Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review
Abstract
:1. Introduction
2. Anaerobic Digestion
2.1. Biochemical Mechanisms Found in Anaerobic Digestion
2.2. Limitations of the Traditional Anaerobic Digestion Process
3. Additives Used in Anaerobic Digestion
3.1. Syntrophic Activity
3.2. Metabolic Activity
3.3. Catalytic Activity
3.4. Enzymatic Activity
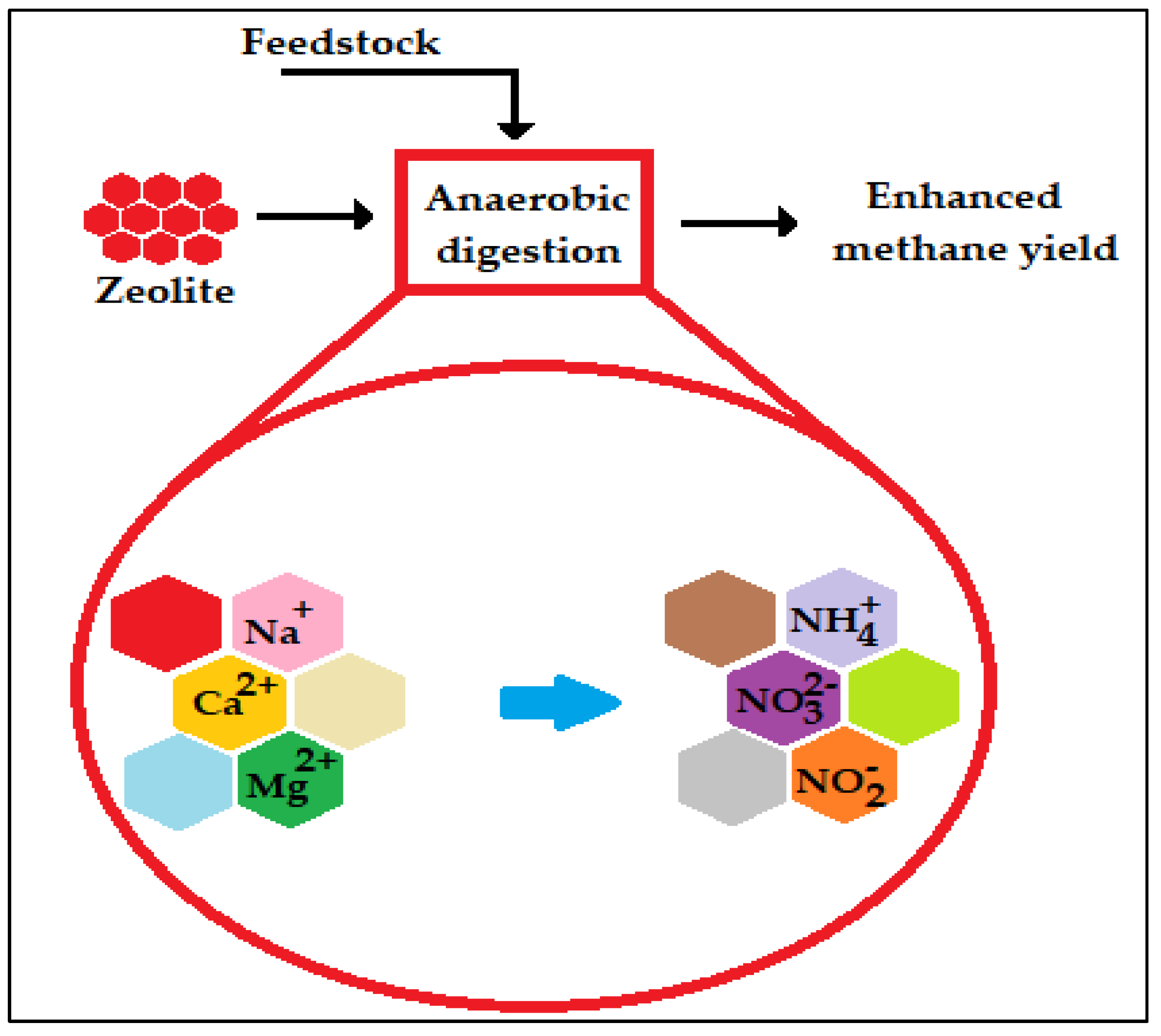
3.5. Cation Exchange Activity
4. Bioelectrochemical System
4.1. Utilization of Bioelectrochemical System on Anaerobic Digestion

4.2. Factors Influencing the Efficiency of the Bioelectrochemical System
4.2.1. Temperature
4.2.2. Electrochemical Efficiencies
4.2.3. Influences of External Resistance on How a Bioelectrochemical System Performs
5. Synergistic Influence of the Bioelectrochemical System and Anaerobic Additives on Anaerobic Digestion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Nhung, T.C.; Ramesh, B.; Srinivasan, S.; Rangasamy, G. A review on biological methodologies in municipal solid waste management and landfilling: Resource and energy recovery. Chemosphere 2022, 309, 136630. [Google Scholar] [CrossRef] [PubMed]
- Al-Dailami, A.; Ahmad, I.; Kamyab, H.; Abdullah, N.; Koti, I.; Veeramuthu, A.; Zabara, B. Sustainable solid waste management in Yemen: Environmental, social aspects, and challenges. Biomass Conv. Bioref. 2022, 12, 1–27. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 25, 38–50. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. Int. Ed. 2021, 61, e202112835. [Google Scholar] [CrossRef]
- Ahmed, A.; Ge, T.; Peng, J.; Yan, W.C.; Tee, B.T.; You, S. Assessment of the renewable energy generation towards net-zero energy buildings: A review. Energy Build. 2022, 256, 111755. [Google Scholar] [CrossRef]
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of Agricultural and Livestock Waste in Anaerobic Digestion (A.D): Applying the Biorefinery Concept in a Circular Economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, M.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114540. [Google Scholar] [CrossRef]
- Jadhav, P.; Muhammad, N.; Bhuyar, P.; Krishnan, S.; Razak, A.S.A.; Zularisam, A.W.; Nasrullah, M. A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: Applications and limitations. Environ. Technol. Innov. 2021, 23, 101526. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J.; Lei, Z. Integrating anaerobic digestion with microbial electrolysis cell for performance enhancement: A review. Bioresour. Technol. 2022, 344, 126321. [Google Scholar] [CrossRef]
- Madondo, N.I.; Kweinor Tetteh, E.; Rathilal, S.; Bakare, B.F. Effect of an Electromagnetic Field on Anaerobic Digestion: Comparing an Electromagnetic System (ES), a Microbial Electrolysis System (MEC), and a Control with No External Force. Molecules 2022, 27, 3372. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chang, Y.; Xie, J.; Adams, M.; Zhao, D.; Chen, C.; Ma, J.; Zhu, G.; Zhang, T.C. Insights into the mechanism, performance and electrode modification of BES-AD combined systems for refractory wastewater treatment: A review. J. Water Process Eng. 2021, 40, 101895. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Liang, B.; Jiang, J.; Wang, A. Microbial fingerprints of methanation in a hybrid electric-biological anaerobic digestion. Water Res. 2022, 226, 119270. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, C.; Kumar, A.; Mondal, P.C. Overview on Induced Chirality in Magnetic Field Controlled Electro-Deposition and Induced Magnetic Moment Originating from Chiral Electrodes. Magnetochemistry 2018, 4, 36. [Google Scholar]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on An-aerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Application of Bioelectrochemical System and Magnetite Nanoparticles on the Anaerobic Digestion of Sewage Sludge: Effect of Electrode Configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F. Utilization of Response Surface Methodology in Optimization and Modelling of a Microbial Electrolysis Cell for Wastewater Treatment Using Box–Behnken Design Method. Catalysts 2022, 12, 1052. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Casale, S.; Chouchane, H.; Askri, R.; Fazi, S.; Cherif, A.; Zeppilli, M.; Aulenta, F. Magnetite nanoparticles enhance the bioelectrochemical treatment of municipal sewage by facilitating the syntrophic oxidation of volatile fatty acids. J. Chem. Technol. Biotechnol. 2019, 94, 3134–3146. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, A.; Im, S.; Song, Y.C.; Ahn, Y.; Kim, D.H. Enhanced Anaerobic Digestion by Stimulating DIET Reaction. Processes 2020, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- El Mashad, H.M.; Barzee, T.J.; Franco, R.B.; Zhang, R.; Kaffka, S.; Mitloehner, F. Anaerobic Digestion and Alternative Manure Management Technologies for Methane Emissions Mitigation on Californian Dairies. Atmosphere 2023, 14, 120. [Google Scholar] [CrossRef]
- Shapovalov, Y.; Zhadan, S.; Bochmann, G.; Salyuk, A.; Nykyforov, V. Dry Anaerobic Digestion of Chicken Manure: A Review. Appl. Sci. 2020, 10, 7825. [Google Scholar] [CrossRef]
- Dalke, R.; Demro, D.; Khalid, Y.; Wu, H.; Urgun-Demirtas, M. Current status of anaerobic digestion of food waste in the United States. Renew. Sustain. Energy Rev. 2021, 151, 111554. [Google Scholar] [CrossRef]
- Singh, R.; Paritosh, K.; Pareek, N.; Vivekanand, V. Integrated system of anaerobic digestion and pyrolysis for valorization of agricultural and food waste towards circular bioeconomy: Review. Bioresour. Technol. 2022, 360, 127596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, C.; Song, L.; Zhang, J. Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 9519. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Singh, A.; Saha, P.; Choi, Y.; Maharana, M.; Patil, S.V.; Kim, B.S. Nano-Biochar as a Sustainable Catalyst for Anaerobic Digestion: A Synergetic Closed-Loop Approach. Catalysts 2022, 12, 186. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability 2022, 14, 10904. [Google Scholar] [CrossRef]
- Ignatowicz, K.; Filipczak, G.; Dybek, B.; Wałowski, G. Biogas Production Depending on the Substrate Used: A Review and Evaluation Study—European Examples. Energies 2023, 16, 798. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Characteristics of Solidified Carbon Dioxide and Perspectives for Its Sustainable Application in Sewage Sludge Management. Int. J. Mol. Sci. 2023, 24, 2324. [Google Scholar] [CrossRef]
- Kesarwani, S.; Panwar, D.; Mal, J.; Pradhan, N.; Rani, R. Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation. Fermentation 2023, 9, 6. [Google Scholar] [CrossRef]
- Naphtali, J.; Chan, A.W.Y.; Saleem, F.; Li, E.; Devries, J.; Schellhorn, H.E. Comparative Metagenomics of Anaerobic Digester Communities Reveals Sulfidogenic and Methanogenic Microbial Subgroups in Conventional and Plug Flow Residential Septic Tank Systems. Processes 2022, 10, 436. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1(ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Xu, Z.X.; Ma, X.Q.; Zhou, J.; Duan, P.G.; Zhou, W.Y.; Ahmad, A.; Luque, R. The influence of key reactions during hydrothermal carbonization of sewage sludge on aqueous phase properties: A review. J. Anal. Appl. Pyrolysis 2022, 167, 105678. [Google Scholar] [CrossRef]
- Uthirakrishnan, U.; Sharmila, V.G.; Merrylin, J.; Kumar, S.A.; Dharmadhas, J.S.; Varjani, S.; Banu, J.R. Current advances and future outlook on pretreatment techniques to enhance biosolids disintegration and anaerobic digestion: A critical review. Chemosphere 2022, 288, 132553. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about bioproduction of Volatile Fatty Acids from wastes and wastewaters: Influence of operating conditions and organic composition of the substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Mohanty, M.K.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Conv. Bioref. 2023, 13, 1503–1527. [Google Scholar] [CrossRef]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A review on key pretreatment approaches for lignocellulosic biomass to produce biofuel and value-added products. Int. J. Environ. Sci. Technol. 2022, 19, 1–16. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Mohan, S.V. Understanding acidogenesis towards green hydrogen and volatile fatty acid production—Critical Analysis and Circular Economy Perspective. Chem. Eng. J. 2023, 461, 141550. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Y.; Liu, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste—Pathways and microbial perspective. Renew. Sustain. Energy Rev. 2023, 175, 113181. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D.; Kesharwani, N.; Pareek, N.; Vivekanand, V. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion: A Review. Front. Energy Res. 2020, 8, 88. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granstrom, K. A review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Gerardi, M.H. Wastewater Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 155–160. [Google Scholar]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Milán, Z.; Montalvo, S.; Ilangovan, K.; Monroy, O.; Chamy, R.; Weiland, P.; Sanchez, E.; Borja, R. The impact of ammonia nitrogen concentration and zeolite addition on the specific methanogenic activity of granular and flocculent anaerobic sludges. J. Environ. Sci. Health Part A 2010, 45, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Impact of trace element addition on biogas production from food industrial waste–linking process to microbial communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, J.; Orphan, V.J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Kiser, M.A.; Ryu, H.; Jang, H.; Hristovski, K.; Westerhoff, P. Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res. 2010, 44, 4105–4114. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving biogas production using additives in anaerobic digestion: A review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Liu, C.; Tong, Q.; Li, Y.; Wang, N.; Liu, B.; Xhang, X. Biogas production and metal passivation analysis during anaerobic digestion of pig manure: Effects of a magnetic Fe3O4/FA composite supplement. R. Soc. Chem. 2019, 9, 4488–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.J.; Yan, J.; Yuan, Q.K.; Wang, X.T.; Yuan, Y.; Ren, N.Q.; Lee, D.J.; Chen, C. Enhanced methane production in anaerobic digestion: A critical review on regulation based on electron transfer. Bioresour. Technol. 2022, 364, 128003. [Google Scholar] [CrossRef] [PubMed]
- Castilho, T.G.; Rodrigues, J.A.D.; García, J.; Subtil, E.L. Recent advances and perspectives in the use of conductive materials to improve anaerobic wastewater treatment: A systematic review approached. J. Water Process Eng. 2022, 50, 103193. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.E.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Engineering Direct Interspecies Electron Transfer for Enhanced Methanogenic Performance. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Sinharoy, A., Lens, P.N.L., Eds.; Applied Environmental Science and Engineering for a Sustainable Future; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Dai, X.; Dai, L. Principles and advancements in improving anaerobic digestion of organic waste via direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 148, 111367. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]
- Wang, Z.K.; Liu, Q.H.; Yang, Z.M. Nano magnetite-loaded biochar boosted methanogenesis through shifting microbial community composition and modulating electron transfer. Sci. Total Environ. 2023, 861, 160597. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Shin, D.C.; Kim, I.-T.; Jung, J.; Jeong, Y.; Lee, Y.-E.; Ahn, K.-H. Increasing Anaerobic Digestion Efficiency Using Food-Waste-Based Biochar. Fermentation 2022, 8, 282. [Google Scholar] [CrossRef]
- Feng, D.; Xia, A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Effects of carbon cloth on anaerobic digestion of high concentration organic wastewater under various mixing conditions. J. Hazard. Mater. 2022, 5, 423. [Google Scholar] [CrossRef]
- Mostafa, A.; Im, S.; Song, Y.-C.; Kang, S.; Kim, D.-H. Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes. Microorganisms 2020, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo, L.; Stams, A.; Sousa, D. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Biotechnol. 2015, 14, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tian, Y.; Zheng, L.; Li, S.; Hao, H.; Yin, M.; Cao, Y.; Huang, H. Process Analysis of Anaerobic Fermentation Exposure to Metal Mixtures. Int. J. Environ. Res. Public Health 2019, 16, 2458. [Google Scholar]
- Bakari, O.; Njau, K.N.; Noubactep, C. Fe0-Supported Anaerobic Digestion for Organics and Nutrients Removal from Domestic Sewage. Water 2022, 14, 1623. [Google Scholar] [CrossRef]
- Rahman, K.; Melville, S.; Imamul Huq, S.; Khoda, S. Understanding bioenergy production and optimisation at the nanoscale—A review. J. Exp. Nanosci. 2016, 11, 762–775. [Google Scholar] [CrossRef] [Green Version]
- Córdova-Lizama, A.; Carrera-Figueiras, C.; Palacios, A.; Castro-Olivera, P.M.; Ruiz-Espinoza, J. Improving hydrogen production from the anaerobic digestion of waste activated sludge: Effects of cobalt and iron zero valent nanoparticles. Int. J. Hydrog. Energy 2022, 47, 30074–30084. [Google Scholar] [CrossRef]
- García, A.; Delgado, L.; Torà, J.A.; Casals, E.; González, E.; Puntes, V.; Font, X.; Carrera, J.; Sánchez, A. Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. J. Hazard. Mater. 2012, 199–200, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, T.; Cunningham, G.; Suresh, D.; Shanks, B.; Kannan, R.; Upendran, A.; Afrasiabi, Z. Wastewater Treatment Using Novel Magnetic Nanosponges. Water 2022, 14, 505. [Google Scholar] [CrossRef]
- Award, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. Chem. Eng. 2021, 5, 34. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Li, Y.Z.; Inoue, D.; Ike, M. Mitigating ammonia-inhibition in anaerobic digestion by bioaugmentation: A review. J. Water Process Eng. 2023, 52, 103506. [Google Scholar] [CrossRef]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Heerenklage, J.; Rechtenbach, D.; Atamaniuk, I.; Alassali, A.; Raga, R.; Koch, K. Development of a method to produce standardised and storable inocula for biomethane potential tests–preliminary steps. Renew. Energy 2019, 143, 753–761. [Google Scholar] [CrossRef]
- Xu, C.; Qin, Y.; Li, Y.; Ji, Y.; Huang, J.; Song, H. Factors influencing cellulosome activity in consolidated bioprocessing of cellulosic ethanol. Bioresour. Technol. 2010, 101, 9560–9569. [Google Scholar] [CrossRef]
- Yu, C.; Li, D.; Wang, Q.; Zhang, Z.; Yang, Y. Improving Anaerobic Methane Production from Ammonium-rich Piggery Waste in a Zeolite-fixed Bioreactor and Evaluation of Ammonium Adsorbed on Zeolite A-3 as Fertilizer. Int. J. Waste Resour. 2014, 4, 1000106. [Google Scholar]
- Ciezkowska, M.; Bajda, T.; Decewicz, P.; Dziewit, D.; Drewniak, L. Effect of Clinoptilolite and Halloysite Addition on Biogas Production and Microbial Community Structure during Anaerobic Digestion. Materials 2020, 13, 4127. [Google Scholar] [CrossRef]
- Lauka, D.; Pastare, L.; Blumberga, D.; Romagnoli, F. Preliminary analysis of anaerobic digestion process using Cerathophyllum demersum and low carbon content additives: A batch test study. In Proceedings of the International Scientific Conference “Environmental and Climate Technologies—CONECT 2014”, Riga, Latvia, 14–16 October 2014; pp. 25–31. [Google Scholar]
- Wang, Q.; Yang, Y.; Yu, C.; Huang, H.; Kim, M.; Feng, C.; Zhang, Z. Study on a fixed zeolite bioreactor for anaerobic digestion of ammonium-rich swine wastes. Bioresour. Technol. 2011, 102, 7064–7068. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A. Mechanism of Zeolite Activity in Biogas Co-Digestion. Master’s Thesis, Linkoping University of Technology, Linkoping, Sweden, 2011. [Google Scholar]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Hamelers, H.M.; Heijne, A.; Sleutels, T.J.A.; Jeremiasse, A.; Strik, D.B.T.B.; Buisman, C.N. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Singh, A.; Van Bogaert, G.; Irving Olsen, S.; Singh Nigam, P.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Vijay, A.; Sonawane, J.M.; Chhabra, M. Denitrification process in microbial fuel cell: A comprehensive review. Bioresour. Technol. Rep. 2022, 17, 100991. [Google Scholar] [CrossRef]
- Gorby, Y.U.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Culley, D.E.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Chae, K.; Choi, M.; Vestraete, W. Microbial Fuel Cells: Recent advances, bacterial communities and application beyond electricity generation. Environ. Eng. Res. 2008, 13, 51–65. [Google Scholar] [CrossRef]
- Aiswaria, P.; Mohamed, S.N.; Singaravelu, D.L.; Brindhadevi, K.; Pugazhendhi, A. A review on graphene/graphene oxide supported electrodes for microbial fuel cell applications: Challenges and prospects. Chemosphere 2022, 296, 133983. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Majone, M. Perspectives of biofuels production from renewable resources with bioelectrochemical systems. Asia Pac. J. Chem. Eng. 2012, 7 (Suppl. 3), S263–S274. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Lu, Y.; Al-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives. Environ. Eng. Res. 2021, 26, 190405. [Google Scholar] [CrossRef]
- Tzelepis, S.; Kavadias, K.A.; Marnellos, G.E.; Xydis, G. A review study on proton exchange membrane fuel cell electrochemical performance focusing on anode and cathode catalyst layer modelling at macroscopic level. Renew. Sustain. Energy Rev. 2021, 151, 111543. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef]
- Radhika, D.; Shivakumar, A.; Kasai, D.R.; Koutavarapu, R.; Peera, S.G. Microbial Electrolysis Cell as a Diverse Technology: Overview of Prospective Applications, Advancements, and Challenges. Energies 2022, 15, 2611. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Dessì, P.; Kokko, M.; Lakaniemi, A.M.; Lens, P. Selective enrichment of biocatalysts for bioelectrochemical systems: A critical review. Renew. Sustain. Energy Rev. 2019, 109. [Google Scholar] [CrossRef]
- Hassanein, A.; Witarsa, F.; Lansing, S.; Qiu, L.; Liang, Y. Bio-Electrochemical Enhancement of Hydrogen and Methane Production in a Combined Anaerobic Digester (AD) and Microbial Electrolysis Cell (MEC) from Dairy Manure. Sustainability 2020, 12, 8491. [Google Scholar] [CrossRef]
- An, Z.; Feng, Q.; Zhao, R.; Wang, X. Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode. Processes 2020, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.L.; He, Y.T.; Yu, P.F.; Sun, H.; Fu, J.X. Effect of temperature on electricity generation of single-chamber microbial fuel cells with proton exchange membrane. Adv. Mater. Res. 2012, 393–395, 1169–1172. [Google Scholar] [CrossRef]
- Trinh, N.T.; Park, J.H.; Kim, B.W. Increased generation of electricity in a microbial fuel cell using geobacter sulfurreducens. Korean J. Chem. Eng. 2009, 26, 748–753. [Google Scholar] [CrossRef]
- Liu, Y.; Climent, V.; Berna, A.; Feliu, J.M. Effect of temperature on the catalytic ability of electrochemically active biofilm as anode catalyst in microbial fuel cells. Electroanalysis 2011, 23, 387–394. [Google Scholar] [CrossRef]
- Li, L.H.; Sun, Y.M.; Yuan, Z.H.; Kong, X.Y.; Li, Y. Effect of temperature change on power generation of microbial fuel cell. Environ. Technol. 2013, 34, 1929–1934. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.A.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Wade, M.; Sloan, W.T.; Quince, C.; Curtis, T.P. Temperature, inocula and substrate: Contrasting electroactive consortia, diversity and performance in microbial fuel cells. Bioelectrochemistry 2018, 119, 43–50. [Google Scholar] [CrossRef]
- Ahn, Y.; Im, S.; Chung, J.W. Optimizing the operating temperature for microbial electrolysis cell treating sewage sludge. Int. J. Hydrogen Energy 2017, 42, 27784–27791. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Guo, L.; Dai, Q.; Ma, X. Electrochemical oxidation of Acid Orange 7 azo dye using a PbO2 electrode: Parameter optimization, reaction mechanism and toxicity evaluation. Chemosphere 2020, 241, 125010. [Google Scholar] [CrossRef] [PubMed]
- Nelabhotla, A.B.T.; Dinamarca, C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Appl. Sci. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gan, L.; Liao, Z.; Hou, R.; Zhou, S.; Zhou, l.; Yuan, Y. Self-produced biophotosensitizers enhance the degradation of organic pollutants in photo-bioelectrochemical systems. J. Hazard. Mater. 2022, 433, 128797. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Molenaar, S.D.; Ter Heijne, A.; Buisman, C.J.N. Low Substrate Loading Limits Methanogenesis and Leads to High Coulombic Efficiency in Bioelectrochemical Systems. Microorganisms 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Bautista, Y.; Paraguay-Delgado, F.; Perez Hernandez, B.; Perez Hernandez, G.; Martinez Pereyra, G.; Ramirez Morales, E. Influence of external resistance and anodic pH on power density in microbial fuel cell operated with B. subtilis BSC-2 strain. Appl. Ecol. Environ. Res. 2018, 16, 1983–1997. [Google Scholar] [CrossRef]
- Konopacki, M.; Rakoczy, R. The analysis of rotating magnetic field as a trigger of Gram-positive and Gram-negative bacteria growth. Biochem. Eng. J. 2019, 141, 259–267. [Google Scholar] [CrossRef]
- Nopharatana, A.; Pullammanappallil, P.C.; Clarke, W.P. Kinetic and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag. 2007, 27, 595–603. [Google Scholar] [CrossRef]
- Mersinkova, Y.; Yemendzhiev, H.; Nenov, V. Comparative study on the metabolic behaviour of anode biofilm in microbial fuel cell under different external resistance. Biotechnol. Biotechnol. Equip. 2022, 36, 142–147. [Google Scholar] [CrossRef]
- Kamau, J.M.; Mbui, D.N.; Mwaniki, J.M.; Mwaura, F.B.; Kamau, G.N. Microbial Fuel Cells: Influence of External Resistors on Power, Current and Power Density. J. Thermodyn. Catal. 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, C.; Liu, X.; Sun, D.; Li, P.; Qiu, P.; Dang, Y.; Karpinski, N.A.; Smith, J.A.; Holmes, D.E. Magnetite enhances anaerobic digestion of high salinity organic wastewater. Environ. Res. 2020, 189, 109884. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, W.A.; Gehring, T.A.; Zaiat, M. Stimulation and inhibition of direct interspecies electron transfer mechanisms within methanogenic reactors by adding magnetite and granular actived carbon. Chem. Eng. J. 2021, 415, 128882. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Sun, J.; Zhang, L.; Jiao, J.; Wang, Q.; Liu, S. Nanomaterials Facilitating Microbial Extracellular Electron Transfer at Interfaces. Adv. Mater. 2021, 33, 2004051. [Google Scholar] [CrossRef]
- Vu, M.T.; Noori, M.T.; Min, B. Magnetite/zeolite nanocomposite-modified cathode for enhancing methane generation in microbial electrochemical systems. Chem. Eng. J. 2020, 393, 124613. [Google Scholar] [CrossRef]
- Hamed, M.S.; Majdi, H.; Hasan, B.O. Effect of Electrode Material and Hydrodynamics on Produced Current in Double Chamber Microbial Fuel Cells. ACS Omega 2020, 5, 10339–10348. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, X.; Cai, T.; Niu, C.; Han, Y.; Zhang, Z.; Zhu, X.; Zhen, G. Magnetite-enhanced bioelectrochemical stimulation for biodegradation and biomethane production of waste activated sludge. Sci. Total Environ. 2021, 789, 147859. [Google Scholar] [CrossRef] [PubMed]









| Inhibitor | Impact on Anaerobic Digestion | Main Feedstock Waste | Reference |
|---|---|---|---|
| VFA (>200 mg/L) | Lower pH Unstable syntrophic reaction Reduced population of Achaea Sour digester | Foodstuff Sugar industry effluent | Capson-Tojo et al. [42]; Gerardi et al. [43] |
| D-Limonene (>2–3.5 gVS−1 day−1) | Higher cell permeability Cell destruction The lower population of microbes | Citrus waste and peelings | Fagbohungbe et al. [44] |
| Nitrogen (>3 g/L) | Proteins that are not balanced May hinder methanogen microorganisms Build-up of acids | Industrial effluent Urine of animals | Milán et al. [45] |
| Sulphur products (10–20 gVS/L·day) | Stimulates sulphur-reducing microorganisms that have competition with acetogenesis microorganisms Reduction of methanogenesis microorganisms | Abattoir Domestic fowl dung | Feng et al. [46] |
| High amounts of light and heavy metals (0.2–2 mM for Fe) | Disturbance of cells Inhibition of acetoclastic microorganisms Competition in cellular structure adsorption Limit cell formation This might result in the destabilization of the buffering capability Might include neutralization impact on the structure of cells | Industrial effluent Domestic | Glass and Orphan [47] |
| Aliphatic that are halogenated | Inhibits the methanogenic stage; COD removal reduced by 20% and VFA reduced by 509 mg/L Troubled energy of cells | Industry effluent Oil & grease | Liu et al. [48]; Kiser et al. [49] |
| Lignin | Resistance in anaerobic digestion Low production of methane Limited access to cellulose | Biomass for lignocellulose Harvest remains | Demirel and Scherer [50] |
| Carbonaceous Additives | Effect in Anaerobic Digestion | Reference |
|---|---|---|
| Bio-char | Is able to increase the population of microorganisms and methane yield by more than 21 L/kg VS Helps in the mitigation of limonene Methyltransferases Mitigation of ammonia ion Reduces lag phase by 27–64% and enhanced the methanogenesis by 22–40% | Fagbohungbe et al. [58]; Wang et al. [59] |
| Activated carbon | Decreases digester delay time by 2 days Improves the DIET process and methane production by 17.4% Improves the consumption of acids | Capson-Tojo et al. [42]; Yang et al. [60] |
| Granular activated carbon | Help in increasing Firmicutes population with sludge reduction increase of 6.1% May tolerate low temperature Increased methane production of 13.1% | Yang et al. [60]; Peng et al. [61] |
| Carbon cloth | Higher methanogenesis activities Enabled 1.34-fold more organic loading than that of the control Volatile fatty acid utilization direct interspecies electron transfer | Lei et al. [62] |
| Magnetite | Enables the interspecies transfer between Archaea and microorganisms Improved bacterial variety in the digester | Wang et al. [63] |
| Additive | Material/Type | Effect on Anaerobic Digestion | Reference |
|---|---|---|---|
| Syntrophic activity | Carbon cloth | Enhanced methane content by 10.1–23.0% | Feng et al. [66] |
| Metabolic activity | Iron (F0) | Highest removals of COD and phosphates were 88.0% and 98.0%, respectively. | Bakari et al. [70] |
| Catalytic activity | Cobalt and iron zero valent nanoparticles | Enhanced the early stages of the anaerobic digestion of waste-activated sludge. | Córdova-Lizama et al. [72] |
| Enzymatic activity | Carbon-based acids | Lactate, formate, and acetate have been found to function like promoters to improve the cellulosome activity for the duration of anaerobic digestion within 50, 100 and 200 mM concentrations, respectively. | Xu et al. [80] |
| Cation exchange activity | Zeolites | Increased the methane content by 19.7% and the overall methane yield by 120.9 CH4/kg VS. | Wang et al. [84] |
| Type of Bioelectrochemical System | Effect of Electrochemical Efficiencies on Bioelectrochemical System | Reference |
|---|---|---|
| Microbial electrosynthesis system |
| Nelabhotla and Dinamarca [112] |
| Photo-Bioelectrochemical system |
| Wang et al. [113] |
| Microbial electrosynthesis system |
| Sleutels et al. [114] |
| MEC |
| Villano et al. [115] |
| MFC |
| Cordova-Bautista [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review. Int. J. Mol. Sci. 2023, 24, 4753. https://doi.org/10.3390/ijms24054753
Madondo NI, Rathilal S, Bakare BF, Tetteh EK. Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review. International Journal of Molecular Sciences. 2023; 24(5):4753. https://doi.org/10.3390/ijms24054753
Chicago/Turabian StyleMadondo, Nhlanganiso Ivan, Sudesh Rathilal, Babatunde Femi Bakare, and Emmanuel Kweinor Tetteh. 2023. "Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review" International Journal of Molecular Sciences 24, no. 5: 4753. https://doi.org/10.3390/ijms24054753







