The Longitudinal Changes in Subcutaneous Abdominal Tissue and Visceral Adipose Tissue Volumetries Are Associated with Iron Status
Abstract
:1. Introduction
2. Results
2.1. Cross-Sectional Findings
2.2. Longitudinal Findings
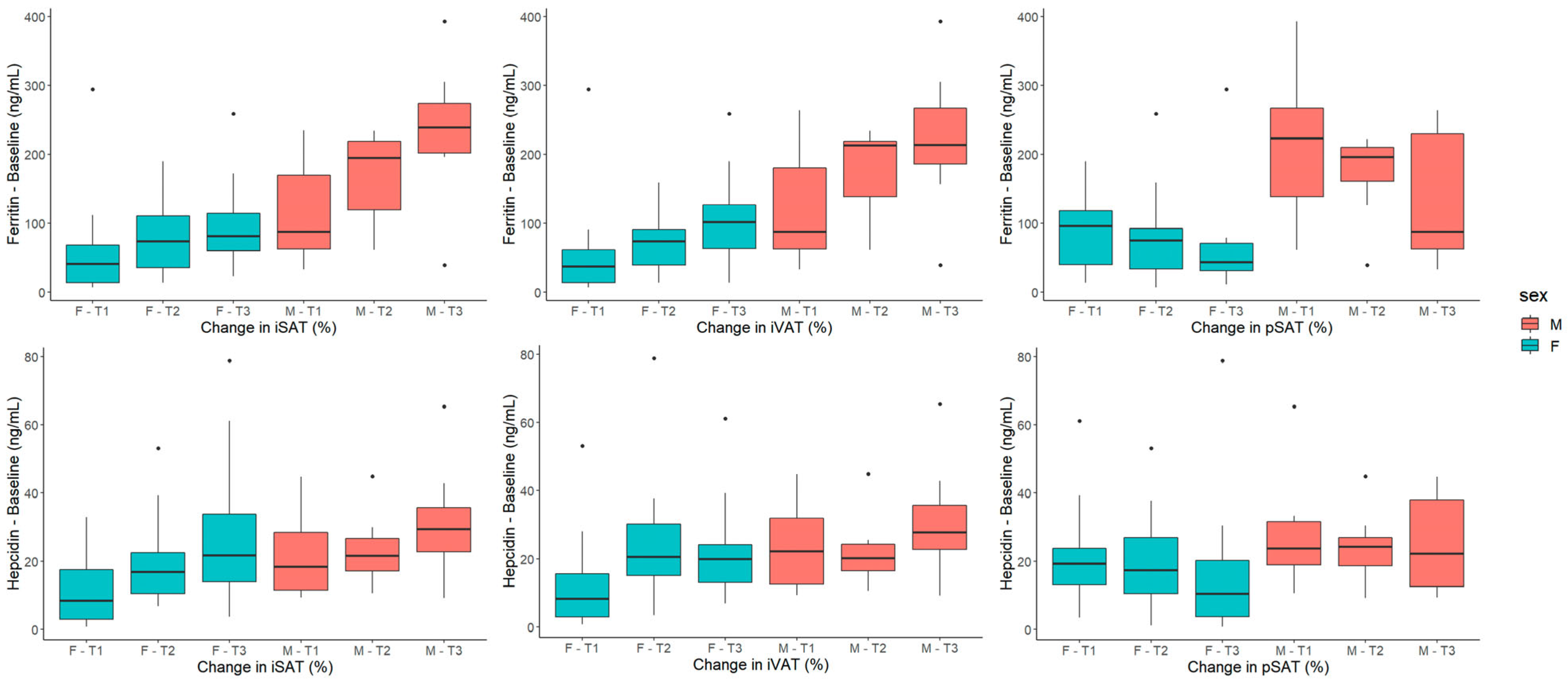
2.2.1. Baseline Parameters of Iron Metabolism and Chronic Inflammation Are Associated with Changes in Adipose Tissue Volumetries
2.2.2. Changes in the Chronic-Inflammation Marker, Ultrasensitive CRP, Are Associated with Changes in Adipose Tissue Volumetries
2.2.3. Baseline Parameters of Iron Metabolism, Chronic Inflammation and Adipose Tissue Volumetries Are Associated with Changes in Insulin Sensitivity
2.2.4. Adrenal- and Gonadal-Steroid Hormones Are Not Associated with Changes in Adipose Tissue Volumetries or Baseline Parameters of Iron Metabolism
2.2.5. Multiple-Linear-Regression Models
2.2.6. Serum Ferritin and Hepatic Iron
3. Discussion
3.1. Iron or Chronic Low-Grade Inflammation: The Role of Iron
3.2. The Role of Chronic Low-Grade Inflammation
3.3. The Role of Insulin Sensitivity
4. Materials and Methods
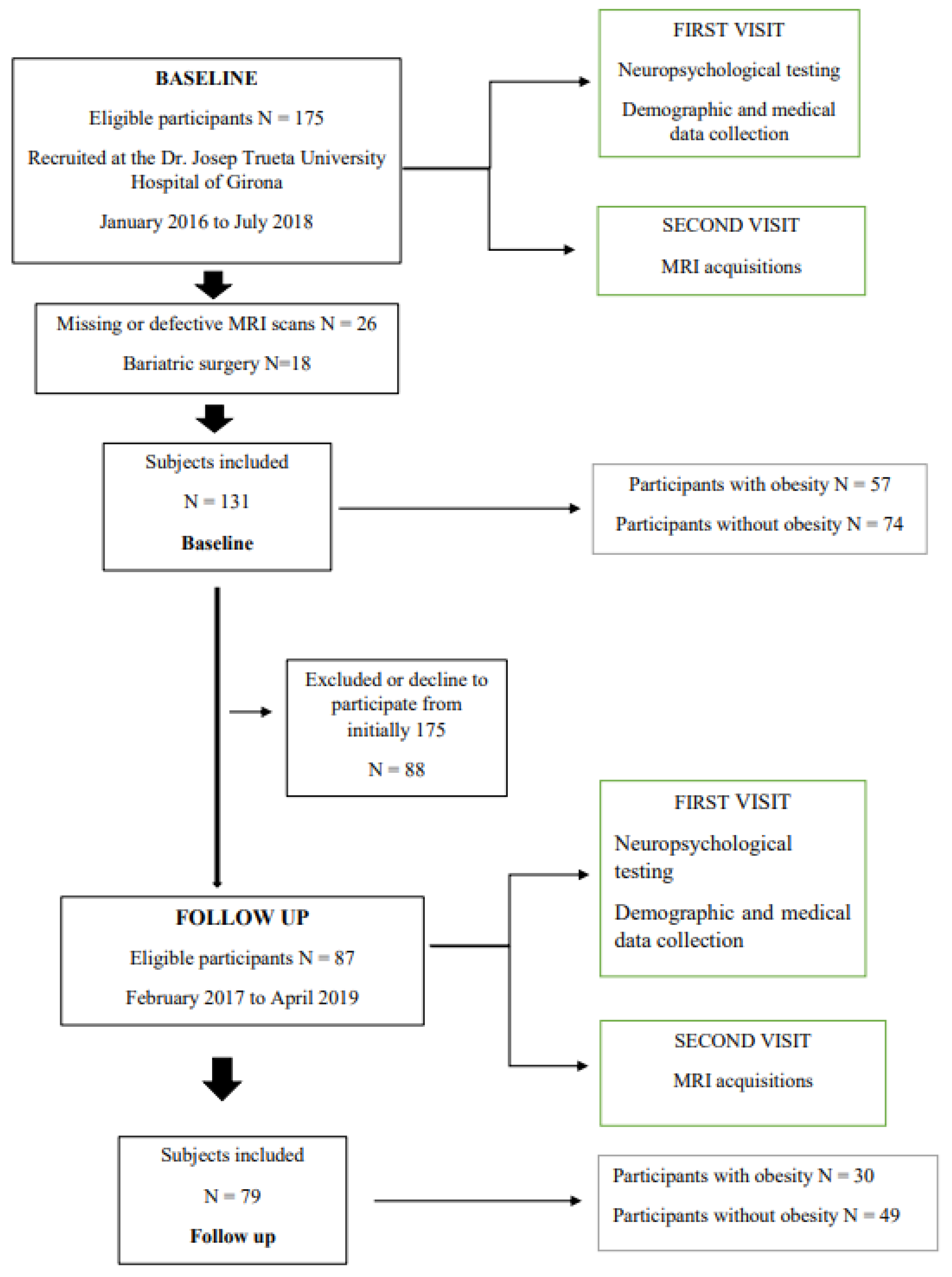
4.1. Study Design
4.2. Clinical and Laboratory Features
4.3. Image Acquisition
4.4. Image Processing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iwasaki, T.; Nakajima, A.; Yoneda, M.; Yamada, Y.; Mukasa, K.; Fujita, K.; Fujisawa, N.; Wada, K.; Terauchi, Y. Serum Ferritin Is Associated With Visceral Fat Area and Subcutaneous Fat Area. Diabetes Care 2005, 28, 2486–2491. [Google Scholar] [CrossRef] [Green Version]
- Kerkadi, A.; Ali, R.M.; Shehada, A.A.H.; AbouHassanein, E.A.; Moawad, J.; Bawadi, H.; Shi, Z. Association between central obesity indices and iron status indicators among Qatari adults. PLoS ONE 2021, 16, e0250759. [Google Scholar] [CrossRef]
- Wu, H.; Qi, Q.; Yu, Z.; Sun, L.; Li, H.; Lin, X. Opposite associations of trunk and leg fat depots with plasma ferritin levels in middle-aged and older Chinese men and women. PLoS ONE 2010, 5, e13316. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Moreno, M.; Puig, J.; Blasco, G.; Ortega, F.J.; Xifra, G.; Ricart, W.; Fernández-Real, J.M. Hepatic iron content is independently associated with serum hepcidin levels in subjects with obesity. Clin. Nutr. 2017, 36, 1434–1439. [Google Scholar] [CrossRef]
- Cheng, H.L.; Bryant, C.; Cook, R.; O’Connor, H.; Rooney, K.; Steinbeck, K. The relationship between obesity and hypoferraemia in adults: A systematic review. Obes. Rev. 2012, 13, 150–161. [Google Scholar] [CrossRef]
- Wlazlo, N.; Van Greevenbroek, M.M.; Ferreira, I.; Jansen, E.H.; Feskens, E.J.; Van Der Kallen, C.J.; Schalkwijk, C.G.; Bravenboer, B.; Stehouwer, C.D. Iron metabolism is associated with adipocyte insulin resistance and plasma adiponectin: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care 2013, 36, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Gabrielsen, J.S.; Gao, Y.; Simcox, J.A.; Huang, J.; Thorup, D.; Jones, D.; Cooksey, R.C.; Gabrielsen, D.; Adams, T.D.; Hunt, S.C.; et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J. Clin. Investig. 2012, 122, 3529–3540. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Novelle, M.G.; Catalán, V.; Ortega, F.; Moreno, M.; Gomez-Ambrosi, J.; Xifra, G.; Serrano, M.; Guerra, E.; Ricart, W.; et al. Insulin resistance modulates iron-related proteins in adipose tissue. Diabetes Care 2014, 37, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Ortega, F.; Moreno, M.; Ricart, W.; Fernández-Real, J.M. Fine-tuned iron availability is essential to achieve optimal adipocyte differentiation and mitochondrial biogenesis. Diabetologia 2014, 57, 1957–1967. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Moreno, M.; Serrano, M.; Ricart, W.; Fernández-Real, J.M. Lactoferrin gene knockdown leads to similar effects to iron chelation in human adipocytes. J. Cell. Mol. Med. 2014, 18, 391–395. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Liu, Z.Y.; Guan, Z.A.; Guo, C. Deferoxamine ameliorates adipocyte dysfunction by modulating iron metabolism in ob/ob mice. Endocr. Connect. 2018, 7, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Funcke, J.-B.; Zi, Z.; Zhao, S.; Straub, L.G.; Zhu, Y.; Zhu, Q.; Crewe, C.; An, Y.A.; Chen, S.; et al. Adipocyte iron levels impinge on a fat-gut crosstalk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab. 2021, 33, 1624–1639.e9. [Google Scholar] [CrossRef] [PubMed]
- Rospleszcz, S.; Dermyshi, D.; Müller-Peltzer, K.; Strauch, K.; Bamberg, F.; Peters, A. Association of serum uric acid with visceral, subcutaneous and hepatic fat quantified by magnetic resonance imaging. Sci. Rep. 2020, 10, 442. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.L.; Koehler, E.; Herring, A.H.; Paynter, L.; Du, S.; Zhang, B.; Popkin, B.; Gordon-Larsen, P. Weight Gain Trajectories Associated With Elevated C-Reactive Protein Levels in Chinese Adults. J. Am. Heart Assoc. 2016, 5, e003262. [Google Scholar] [CrossRef]
- Holz, T.; Thorand, B.; Döring, A.; Schneider, A.; Meisinger, C.; Koenig, W. Markers of Inflammation and Weight Change in Middle-Aged Adults: Results From the Prospective MONICA/KORA S3/F3 Study. Obesity 2010, 18, 2347–2353. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Brandon, D.D.; Isabelle, L.M.; Loriaux, D.L.; Samuels, M.H. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J. Clin. Endocrinol. Metab. 2004, 89, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Bracht, J.R.; Vieira-Potter, V.J.; De Souza Santos, R.; Öz, O.K.; Palmer, B.F.; Clegg, D.J. The role of estrogens in the adipose tissue milieu. Ann. N. Y. Acad. Sci. 2020, 1461, 127–143. [Google Scholar] [CrossRef]
- De Maddalena, C.; Vodo, S.; Petroni, A.; Aloisi, A.M. Impact of testosterone on body fat composition. J. Cell. Physiol. 2012, 227, 3744–3748. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Berryman, C.E.; Harris, M.N.; Karl, J.P.; Lieberman, H.R.; McClung, J.P.; Rood, J.C.; Pasiakos, S.M. Testosterone Administration During Energy Deficit Suppresses Hepcidin and Increases Iron Availability for Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, dgz316. [Google Scholar] [CrossRef] [Green Version]
- Beggs, L.A.; Yarrow, J.F.; Conover, C.F.; Meuleman, J.R.; Beck, D.; Morrow, M.; Zou, B.; Shuster, J.J.; Borst, S.E. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E456–E461. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin—A regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Miller, F.; Worwood, M.; Beamish, M.R.; Wardrop, C.A.J. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Br. Med. J. 1972, 4, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, J.; Bai, Z.; Sink, S.; Zhao, C.; Lorenzo, F.R.; McClain, D.A. Iron down-regulates leptin by suppressing protein O-GlcNAc modification in adipocytes, resulting in decreased levels of O-glycosylated CREB. J. Biol. Chem. 2019, 294, 5487–5495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.-H.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte iron regulates leptin and food intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koperdanova, M.; Cullis, J.O. Interpreting raised serum ferritin levels. Br. Med. J. 2015, 351, h3692–h3695. [Google Scholar] [CrossRef] [Green Version]
- Tolosano, E. Increasing serum transferrin to reduce tissue iron overload due to ineffective erythropoiesis. Haematologica 2015, 100, 565. [Google Scholar] [CrossRef] [Green Version]
- Faruqi, A.; Mukkamalla, S.K.R. Iron Binding Capacity. In StatPearls; StatPearls Publishing LLC.: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini–Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased Adipose Tissue Expression of Hepcidin in Severe Obesity Is Independent From Diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef]
- Fleming, R.E. Hepcidin Activation During Inflammation: Make It STAT. Gastroenterology 2007, 132, 447–449. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Bekri, S.; Luciani, N.; Saint-Paul, M.-C.; Dahman, M.; Iannelli, A.; Ben Amor, I.; Staccini-Myx, A.; Huet, P.-M.; Gugenheim, J.; et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am. J. Gastroenterol. 2006, 101, 1824–1833. [Google Scholar] [CrossRef]
- Rushton, D.H.; Barth, J.H. What is the evidence for gender differences in ferritin and haemoglobin? Crit. Rev. Oncol. Hematol. 2010, 73, 1–9. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Heijer, M.D.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [Green Version]
- Wrede, C.E.; Buettner, R.; Bollheimer, L.C.; Schölmerich, J.; Palitzsch, K.D.; Hellerbrand, C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur. J. Endocrinol. 2006, 154, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Shimobayashi, M.; Albert, V.; Wölnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Hayashi, T.; Boyko, E.J.; McNeely, M.J.; Leonetti, D.L.; Kahn, S.E.; Fujimoto, W.Y. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 2008, 57, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential Fat Deposition in Subcutaneous Versus Visceral Depots Is Associated with Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.W.; Song, S.O.; Hayashi, T.; Sato, K.K.; Kahn, S.E.; Leonetti, D.L.; Fujimoto, W.Y.; Boyko, E. Change in CT-Measured Abdominal Subcutaneous and Visceral but Not Thigh Fat Areas Predict Future Insulin Sensitivity. Diabetes Res. Clin. Pract. 2019, 154, 17. [Google Scholar] [CrossRef]
- Tang, A.; Coster, A.C.F.; Tonks, K.T.; Heilbronn, L.K.; Pocock, N.; Purtell, L.; Govendir, M.; Blythe, J.; Zhang, J.; Xu, A.; et al. Longitudinal Changes in Insulin Resistance in Normal Weight, Overweight and Obese Individuals. J. Clin. Med. 2019, 8, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Navarrete, J.M.; Blasco, G.; Xifra, G.; Karczewska-Kupczewska, M.; Stefanowicz, M.; Matulewicz, N.; Puig, J.; Ortega, F.J.; Ricart, W.; Straczkowski, M.; et al. Obesity Is Associated With Gene Expression and Imaging Markers of Iron Accumulation in Skeletal Muscle. J. Clin. Endocrinol. Metab. 2016, 101, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Córdoba, D.; Priego-Capote, F. Fully automated method for quantitative determination of steroids in serum: An approach to evaluate steroidogenesis. Talanta 2021, 224, 121923. [Google Scholar] [CrossRef] [PubMed]

| Subjects without Obesity | Subjects with Obesity | p * | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | ||
| N | 74 | 49 | 57 | 30 | |||
| Clinical | |||||||
| Age (years) | 50.4 (40.4, 58. 8) | 54.7 (42.7, 60.2) | <0.001 | 48.3 (41.8, 54.9) | 47.88 ± 10.4 | <0.001 | 0.428 |
| Sex (men/women) | 25/49 | 16/33 | 16/41 | 8/22 | 0.865 | ||
| Height (cm) | 166.3 ± 9.0 | 165.7 ± 8.4 | 0.204 | 162.9 ± 8.4 | 162.4 ± 7.5 | 1 | 0.029 |
| Weight (kg) | 66.2 (60.7, 77.4) | 67 (60, 78.2) | 0.395 | 112.1 ± 20.8 | 105. 8 ± 19.5 | 0.711 | <0.001 |
| BMI (kg/m2) | 24.9 ± 2.7 | 24.9 ± 2.9 | 0.261 | 42. 7 ± 6.4 | 40.5 ± 7.7 | 0.703 | <0.001 |
| SBP (mmHg) | 124.6 ± 16.04 | 125 (115, 132) | 1 | 138.25 ± 20.13 | 131.82 ± 9.98 | 0.414 | <0.001 |
| DBP (mmHg) | 71.99 ± 10.73 | 71.71 ± 8.56 | 0.908 | 76.76 ± 11.3 | 73.5 ± 10.5 | 0.062 | 0.016 |
| Laboratory | |||||||
| Hb (g/dL) | 13.9 ± 1.3 | 13.8 ± 1.37 | 0.594 | 13.7 ± 1.4 | 13.8 (13, 14.6) | 0.362 | 0.661 |
| Hct (%) | 42.1 ± 3.4 | 41.2 ± 3.4 | 0.074 | 42.47 ± 3.67 | 42.37 ± 2.86 | 0.104 | 0.564 |
| Total WBC (K/μL) a | 5.49 (4.87, 6.71) | 4.80 (4.08, 6.04) | 0.043 | 6.36 ± 1.96 | 6.04 (4.92, 6.71) | 0.918 | 0.402 |
| HbA1c (%) | 5.4 (5.23, 5.5) | 5.47 ± 0.3 | 0.249 | 5.6 ± 0.31 | 5.61 ± 0.27 | 0.301 | 0.001 |
| HbA1c (mmol/mol) | 35.6 (33.7, 36.7) | 36.3 ± 3.3 | 0.249 | 37.8 ± 3.4 | 37.9 ± 2.9 | 0.351 | 0.001 |
| Iron (μg/dL) | 85 (67, 113) | 84 (71, 106) | 0.243 | 77.2 ± 24.6 | 87 (60.2, 104.2) | 0.094 | 0.008 |
| hsCRP (mg/dL) | 0.6 (0.4, 1.6) | 0.9 (0.6, 1.7) | 0.366 | 4.2 (2.6, 7.7) | 3.1 (1.9, 6.6) | 0.198 | <0.001 |
| Tf (mg/dL) | 246 (224.2, 283. 2) | 259.1 ± 36.3 | 0.522 | 275.9 ± 36.3 | 278.5 ± 37.8 | 0.449 | 0.001 |
| TIBC (μg/dL) | 310.5 (284.8, 352.6) | 326.4 ± 41.9 | 0.621 | 350.4 ± 46.1 | 353.6 ± 48.1 | 0.462 | 0.001 |
| Uric Acid (mg/dL) | 4.49 ± 1.35 | 4.52 ± 1.32 | 0.332 | 5.55 ± 1.2 | 5.09 ± 0.94 | 0.509 | <0.001 |
| Ferritin (ng/mL) | 83 (42, 181.5) | 93 (33, 178) | 0.274 | 79 (39, 138) | 73 (40.2, 126.2) | 0.130 | 0.601 |
| Hepcidin (ng/mL) | 19.1 (10.5, 26.5) | 18.0 (9.5, 27.2) | 0.623 | ||||
| Hep:Fer ratio | 0.2 (0.12, 0.24) | 0.2 (0.14, 0.28) | 0.845 | ||||
| Glucose (mg/dL) | 93 (89, 99) | 95 (90, 97.2) | 0.894 | 98.6 ± 12.3 | 102.2 ± 8.9 | 0.003 | 0.061 |
| Insulin (μU/mL) | 9.4 ± 5.3 | 9.5 (8.1, 14.2) | 0.001 | 24.7 ± 11.5 | 20.3 ± 9.4 | 0.153 | <0.001 |
| M (mg/kg/min) | 10.2 ± 3.5 | 9.6 ± 3.12 | 0.957 | 3.7 (2.3, 6.02) | 4.09 ± 2.1 | 0.300 | <0.001 |
| HOMA-IR | 2.1 (1.3, 3.0) | 2.3 (1.8, 3.6) | <0.001 | 5.5 (3.6, 7.3) | 4.4 (3.4, 6.7) | 0.319 | <0.001 |
| TC (mg/dL) | 202.68 ± 40.81 | 203.08 ± 34.64 | 0.543 | 200.25 ± 42.37 | 196.6 ± 40.94 | 0.063 | 0.741 |
| LDL-C (mg/dL) | 122.24 ± 34.74 | 121.37 ± 30.86 | 0.751 | 124 (104, 149) | 116.7 ± 32.64 | 0.006 | 0.364 |
| HDL-C (mg/dL) | 61 (51.75, 76.5) | 60 (49, 78) | 0.315 | 51.09 ± 12.91 | 56.17 ± 13.3 | 0.501 | <0.001 |
| TG (mg/dL) | 77.5 (58.5, 97.75) | 77 (61, 108) | 0.680 | 121 (81, 153) | 118.63 ± 57.1 | 0.299 | 0.001 |
| Cortisol (ng/mL) | 99.0 (51.0, 161.1) | 72.6 (33.6, 155.3) | 0.7 | ||||
| Estradiol (ng/mL) | 0.058 (0.001, 0.112) | 0.062 (0.001, 0.115) | 0.6 | ||||
| DHEA (ng/mL) | 3.09 (1.95, 5.08) | 1.61 (0.94, 2.74) | <0.001 | ||||
| Testosterone (ng/mL) | 0.14 (0.06, 1.99) | 0.11 (0.06, 0.98) | 0.04 | ||||
| Progesterone (ng/mL) | 0.016 (0.010, 0.031) | 0.013 (0.005, 0.023) | 0.3 | ||||
| Corticosterone (ng/mL) | 0.55 (0.21, 1.86) | 0.29 (0.09, 1.17) | 0.01 | ||||
| Aldosterone (ng/mL) | 0.037 (0.024, 0.054) | 0.026 (0.015, 0.038) | 0.03 | ||||
| Imaging | |||||||
| SAT volume (L) | 5.82 (4.68, 7.6) | 6.35 ± 2 | 0.030 | 17.61 ± 4.02 | 16.64 ± 4.55 | 0.869 | <0.001 |
| VAT volume (L) | 1.81 (1.13, 3) | 2.12 (1.16, 3.06) | <0.001 | 5.23 (4.15, 6.36) | 5.58 ± 2.18 | 0.241 | <0.001 |
| pSAT (%) | 76.9 (68.2, 83.7) | 73.4 ± 12.1 | 0.001 | 76.5 (72.8, 81.6) | 74.3 ± 9.6 | 0.053 | 0.636 |
| Height of VOI (m) | 0.35 (0.34, 0.37) | 0.357 ± 0.019 | 0.176 | 0.36 (0.34, 0.37) | 0.35 (0.34, 0.37) | 0.428 | 0.456 |
| iSAT (L/m2) | 49.3 ± 17.3 | 50.1 ± 16.5 | 0.122 | 138.0 ± 29.2 | 130.7 ± 34.5 | 0.334 | <0.001 |
| iVAT (L/m2) | 14.0 (8.8, 23.8) | 17.6 (9.1, 23.6) | <0.001 | 39.8 (32.1, 56.4) | 38.1 (30.9, 56.6) | 0.156 | <0.001 |
| Leptin (ng/mL) | 1.9 (0.4, 6.1) | 23.2 (14.8, 31.7) | <0.001 | ||||
| HIC (μg/g) | 10.8 (9.7, 13.1) | 12.9 ± 2.31 | 0.032 | ||||
| Changes iSAT | Changes iVAT | Changes pSAT | Hepcidin (ng/mL) | Transferrin (mg/dL) | Ferritin (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Cortisol (ng/mL) | r | −0.08 | −0.15 | 0.12 | 0.01 | −0.07 | 0.002 |
| p-value | 0.43 | 0.18 | 0.30 | 0.91 | 0.42 | 0.98 | |
| Estradiol (ng/mL) | r | −0.16 | −0.02 | −0.06 | −0.11 | 0.16 | −0.20 |
| p-value | 0.15 | 0.83 | 0.56 | 0.22 | 0.06 | 0.02 | |
| DHEA (ng/mL) | r | −0.14 | −0.02 | −0.13 | −0.12 | −0.07 | −0.06 |
| p-value | 0.19 | 0.85 | 0.25 | 0.17 | 0.39 | 0.45 | |
| Testosterone (ng/mL) | r | −0.16 | −0.07 | −0.15 | 0.14 | −0.05 | 0.38 |
| p-value | 0.16 | 0.51 | 0.19 | 0.12 | 0.59 | <0.001 | |
| Progesterone (ng/mL) | r | −0.09 | 0.05 | −0.05 | −0.13 | 0.05 | −0.22 |
| p-value | 0.42 | 0.63 | 0.64 | 0.16 | 0.59 | 0.01 | |
| Corticosterone (ng/mL) | r | −0.15 | −0.15 | 0.07 | −0.08 | −0.04 | −0.06 |
| p-value | 0.18 | 0.17 | 0.50 | 0.4 | 0.61 | 0.50 | |
| Aldosterone (ng/mL) | r | 0.05 | 0.08 | −0.04 | −0.10 | −0.10 | 0.02 |
| p-value | 0.64 | 0.47 | 0.71 | 0.28 | 0.24 | 0.81 | |
| Changes iSAT | Changes iVAT | Changes pSAT | ||||
|---|---|---|---|---|---|---|
| A | β | p-Value | β | p-Value | β | p-Value |
| Age (years) | 0.004 | 0.98 | −0.089 | 0.57 | 0.129 | 0.42 |
| Sex | 0.090 | 0.81 | 0.097 | 0.80 | 0.225 | 0.57 |
| BMI (kg/m2) | −0.248 | 0.19 | −0.292 | 0.12 | 0.207 | 0.29 |
| Ferritin (ng/mL) | −0.091 | 0.61 | 0.010 | 0.95 | −0.076 | 0.67 |
| Hepcidin (ng/mL) | 0.392 | 0.008 | 0.283 | 0.05 | −0.064 | 0.66 |
| Transferrin (mg/dL) | −0.120 | 0.38 | −0.222 | 0.11 | 0.206 | 0.15 |
| hsCRP (mg/dL) | 0.011 | 0.94 | −0.196 | 0.21 | 0.309 | 0.06 |
| M value (mg/kg/min) | −0.329 | 0.06 | −0.354 | 0.04 | 0.236 | 0.18 |
| TG (mg/dL) | −0.056 | 0.66 | 0.037 | 0.78 | −0.278 | 0.04 |
| Testosterone (ng/mL) | 0.052 | 0.88 | −0.127 | 0.72 | 0.414 | 0.25 |
| Estradiol (ng/mL) | −0.154 | 0.27 | −0.021 | 0.88 | −0.082 | 0.57 |
| B | β | p-Value | β | p-Value | β | p-Value |
| Age (years) | −0.044 | 0.78 | −0.151 | 0.35 | 0.193 | 0.22 |
| Sex | 0.336 | 0.41 | 0.359 | 0.38 | −0.087 | 0.83 |
| BMI (kg/m2) | −0.145 | 0.40 | −0.183 | 0.30 | 0.180 | 0.30 |
| Ferritin (ng/mL) | −0.103 | 0.57 | 0.004 | 0.98 | −0.102 | 0.57 |
| Hepcidin (ng/mL) | 0.406 | 0.007 | 0.306 | 0.04 | −0.066 | 0.65 |
| Transferrin (mg/dL) | −0.069 | 0.62 | −0.150 | 0.29 | 0.140 | 0.31 |
| hsCRP (mg/dL) | 0.136 | 0.40 | −0.060 | 0.71 | 0.166 | 0.31 |
| M value (Change) | −0.222 | 0.09 | −0.218 | 0.10 | 0.287 | 0.03 |
| TG (mg/dL) | −0.021 | 0.87 | 0.076 | 0.56 | −0.285 | 0.03 |
| Testosterone (ng/mL) | 0.303 | 0.41 | 0.144 | 0.70 | 0.118 | 0.75 |
| Estradiol (ng/mL) | −0.258 | 0.07 | −0.134 | 0.35 | 0.025 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojosa-Moscoso, A.; Motger-Albertí, A.; De la Calle-Vargas, E.; Martí-Navas, M.; Biarnés, C.; Arnoriaga-Rodríguez, M.; Blasco, G.; Puig, J.; Luque-Córdoba, D.; Priego-Capote, F.; et al. The Longitudinal Changes in Subcutaneous Abdominal Tissue and Visceral Adipose Tissue Volumetries Are Associated with Iron Status. Int. J. Mol. Sci. 2023, 24, 4750. https://doi.org/10.3390/ijms24054750
Hinojosa-Moscoso A, Motger-Albertí A, De la Calle-Vargas E, Martí-Navas M, Biarnés C, Arnoriaga-Rodríguez M, Blasco G, Puig J, Luque-Córdoba D, Priego-Capote F, et al. The Longitudinal Changes in Subcutaneous Abdominal Tissue and Visceral Adipose Tissue Volumetries Are Associated with Iron Status. International Journal of Molecular Sciences. 2023; 24(5):4750. https://doi.org/10.3390/ijms24054750
Chicago/Turabian StyleHinojosa-Moscoso, Alejandro, Anna Motger-Albertí, Elena De la Calle-Vargas, Marian Martí-Navas, Carles Biarnés, María Arnoriaga-Rodríguez, Gerard Blasco, Josep Puig, Diego Luque-Córdoba, Feliciano Priego-Capote, and et al. 2023. "The Longitudinal Changes in Subcutaneous Abdominal Tissue and Visceral Adipose Tissue Volumetries Are Associated with Iron Status" International Journal of Molecular Sciences 24, no. 5: 4750. https://doi.org/10.3390/ijms24054750





