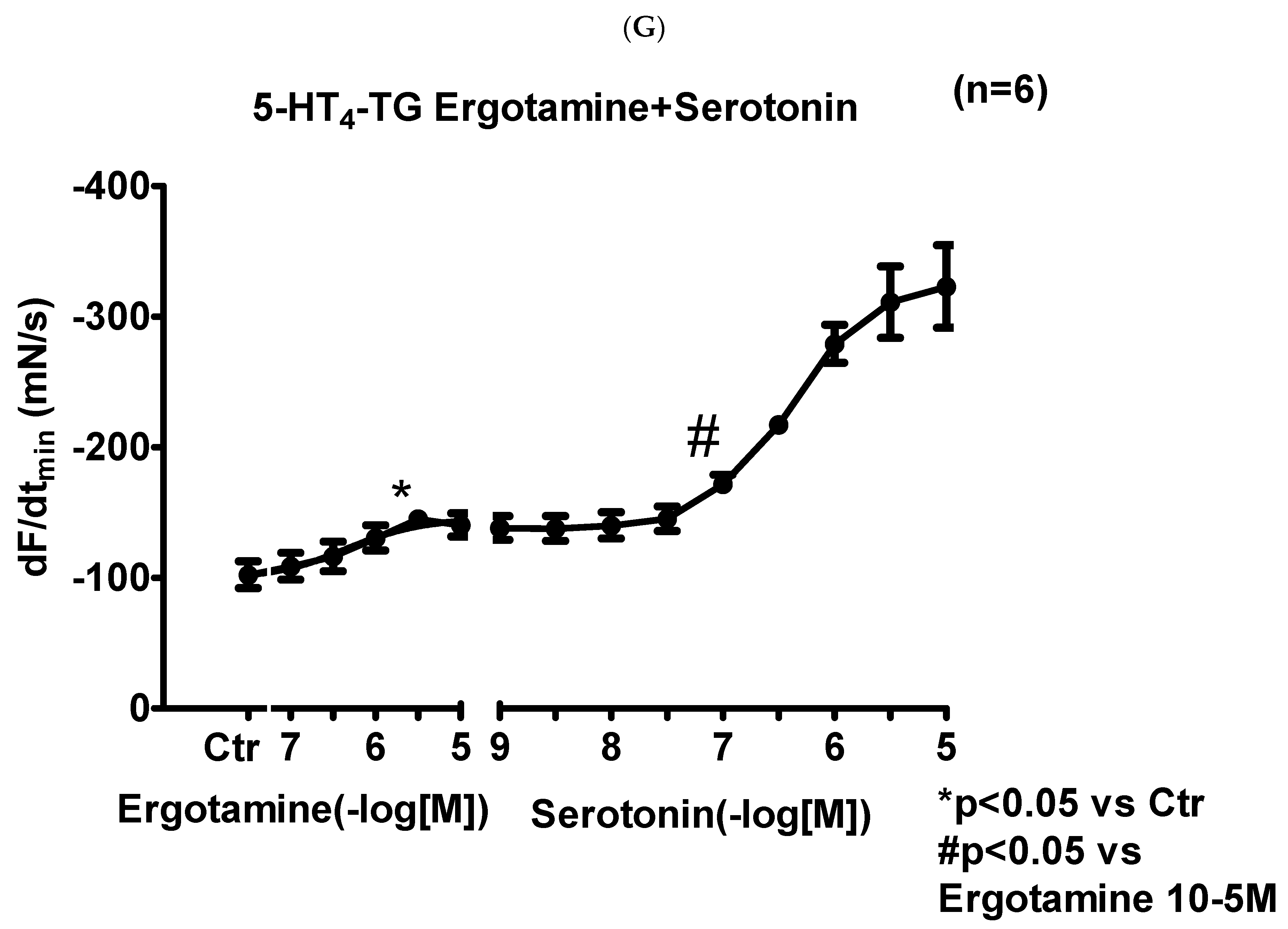
2.2. 5-HT4-TG
2.2.1. Left Atrium
2.2.2. Right Atrium
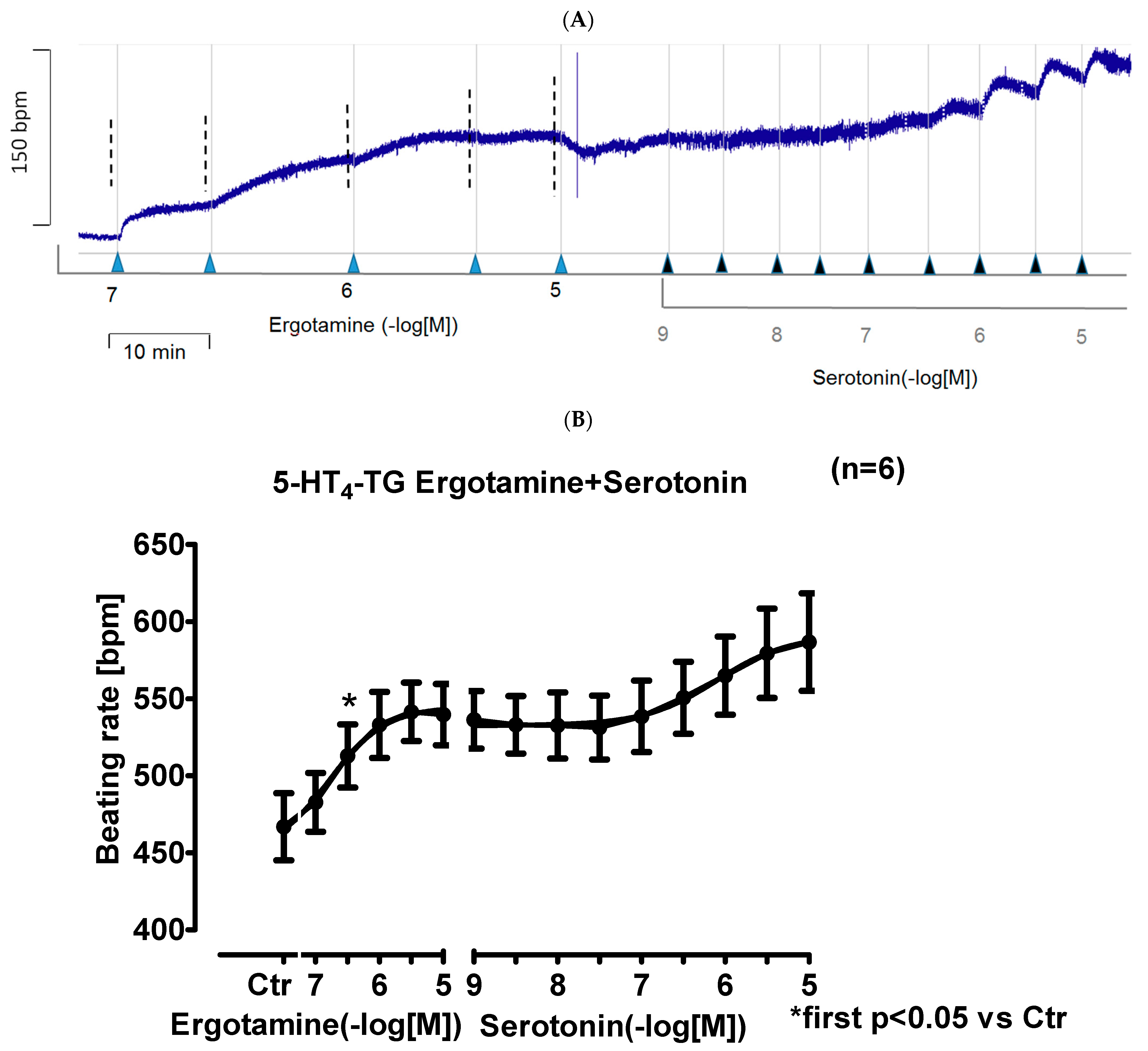
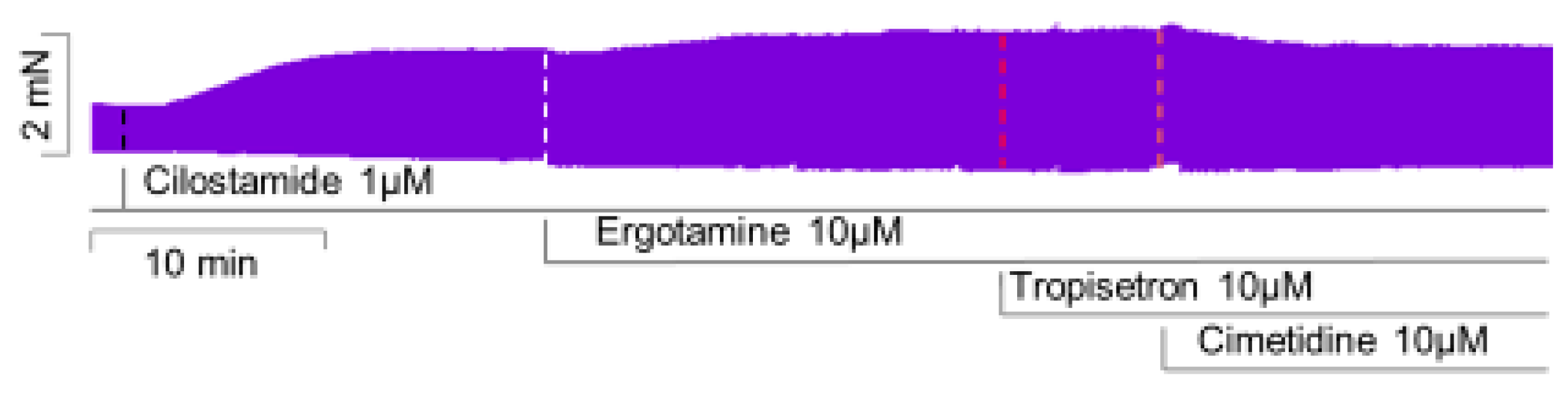
2.3. Isolated Perfused Hearts
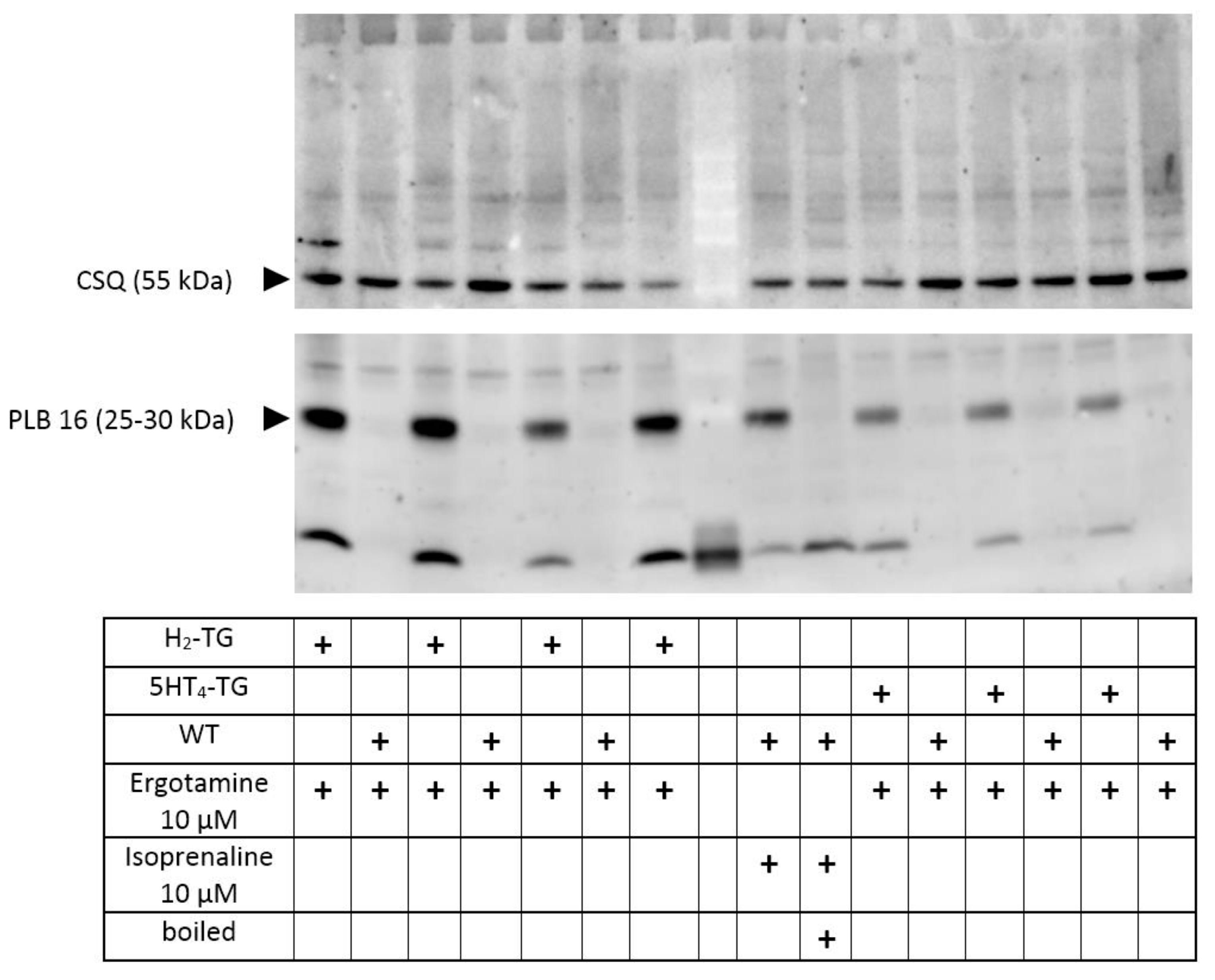
2.4. Phosphorylation
2.5. Human Atrial Contraction
3. Discussion
3.1. Main New Findings
3.2. 5-HT4-Receptors
3.3. H2-Receptors
3.4. Mechanism of Ergotamine
3.5. Role of Phosphorylation of Regulatory Proteins
3.6. Contractile Role of Ergotamine in Human Atrium
3.7. Clinical Relevance
3.8. Limitations of the Study
4. Materials and Methods
4.1. Transgenic Mice
4.2. Contractile Studies in Mice
4.3. Contractile Studies on Human Preparations
4.4. Isolated Perfused Hearts
4.5. Western Blotting
4.6. Data Analysis
4.7. Drugs and Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, J.L.; Munshi, K.; Peasah, S.K.; Swart, E.C.S.; Kohli, M.; Henderson, R.; Good, C.B. Trends in utilization and costs of migraine medications, 2017–2020. J. Headache Pain 2022, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.; Tadi, P. Ergotamine/Caffeine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gulbranson, S.H.; Mock, R.E.; Wolfrey, J.D. Possible Ergotamine-Caffeine–Associated Delirium. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; McCrory, D. Ergotamine and Dihydroergotamine: History, Pharmacology, and Efficacy. Headache 2003, 43, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Stange, K.; Pohlmeier, H.; Lübbesmeyer, A.; Gumbinger, G.; Schmitz, W.; Baumgart, P. Vaskulärer Ergotismus durch Getreidestaubinhalation [Vascular ergotism through inhalation of grain dust]. Dtsch. Med. Wochenschr. 1998, 123, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.A.; Liegl, C.A. Ergotism: Case Report and Review of the Literature. Int. J. Angiol. 2016, 25, e8–e11. [Google Scholar] [CrossRef] [Green Version]
- Cervellin, G.; Longobardi, U.; Lippi, G. One holy man, one eponym, three distinct diseases. St. Anthonys Fire Revisited. Acta Biomed. 2020, 92, e2021008. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, B.; Malysheva, S.V.; Masquelier, J. A Targeted UHPLC-MS/MS Method Validated for the Quantification of Ergot Alkaloids in Cereal-Based Baby Food from the Belgian Market. Toxins 2021, 13, 531. [Google Scholar] [CrossRef]
- Jamieson, C.S.; Misa, J.; Tang, Y.; Billingsley, J.M. Biosynthesis and synthetic biology of psychoactive natural products. Chem. Soc. Rev. 2021, 50, 6950–7008. [Google Scholar] [CrossRef]
- Rabinowitz, B.; Chuck, L.; Kligerman, M.; Parmley, W.W. Positive inotropic effects of methoxamine: Evidence for alpha-adrenergic receptors in ventricular myocardium. Am. J. Physiol. Content 1975, 229, 582–585. [Google Scholar] [CrossRef] [Green Version]
- Laher, I.; McNeill, J.H. Effects of histamine on rat isolated atria. Can. J. Physiol. Pharmacol. 1980, 58, 1114–1116. [Google Scholar] [CrossRef]
- Laher, I.; McNeill, J.H. Effects of histamine in the isolated kitten heart. Can. J. Physiol. Pharmacol. 1980, 58, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. The Roles of Cardiovascular H2-Histamine Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 12, 732842. [Google Scholar] [CrossRef] [PubMed]
- Bongrani, S.; Di Donato, M.; Visioli, O.; Mantovani, P. Effect of ergometrine on contractile force of guinea-pig isolated heart: Antagonism by cimetidine. Agents Actions 1979, 9, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Angus, J.A.; Black, J.W. Pharmacological assay of cardiac H2-receptor blockade by amitriptyline and lysergic acid diethylamide. Circ. Res. 1980, 46 Pt 2, I64–I69. [Google Scholar]
- Cortijo, J.; Martí-Cabrera, M.; Bernabeu, E.; Domènech, T.; Bou, J.; Fernández, A.G.; Beleta, J.; Palacios, J.M.; Morcillo, E. Characterization of 5-HT receptors on human pulmonary artery and vein: Functional and binding studies. Br. J. Pharmacol. 1997, 122, 1455–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaumann, A.J.; Levy, F.O. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Hofmann, B.; Gergs, U. Production and function of serotonin in cardiac cells. In Serotonin—A Chemical Messenger Between All Types of Living Cells; Kaneez, F.S., Ed.; Intech: Rijeka, Croatia, 2017; Chapter 13; pp. 271–305. ISBN 978-953-51-3361-2. [Google Scholar]
- Gergs, U.; Baumann, M.; Böckler, A.; Buchwalow, I.B.; Ebelt, H.; Fabritz, L.; Hauptmann, S.; Keller, N.; Kirchhof, P.; Klöckner, U.; et al. Cardiac overexpression of the human 5-HT4receptor in mice. Am. J. Physiol. Circ. Physiol. 2010, 299, H788–H798. [Google Scholar] [CrossRef] [Green Version]
- Gergs, U.; Böckler, A.; Ebelt, H.; Hauptmann, S.; Keller, N.; Otto, V.; Pönicke, K.; Schmitz, W.; Neumann, J. Human 5-HT4 receptor stimulation in atria of transgenic mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2013, 386, 357–367. [Google Scholar] [CrossRef]
- Neumann, J.; Käufler, B.; Gergs, U. Which phosphodiesterase can decrease cardiac effects of 5-HT4 receptor activation in transgenic mice? Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 991–1004. [Google Scholar] [CrossRef]
- Neumann, J.; Schwarzer, D.; Fehse, C.; Schwarz, R.; Marusakova, M.; Kirchhefer, U.; Hofmann, B.; Gergs, U. Functional interaction of H2-receptors and 5HT4-receptors in atrial tissues isolated from double transgenic mice and from human patients. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 2401–2418. [Google Scholar] [CrossRef]
- Gergs, U.; Bernhardt, G.; Buchwalow, I.B.; Edler, H.; Fröba, J.; Keller, M.; Kirchhefer, U.; Köhler, F.; Mißlinger, N.; Wache, H.; et al. Initial Characterization of Transgenic Mice Overexpressing Human Histamine H2Receptors. J. Pharmacol. Exp. Ther. 2019, 369, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gergs, U.; Kirchhefer, U.; Bergmann, F.; Künstler, B.; Mißlinger, N.; Au, B.; Mahnkopf, M.; Wache, H.; Neumann, J. Characterization of Stressed Transgenic Mice Overexpressing H2-Histamine Receptors in the Heart. J. Pharmacol. Exp. Ther. 2020, 374, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Gergs, U.; Büxel, M.L.; Bresinsky, M.; Kirchhefer, U.; Fehse, C.; Höring, C.; Hofmann, B.; Marušáková, M.; Čináková, A.; Schwarz, R.; et al. Cardiac Effects of Novel Histamine H2 Receptor Agonists. J. Pharm. Exp. Ther. 2021, 379, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Voss, R.; Laufs, U.; Werner, C.; Gergs, U. Phosphodiesterases 2, 3 and 4 can decrease cardiac effects of H2-histamine-receptor activation in isolated atria of transgenic mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 1215–1229. [Google Scholar] [CrossRef]
- Neumann, J.; Binter, M.B.; Fehse, C.; Marušáková, M.; Büxel, M.L.; Kirchhefer, U.; Hofmann, B.; Gergs, U. Amitriptyline functionally antagonizes cardiac H2 histamine receptors in transgenic mice and human atria. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 1251–1262. [Google Scholar] [CrossRef]
- Neumann, J.; Grobe, J.M.; Weisgut, J.; Schwelberger, H.G.; Fogel, W.A.; Marušáková, M.; Wache, H.; Bähre, H.; Buchwalow, I.B.; Dhein, S.; et al. Histamine can be Formed and Degraded in the Human and Mouse Heart. Front. Pharmacol. 2021, 12, 582916. [Google Scholar] [CrossRef]
- Flacke, W.; Atanacković, D.; Gillis, R.A.; Alper, M.H. The actions of histamine on the mammalian heart. J. Pharmacol. Exp. Ther. 1967, 155, 271–278. [Google Scholar]
- Dai, S. A study of the actions of histamine on the isolated rat heart. Clin. Exp. Pharm. Physiol. 1976, 3, 359–367. [Google Scholar] [CrossRef]
- Bartlet, A.L. The action of histamine in the isolated heart. Br. J. Pharm. Chemother. 1963, 21, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Wellner-Kienitz, M.C.; Bender, K.; Meyer, T.; Pott, L. Coupling to Gs and G(q/11) of histamine H2 receptors heterologously expressed in adult rat atrial myocytes. Biochim. Biophys. Acta 2003, 1642, 67–77. [Google Scholar] [CrossRef]
- Baumann, G.; Mercader, D.; Busch, U.; Felix, S.B.; Loher, U.; Ludwig, L.; Sebening, H.; Heidecke, C.D.; Hagl, S.; Sebening, F.; et al. Effects of the H2-receptor agonist impromidine in human myocardium from patients with heart failure due to mitral and aortic valve disease. J. Cardiovasc. Pharmacol. 1983, 5, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Jesmin, S.; Takahashi, Y.; Hatta, E.; Kobayashi, M.; Matsuyama, K.; Kawakami, N.; Sakuma, I.; Gando, S.; Fukui, H.; et al. Histamine H1 and H2 receptor gene and protein levels are differentially expressed in the hearts of rodents and humans. J. Pharm. Exp. Ther. 2004, 309, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Levi, R.; Malm, J.R.; Bowman, F.O.; Rosen, M.R. The arrhythmogenic actions of histamine on human atrial fibers. Circ. Res. 1981, 49, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, A.; Gross, S.S.; Sakuma, I.; Levi, R. Adenosine promotes histamine H1-mediated negative chronotropic and inotropic effects on human atrial myocardium. J. Pharm. Exp. Ther. 1988, 247, 844–849. [Google Scholar]
- Zerkowski, H.R.; Broede, A.; Kunde, K.; Hillemann, S.; Schäfer, E.; Vogelsang, M.; Michel, M.C.; Brodde, O.E. Comparison of the positive inotropic effects of serotonin, histamine, angiotensin II, endothelin and isoprenaline in the isolated human right atrium. Naunyn-Schmiedebergs Arch. Pharmacol. 1993, 347, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.; Braekow, P.; Höhm, C.; Gergs, U.; Hofmann, B.; Kirchhefer, U.; Humphrys, L.; Pockes, S.; Neumann, J. Effects of ergotamine, ergometrine and LSD on mouse and human atrial preparations. Naunyn-Schmiedebergs Arch. Pharm. 2023, in press. [Google Scholar]
- Wang, C.; Jiang, Y.; Ma, J.; Wu, H.; Wacker, D.; Katritch, V.; Han, G.W.; Liu, W.; Huang, X.P.; Vardy, E.; et al. Structural basis for molecular recognition at serotonin receptors. Science 2013, 340, 610–614. [Google Scholar] [CrossRef] [Green Version]
- Simmerman, H.K.; Collins, J.H.; Theibert, J.L.; Wegener, A.D.; Jones, L.R. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 1986, 261, 13333–13341. [Google Scholar] [CrossRef]
- Christ, T.; Rozmaritsa, N.; Engel, A.; Berk, E.; Knaut, M.; Metzner, K.; Canteras, M.; Ravens, U.; Kaumann, A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 11193–11198, Erratum in Proc. Natl. Acad. Sci. USA 2014, 111, 14003. [Google Scholar] [CrossRef] [Green Version]
- Gergs, U.; Neumann, J.; Simm, A.; Silber, R.E.; Remmers, F.O.; Läer, S. Phosphorylation of phospholamban and troponin I through 5-HT4 receptors in the isolated human atrium. Naunyn-Schmiedebergs Arch. Pharmacol. 2009, 379, 349–359. [Google Scholar] [CrossRef]
- Brattelid, T.; Qvigstad, E.; Lynham, J.A.; Molenaar, P.; Aass, H.; Geiran, O.; Skomedal, T.; Osnes, J.B.; Levy, F.O.; Kaumann, A.J. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn-Schmiedebergs Arch. Pharmacol. 2004, 370, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.W.; Haering, N.; Mosberg, H.; Jaeger, H. Pharmacokinetics of ergotamine in healthy volunteers following oral and rectal dosing. Eur. J. Clin. Pharmacol. 1986, 30, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Mohamedi, N.; Mirault, T.; Durivage, A.; Di Primio, M.; Khider, L.; Detriche, G.; El Batti, S.; Sapoval, M.; Messas, E.; Goudot, G. Ergotism with acute limb ischemia, provoked by HIV protease inhibitors interaction with ergotamine, rescued by multisite transluminal balloon angioplasty. J. Med. Vasc. 2021, 46, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Velez Roa, S.; Preumont, N.; Mols, P.; Stoupel, E.; Renard, M. Ventricular fibrillation secondary to ergotamine in a healthy young woman. Acta Cardiol. 1999, 54, 283–286. [Google Scholar]
- Okutucu, S.; Karakulak, U.N.; Kabakcı, G.; Aytemir, K. An unusual cause of chest pain: Acute coronary syndrome following administration of ergotamine tartrate. Exp. Clin. Cardiol. 2012, 17, 43–44. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]




| WT | H2-TG | 5-HT4-TG | |
|---|---|---|---|
| N= | 7 | 7 | 4 |
| Basal force (mN) | 11.3 ± 1.2 | 11.3 ± 1.5 | 14.9 ± 2.1 |
| Force after ergotamine (mN) | 12.3 ± 1.2 | 15.8 ± 1.8 # | 18.5 ± 2.9 # |
| Basal rate of relaxation (mN/s) | 224 ± 14.1 | 208 ± 28.4 | 218 ± 14.0 |
| Rate of relaxation after ergotamine (mN/s) | 250 ± 18.6 | 315 ± 44.8 # | 287 ± 27.8 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, H.; Braekow, P.; Schwarz, R.; Höhm, C.; Kirchhefer, U.; Hofmann, B.; Neumann, J.; Gergs, U. Ergotamine Stimulates Human 5-HT4-Serotonin Receptors and Human H2-Histamine Receptors in the Heart. Int. J. Mol. Sci. 2023, 24, 4749. https://doi.org/10.3390/ijms24054749
Jacob H, Braekow P, Schwarz R, Höhm C, Kirchhefer U, Hofmann B, Neumann J, Gergs U. Ergotamine Stimulates Human 5-HT4-Serotonin Receptors and Human H2-Histamine Receptors in the Heart. International Journal of Molecular Sciences. 2023; 24(5):4749. https://doi.org/10.3390/ijms24054749
Chicago/Turabian StyleJacob, Hannes, Pauline Braekow, Rebecca Schwarz, Christian Höhm, Uwe Kirchhefer, Britt Hofmann, Joachim Neumann, and Ulrich Gergs. 2023. "Ergotamine Stimulates Human 5-HT4-Serotonin Receptors and Human H2-Histamine Receptors in the Heart" International Journal of Molecular Sciences 24, no. 5: 4749. https://doi.org/10.3390/ijms24054749
APA StyleJacob, H., Braekow, P., Schwarz, R., Höhm, C., Kirchhefer, U., Hofmann, B., Neumann, J., & Gergs, U. (2023). Ergotamine Stimulates Human 5-HT4-Serotonin Receptors and Human H2-Histamine Receptors in the Heart. International Journal of Molecular Sciences, 24(5), 4749. https://doi.org/10.3390/ijms24054749






