Benefits of the Neurogenic Potential of Melatonin for Treating Neurological and Neuropsychiatric Disorders
Abstract
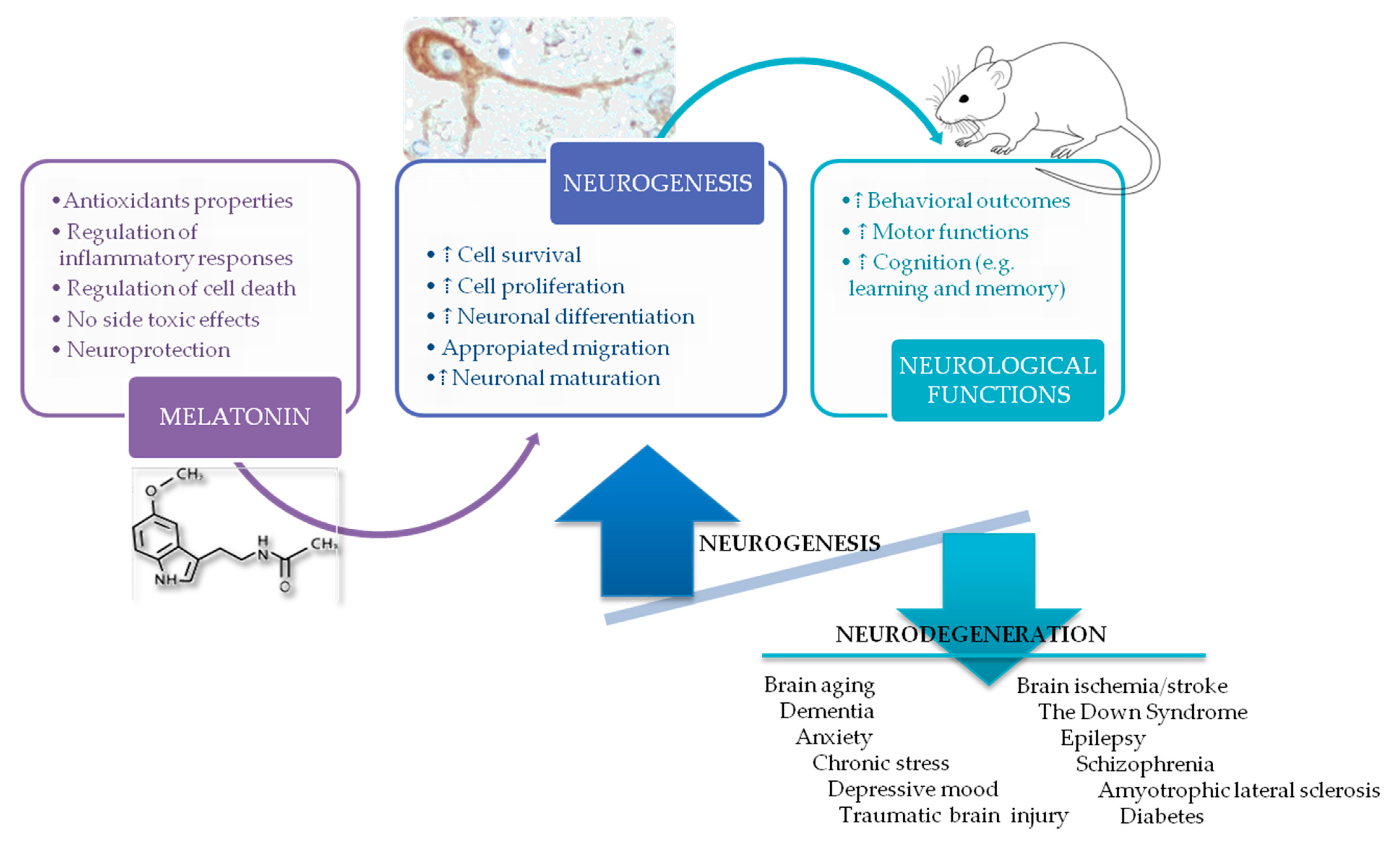
1. Melatonin
2. Regulatory Role of Melatonin in Physiological Processes
2.1. Antioxidant Activity
2.2. Immune System Properties
2.3. Anti-apoptotic Activity
3. Neurogenesis in the Adult Brain
4. Melatonin and Neurogenesis: Impact on Different Neurological Disorders
4.1. Aging and Dementia
4.2. Stress, Anxiety and Depression
4.3. Acquired Brain Damage
4.4. Down Syndrome
4.5. Epilepsy
4.6. Schizophrenia
4.7. Amyotrophic Lateral Sclerosis (ALS)
4.8. Other Common Diseases Related to Neurogenesis Impairments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, V.; Spence, W.D.; Pandi-Perumal, S.R.; Zakharia, R.; Bhatnagar, K.P.; Brzezinski, A. Melatonin and human reproduction: Shedding light on the darkness hormone. Gynecol. Endocrinol. 2009, 25, 779–785. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Pelaez, A.; Howes, K.A.; Gonzalez-Brito, A.; Reiter, R.J. N-acetyltransferase activity, hydroxyindole-O-methyltransferase activity, and melatonin levels in the Harderian glands of the female Syrian hamster: Changes during the light:dark cycle and the effect of 6-parachlorophenylalanine administration. Biochem. Biophys. Res. Commun. 1987, 145, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.T.; Tipoe, G.L.; Tam, S.; Ma, E.S.; Zou, L.Y.; Chan, P.S. Preclinical evaluation of pharmacokinetics and safety of melatonin in propylene glycol for intravenous administration. J. Pineal Res. 2006, 41, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochimica Biophysica Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef]
- Bañón-Arnao, M.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Esparza, J.L.; Gomez, M.; Rosa Nogues, M.; Paternain, J.L.; Mallol, J.; Domingo, J.L. Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J. Pineal Res. 2005, 39, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Esparza, J.L.; Nogues, M.R.; Giralt, M.; Cabre, M.; Domingo, J.L. Pro-oxidant activity of aluminum in the rat hippocampus: Gene expression of antioxidant enzymes after melatonin administration. Free Radic. Biol. Med. 2005, 38, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bettahi, I.; Pozo, D.; Osuna, C.; Reiter, R.J.; Acuna-Castroviejo, D.; Guerrero, J.M. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J. Pineal Res. 1996, 20, 205–210. [Google Scholar] [CrossRef]
- Leon, J.; Escames, G.; Rodriguez, M.I.; Lopez, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrion, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef]
- Teixeira, A.; Morfim, M.P.; de Cordova, C.A.; Charao, C.C.; de Lima, V.R.; Creczynski-Pasa, T.B. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J. Pineal Res. 2003, 35, 262–268. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, C.; Hu, W.; Jiang, S.; Ma, Z.; Yan, X.; Deng, C.; Di, S.; Xin, Z.; Wu, G.; et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016, 60, 253–262. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- McKinney, T.D.; Vaughan, M.K.; Reiter, R.J. Pineal influence on intermale aggression in adult house mice. Physiol. Behav. 1975, 15, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin. Exp. Immunol. 1987, 68, 384–391. [Google Scholar]
- Carrillo-Vico, A.; Reiter, R.J.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 2006, 7, 423–431. [Google Scholar] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, J.; Chongthammakun, S.; Govitrapong, P. Melatonin inhibits amphetamine-induced nitric oxide synthase mRNA overexpression in microglial cell lines. Neurosci. Lett. 2008, 439, 134–137. [Google Scholar] [CrossRef]
- Tamura, E.K.; Cecon, E.; Monteiro, A.W.; Silva, C.L.; Markus, R.P. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J. Pineal Res. 2009, 46, 268–274. [Google Scholar] [CrossRef]
- Rahim, I.; Djerdjouri, B.; Sayed, R.K.; Fernandez-Ortiz, M.; Fernandez-Gil, B.; Hidalgo-Gutierrez, A.; Lopez, L.C.; Escames, G.; Reiter, R.J.; Acuna-Castroviejo, D. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. 2017, 63, e12410. [Google Scholar] [CrossRef]
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3863–3875. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.Y.; Lin, X.; Mao, L.Z.; Ge, W.H.; Zhang, L.M.; Huang, Y.L.; Gu, J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 2002, 33, 48–56. [Google Scholar] [CrossRef]
- Shi, L.; Liang, F.; Zheng, J.; Zhou, K.; Chen, S.; Yu, J.; Zhang, J. Melatonin Regulates Apoptosis and Autophagy Via ROS-MST1 Pathway in Subarachnoid Hemorrhage. Front. Mol. Neurosci. 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 55, 26–35. [Google Scholar] [CrossRef]
- Jalili, S.; Ehsanpour, A.A.; Javadirad, S.M. The role of melatonin on caspase-3-like activity and expression of the genes involved in programmed cell death (PCD) induced by in vitro salt stress in alfalfa (Medicago sativa L.) roots. Bot. Stud. 2022, 63, 19. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Fan, Y.; Tian, H.; Li, K.; Mei, X. Melatonin Enhances Autophagy and Reduces Apoptosis to Promote Locomotor Recovery in Spinal Cord Injury via the PI3K/AKT/mTOR Signaling Pathway. Neurochem. Res. 2019, 44, 2007–2019. [Google Scholar] [CrossRef]
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed Res. Int. 2015, 2015, 727542. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 2018, 38, 10401–10410. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020, 49, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jimenez, E.P.; Terreros-Roncal, J.; Flor-Garcia, M.; Rabano, A.; Llorens-Martin, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Cachan-Vega, C.; Vega-Naredo, I.; Potes, Y.; Bermejo-Millo, J.C.; Rubio-Gonzalez, A.; Garcia-Gonzalez, C.; Antuna, E.; Bermudez, M.; Gutierrez-Rodriguez, J.; Boga, J.A.; et al. Chronic Treatment with Melatonin Improves Hippocampal Neurogenesis in the Aged Brain and Under Neurodegeneration. Molecules 2022, 27, 5543. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernandez, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Gutierrez-Cuesta, J.; Pallas, M.; Camins, A.; et al. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in senescence-accelerated mice. J. Pineal Res. 2008, 45, 302–311. [Google Scholar] [CrossRef]
- Gutierrez-Cuesta, J.; Sureda, F.X.; Romeu, M.; Canudas, A.M.; Caballero, B.; Coto-Montes, A.; Camins, A.; Pallas, M. Chronic administration of melatonin reduces cerebral injury biomarkers in SAMP8. J. Pineal Res. 2007, 42, 394–402. [Google Scholar] [CrossRef]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernandez, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Pallas, M.; Camins, A.; Rodriguez-Colunga, M.J.; et al. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J. Pineal Res. 2009, 46, 106–114. [Google Scholar] [CrossRef]
- Gutierrez-Cuesta, J.; Tajes, M.; Jimenez, A.; Camins, A.; Pallas, M. Effects of melatonin in the brain of the senescence-accelerated mice-prone 8 (SAMP8) model. Rev. Neurol. 2011, 52, 618–622. [Google Scholar]
- Chu, J.; Tu, Y.; Chen, J.; Tan, D.; Liu, X.; Pi, R. Effects of melatonin and its analogues on neural stem cells. Mol. Cell. Endocrinol. 2016, 420, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Zheng, H.; Ho, J.; Chan, M.T.; Wu, W.K. Protective roles of melatonin in central nervous system diseases by regulation of neural stem cells. Cell Prolif. 2017, 50, e12323. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Morales-Garcia, J.A.; Ramos, E. Melatonin: A multitasking indoleamine to modulate hippocampal neurogenesis. Neural Regen. Res. 2023, 18, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Benitez-King, G.; Castro-Garcia, M.; Ortiz-Lopez, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef]
- Leung, J.W.; Cheung, K.K.; Ngai, S.P.; Tsang, H.W.; Lau, B.W. Protective Effects of Melatonin on Neurogenesis Impairment in Neurological Disorders and Its Relevant Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5645. [Google Scholar] [CrossRef]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009, 47, 313–317. [Google Scholar] [CrossRef]
- Ortiz-Lopez, L.; Perez-Beltran, C.; Ramirez-Rodriguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ocana-Fernandez, M.A.; Vega-Rivera, N.M.; Torres-Perez, O.M.; Gomez-Sanchez, A.; Estrada-Camarena, E.; Ortiz-Lopez, L. Environmental enrichment induces neuroplastic changes in middle age female Balb/c mice and increases the hippocampal levels of BDNF, p-Akt and p-MAPK1/2. Neuroscience 2014, 260, 158–170. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.B.; Olvera-Hernandez, S.; Vega-Rivera, N.M.; Ortiz-Lopez, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2018, 20, 73. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the Programming of Stem Cells. Int. J. Mol. Sci. 2022, 23, 1971. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Z.; Sheng, P.; Rui, Y.; Cao, J.; Zhang, J.; Gao, M.; Wang, L.; Yu, D.; Yan, B.C. Targeting MicroRNA-144/451-AKT-GSK3beta Axis Affects the Proliferation and Differentiation of Radial Glial Cells in the Mouse Hippocampal Dentate Gyrus. ACS Chem. Neurosci. 2022, 13, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Gomez-Sanchez, A.; Ortiz-Lopez, L. Melatonin maintains calcium-binding calretinin-positive neurons in the dentate gyrus during aging of Balb/C mice. Exp. Gerontol. 2014, 60, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alonso, A.; Valdes-Tovar, M.; Solis-Chagoyan, H.; Benitez-King, G. Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: Participation of the Ca++/Calmodulin complex. Int. J. Mol. Sci. 2015, 16, 1907–1927. [Google Scholar] [CrossRef] [PubMed]
- Argueta, J.; Solis-Chagoyan, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velazquez-Moctezuma, J.; Benitez-King, G. Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity. Int. J. Mol. Sci. 2022, 23, 2479. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.B.; Palacios-Cabriales, D.M.; Ortiz-Lopez, L.; Estrada-Camarena, E.M.; Vega-Rivera, N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal- and Ventral- Dentate Gyrus Concomitantly with Its Antidepressant-Like Effect in Male Balb/C Mice. Int. J. Mol. Sci. 2020, 21, 1724. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-Lopez, L.; Granados-Juarez, A.; Estrada-Camarena, E.M.; Ramirez-Rodriguez, G.B. Melatonin Reverses the Depression-associated Behaviour and Regulates Microglia, Fractalkine Expression and Neurogenesis in Adult Mice Exposed to Chronic Mild Stress. Neuroscience 2020, 440, 316–336. [Google Scholar] [CrossRef]
- Aranarochana, A.; Chaisawang, P.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Protective effects of melatonin against valproic acid-induced memory impairments and reductions in adult rat hippocampal neurogenesis. Neuroscience 2019, 406, 580–593. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H.; et al. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, e01388. [Google Scholar] [CrossRef]
- Sirichoat, A.; Krutsri, S.; Suwannakot, K.; Aranarochana, A.; Chaisawang, P.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin protects against methotrexate-induced memory deficit and hippocampal neurogenesis impairment in a rat model. Biochem. Pharm. 2019, 163, 225–233. [Google Scholar] [CrossRef]
- Sirichoat, A.; Suwannakot, K.; Chaisawang, P.; Pannangrong, W.; Aranarochana, A.; Wigmore, P.; Welbat, J.U. Melatonin attenuates 5-fluorouracil-induced spatial memory and hippocampal neurogenesis impairment in adult rats. Life Sci. 2020, 248, 117468. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, K.; Sritawan, N.; Prajit, R.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Protects against the Side-Effects of 5-Fluorouracil on Hippocampal Neurogenesis and Ameliorates Antioxidant Activity in an Adult Rat Hippocampus and Prefrontal Cortex. Antioxidants 2021, 10, 615. [Google Scholar] [CrossRef]
- Suwannakot, K.; Sritawan, N.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Attenuates Methotrexate-Induced Reduction of Antioxidant Activity Related to Decreases of Neurogenesis in Adult Rat Hippocampus and Prefrontal Cortex. Oxidative Med. Cell. Longev. 2022, 2022, 1596362. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982. [Google Scholar] [CrossRef]
- Disouky, A.; Lazarov, O. Adult hippocampal neurogenesis in Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2021, 177, 137–156. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Kim, W.; Lee, C.H.; Shin, B.N.; Nam, S.M.; Choi, J.H.; Won, M.H.; Yoon, Y.S.; Hwang, I.K. Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J. Pineal Res. 2012, 52, 21–28. [Google Scholar] [CrossRef]
- Pallas, M.; Camins, A.; Smith, M.A.; Perry, G.; Lee, H.G.; Casadesus, G. From aging to Alzheimer’s disease: Unveiling "the switch" with the senescence-accelerated mouse model (SAMP8). J. Alzheimers Dis. 2008, 15, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Quero-Chavez, D.B.; Trueta, C.; Miranda, A.; Valdes-Tovar, M.; Alarcon-Elizalde, S.; Oikawa-Sala, J.; Argueta, J.; Constantino-Jonapa, L.A.; Munoz-Estrada, J.; et al. Low Doses of Ketamine and Melatonin in Combination Produce Additive Antidepressant-like Effects in Mice. Int. J. Mol. Sci. 2021, 22, 9225. [Google Scholar] [CrossRef]
- Boiko, D.I.; Shkodina, A.D.; Hasan, M.M.; Bardhan, M.; Kazmi, S.K.; Chopra, H.; Bhutra, P.; Baig, A.A.; Skrypnikov, A.M. Melatonergic Receptors (Mt1/Mt2) as a Potential Additional Target of Novel Drugs for Depression. Neurochem. Res. 2022, 47, 2909–2924. [Google Scholar] [CrossRef] [PubMed]
- Kholghi, G.; Eskandari, M.; Shokouhi Qare Saadlou, M.S.; Zarrindast, M.R.; Vaseghi, S. Night shift hormone: How does melatonin affect depression? Physiol. Behav. 2022, 252, 113835. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Oikawa-Sala, J.; Gomez-Sanchez, A.; Ortiz-Lopez, L.; Estrada-Camarena, E. Melatonin synergizes with citalopram to induce antidepressant-like behavior and to promote hippocampal neurogenesis in adult mice. J. Pineal Res. 2014, 56, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Tovar, M.; Estrada-Reyes, R.; Solis-Chagoyan, H.; Argueta, J.; Dorantes-Barron, A.M.; Quero-Chavez, D.; Cruz-Garduno, R.; Cercos, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian modulation of neuroplasticity by melatonin: A target in the treatment of depression. Br. J. Pharm. 2018, 175, 3200–3208. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K.; Kim, B.S.; Yim, S.V. Melatonin increases cell proliferation in the dentate gyrus of maternally separated rats. J. Pineal Res. 2004, 37, 193–197. [Google Scholar] [CrossRef]
- Yucel, A.; Yucel, N.; Ozkanlar, S.; Polat, E.; Kara, A.; Ozcan, H.; Gulec, M. Effect of agomelatine on adult hippocampus apoptosis and neurogenesis using the stress model of rats. Acta Histochem. 2016, 118, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, D.; Lazarov, N.; Stoyanov, D.S.; Spassov, R.H.; Tonchev, A.B.; Tchekalarova, J. Reduced neuroinflammation and enhanced neurogenesis following chronic agomelatine treatment in rats undergoing chronic constant light. Neuropharmacology 2021, 197, 108706. [Google Scholar] [CrossRef]
- Fang, X.Y.; Zhao, D.W.; Zhang, C.; Ge, H.F.; Zhang, X.Y.; Zhao, F.C.; Jiang, Y.B.; Feng, H.; Hu, R. A three-dimensional matrix system containing melatonin and neural stem cells repairs damage from traumatic brain injury in rats. Neural Regen. Res. 2022, 17, 2512–2517. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transpl. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina 2020, 56, 137. [Google Scholar] [CrossRef]
- Osier, N.; McGreevy, E.; Pham, L.; Puccio, A.; Ren, D.; Conley, Y.P.; Alexander, S.; Dixon, C.E. Melatonin as a Therapy for Traumatic Brain Injury: A Review of Published Evidence. Int. J. Mol. Sci. 2018, 19, 1539. [Google Scholar] [CrossRef] [PubMed]
- Barlow, K.M.; Esser, M.J.; Veidt, M.; Boyd, R. Melatonin as a Treatment after Traumatic Brain Injury: A Systematic Review and Meta-Analysis of the Pre-Clinical and Clinical Literature. J. Neurotrauma 2019, 36, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.; Kaushal, S.; Khan, S.; Kim, J.H.; Alvarez Villalba, C.L. Melatonin in Traumatic Brain Injury and Cognition. Cureus 2021, 13, e17776. [Google Scholar] [CrossRef]
- Lin, X.J.; Liu, R.; Li, C.; Yi, X.; Fu, B.; Walker, M.J.; Xu, X.M.; Sun, G.; Lin, C.H. Melatonin ameliorates spatial memory and motor deficits via preserving the integrity of cortical and hippocampal dendritic spine morphology in mice with neurotrauma. Inflammopharmacology 2020, 28, 1553–1566. [Google Scholar] [CrossRef]
- Kilic, E.; Kilic, U.; Bacigaluppi, M.; Guo, Z.; Abdallah, N.B.; Wolfer, D.P.; Reiter, R.J.; Hermann, D.M.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008, 45, 142–148. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Patino, P.; Reiter, R.J.; Gil-Martin, E.; Marco-Contelles, J.; Parada, E.; de Los Rios, C.; Romero, A.; Egea, J. Ischemic brain injury: New insights on the protective role of melatonin. Free Radic. Biol. Med. 2017, 104, 32–53. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin Receptor as a Drug Target for Neuroprotection. Curr. Mol. Pharm. 2021, 14, 150–164. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, B.; Yuan, F.; He, X.; Lin, X.; Wang, J.; Wang, Y.; Yang, G.Y. Melatonin Pretreatment Improves the Survival and Function of Transplanted Mesenchymal Stem Cells after Focal Cerebral Ischemia. Cell Transpl. 2014, 23, 1279–1291. [Google Scholar] [CrossRef]
- Corrales, A.; Martinez, P.; Garcia, S.; Vidal, V.; Garcia, E.; Florez, J.; Sanchez-Barcelo, E.J.; Martinez-Cue, C.; Rueda, N. Long-term oral administration of melatonin improves spatial learning and memory and protects against cholinergic degeneration in middle-aged Ts65Dn mice, a model of Down syndrome. J. Pineal Res. 2013, 54, 346–358. [Google Scholar] [CrossRef]
- Corrales, A.; Vidal, R.; Garcia, S.; Vidal, V.; Martinez, P.; Garcia, E.; Florez, J.; Sanchez-Barcelo, E.J.; Martinez-Cue, C.; Rueda, N. Chronic melatonin treatment rescues electrophysiological and neuromorphological deficits in a mouse model of Down syndrome. J. Pineal Res. 2014, 56, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Parisotto, E.B.; Vidal, V.; Garcia-Cerro, S.; Lantigua, S.; Diego, M.; Wilhem Filho, D.; Sanchez-Barcelo, E.J.; Martinez-Cue, C.; Rueda, N. Pre- and post-natal melatonin administration partially regulates brain oxidative stress but does not improve cognitive or histological alterations in the Ts65Dn mouse model of Down syndrome. Behav. Brain Res. 2017, 334, 142–154. [Google Scholar] [CrossRef]
- Parisotto, E.B.; Vidal, V.; Garcia-Cerro, S.; Lantigua, S.; Wilhelm Filho, D.; Sanchez-Barcelo, E.J.; Martinez-Cue, C.; Rueda, N. Chronic Melatonin Administration Reduced Oxidative Damage and Cellular Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochem. Res. 2016, 41, 2904–2913. [Google Scholar] [CrossRef]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Oto, M.M. The misdiagnosis of epilepsy: Appraising risks and managing uncertainty. Seizure 2017, 44, 143–146. [Google Scholar] [CrossRef]
- Consales, A.; Casciato, S.; Asioli, S.; Barba, C.; Caulo, M.; Colicchio, G.; Cossu, M.; de Palma, L.; Morano, A.; Vatti, G.; et al. The surgical treatment of epilepsy. Neurol. Sci. 2021, 42, 2249–2260. [Google Scholar] [CrossRef]
- Sorensen, A.T.; Kokaia, M. Novel approaches to epilepsy treatment. Epilepsia 2013, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bittigau, P.; Sifringer, M.; Genz, K.; Reith, E.; Pospischil, D.; Govindarajalu, S.; Dzietko, M.; Pesditschek, S.; Mai, I.; Dikranian, K.; et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15089–15094. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Sander, J.W.; Sisodiya, S.M.; Walker, M.C. Adult epilepsy. Lancet 2006, 367, 1087–1100. [Google Scholar] [CrossRef]
- Gupta, M.; Aneja, S.; Kohli, K. Add-on melatonin improves quality of life in epileptic children on valproate monotherapy: A randomized, double-blind, placebo-controlled trial. Epilepsy Behav. 2004, 5, 316–321. [Google Scholar] [CrossRef]
- Mi, Q.; Yao, G.; Zhang, G.Y.; Zhang, J.; Wang, J.; Zhao, P.; Liu, J. Disruption of GluR2/GAPDH Complex Interaction by TAT-GluR2(NT1-3-2) Peptide Protects against Neuronal Death Induced by Epilepsy. Ann. Clin. Lab. Sci. 2018, 48, 460–468. [Google Scholar]
- Molina-Carballo, A.; Munoz-Hoyos, A.; Sanchez-Forte, M.; Uberos-Fernandez, J.; Moreno-Madrid, F.; Acuna-Castroviejo, D. Melatonin increases following convulsive seizures may be related to its anticonvulsant properties at physiological concentrations. Neuropediatrics 2007, 38, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Kwon, J.E.; Durrance, E.S.; Jo, S.H.; Choi, S.Y.; Kim, K.T. Melatonin inhibits voltage-sensitive Ca(2+) channel-mediated neurotransmitter release. Brain Res. 2014, 1557, 34–42. [Google Scholar] [CrossRef]
- Uyanikgil, Y.; Turgut, M.; Ates, U.; Baka, M.; Yurtseven, M.E. Beneficial effects of melatonin on morphological changes in postnatal cerebellar tissue owing to epileptiform activity during pregnancy in rats: Light and immunohistochemical study. Dev. Brain Res. 2005, 159, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Lebkuechner, I.; Leke, R.; Marasek, P.; Yang, X.; Antfolk, D.; Chen, M.; Mohseni, P.; Lasic, E.; Bobnar, S.T.; et al. Nestin Regulates Neurogenesis in Mice Through Notch Signaling From Astrocytes to Neural Stem Cells. Cereb. Cortex 2019, 29, 4050–4066. [Google Scholar] [CrossRef]
- Turgut, M.; Uyanikgil, Y.; Ates, U.; Baka, M.; Yurtseven, M.E. Pinealectomy stimulates and exogenous melatonin inhibits harmful effects of epileptiform activity during pregnancy in the hippocampus of newborn rats: An immunohistochemical study. Childs Nerv. Syst. 2006, 22, 481–488. [Google Scholar] [CrossRef]
- Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Ameliorates Valproic Acid-Induced Neurogenesis Impairment: The Role of Oxidative Stress in Adult Rats. Oxidative Med. Cell. Longev. 2021, 2021, 9997582. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, S.W. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur. J. Obs. Gynecol. Reprod. Biol. 2008, 137, 198–203. [Google Scholar] [CrossRef]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, H.J.; Kang, H.J.; Samuels, T.L.; Johnston, N.; Lim, Y.C. Valproic acid suppresses the self-renewal and proliferation of head and neck cancer stem cells. Oncol. Rep. 2015, 34, 2065–2071. [Google Scholar] [CrossRef]
- Ferri, A.L.; Cavallaro, M.; Braida, D.; Di Cristofano, A.; Canta, A.; Vezzani, A.; Ottolenghi, S.; Pandolfi, P.P.; Sala, M.; DeBiasi, S.; et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 2004, 131, 3805–3819. [Google Scholar] [CrossRef]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Rizak, J.D.; Li, X.; Li, J.; Ma, Y. Melatonin treatment increases the transcription of cell proliferation-related genes prior to inducing cell death in C6 glioma cells in vitro. Oncol. Lett. 2013, 6, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.A.; Vos, T.; Hogan, D.R.; Gagnon, M.; Naghavi, M.; Mokdad, A.; Begum, N.; Shah, R.; Karyana, M.; Kosen, S.; et al. Common values in assessing health outcomes from disease and injury: Disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2129–2143. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2022. [Google Scholar]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Yang, A.C.; Tsai, S.J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef]
- Khan, Z.U.; Martin-Montanez, E.; Muly, E.C. Schizophrenia: Causes and treatments. Curr. Pharm. Des. 2013, 19, 6451–6461. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y. Season of birth and schizophrenia: Evidence from China. Psychiatry Res. 2017, 253, 189–196. [Google Scholar] [CrossRef]
- Schwartz, P.J. Season of birth in schizophrenia: A maternal-fetal chronobiological hypothesis. Med. Hypotheses 2011, 76, 785–793. [Google Scholar] [CrossRef]
- Gomes, P.R.L.; Motta-Teixeira, L.C.; Gallo, C.C.; Carmo Buonfiglio, D.D.; Camargo, L.S.; Quintela, T.; Reiter, R.J.; Amaral, F.G.D.; Cipolla-Neto, J. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen. Comp. Endocrinol. 2021, 300, 113633. [Google Scholar] [CrossRef]
- Galvan-Arrieta, T.; Trueta, C.; Cercos, M.G.; Valdes-Tovar, M.; Alarcon, S.; Oikawa, J.; Zamudio-Meza, H.; Benitez-King, G. The role of melatonin in the neurodevelopmental etiology of schizophrenia: A study in human olfactory neuronal precursors. J. Pineal Res. 2017, 63, e12421. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, L. Adult neural stem cells and schizophrenia. World J. Stem Cells 2022, 14, 219–230. [Google Scholar] [CrossRef]
- Cercos, M.G.; Galvan-Arrieta, T.; Valdes-Tovar, M.; Solis-Chagoyan, H.; Argueta, J.; Benitez-King, G.; Trueta, C. Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 1439. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Fritzen, S.; Finger, M.; Strobel, A.; Lauer, M.; Schmitt, A.; Lesch, K.P. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 2006, 11, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Zhou, Y.; Kogan, J.H.; Shin, R.; Webster, M.; Gross, A.K.; Heusner, C.L.; Chen, Q.; Miyake, S.; Tajinda, K.; et al. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl. Psychiatry 2012, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, D.; Juckel, G.; Faissner, A. Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models. Int. J. Mol. Sci. 2022, 23, 5482. [Google Scholar] [CrossRef]
- Pipova Kokosova, N.; Kiskova, T.; Vilhanova, K.; Stafurikova, A.; Jendzelovsky, R.; Racekova, E.; Smajda, B. Melatonin mitigates hippocampal and cognitive impairments caused by prenatal irradiation. Eur. J. Neurosci. 2020, 52, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.; Zhan, J.; Xie, K.; Wang, X.; Xian, X.; Gu, J.; Chen, W.; Hao, A. Melatonin improves short and long-term neurobehavioral deficits and attenuates hippocampal impairments after hypoxia in neonatal mice. Pharm. Res. 2013, 76, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.A.V., Jr.; Oliveira Bastos, P.R.H.; Portella, R.B.; Soares, L.F.G.; Conde, R.B.; Rodrigues, P.M.F., Jr.; Lucchetti, G. Pineal gland and schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology 2019, 104, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Mishra, B.R.; Jena, M.; Mishra, A.; Nath, S. Effect of Haloperidol and Risperidone on Serum Melatonin and GAP-43 in Patients with Schizophrenia: A Prospective Cohort Study. Clin. Psychopharmacol. Neurosci. 2021, 19, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Jenkins, Z.M.; Castle, D. Therapeutic use of melatonin in schizophrenia: A systematic review. World J. Psychiatry 2021, 11, 463–476. [Google Scholar] [CrossRef]
- Chio, A.; Logroscino, G.; Traynor, B.J.; Collins, J.; Simeone, J.C.; Goldstein, L.A.; White, L.A. Global epidemiology of amyotrophic lateral sclerosis: A systematic review of the published literature. Neuroepidemiology 2013, 41, 118–130. [Google Scholar] [CrossRef]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef]
- Lacomblez, L.; Bensimon, G.; Leigh, P.N.; Guillet, P.; Meininger, V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996, 347, 1425–1431. [Google Scholar] [CrossRef]
- Saitoh, Y.; Takahashi, Y. Riluzole for the treatment of amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2020, 10, 343–355. [Google Scholar] [CrossRef]
- Berdynski, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Zekanowski, C.; Kuzma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef]
- Boyd, S.D.; Ullrich, M.S.; Calvo, J.S.; Behnia, F.; Meloni, G.; Winkler, D.D. Mutations in Superoxide Dismutase 1 (Sod1) Linked to Familial Amyotrophic Lateral Sclerosis Can Disrupt High-Affinity Zinc-Binding Promoted by the Copper Chaperone for Sod1 (Ccs). Molecules 2020, 25, 1086. [Google Scholar] [CrossRef] [PubMed]
- DiFiglia, M.; Sena-Esteves, M.; Chase, K.; Sapp, E.; Pfister, E.; Sass, M.; Yoder, J.; Reeves, P.; Pandey, R.K.; Rajeev, K.G.; et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. USA 2007, 104, 17204–17209. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, F.; West, P.K.; Brennan, S.; Petrovic, B.; Hooshmand, K.; Akkari, P.A.; Keon, M.; Guennewig, B. New perspectives on cytoskeletal dysregulation and mitochondrial mislocalization in amyotrophic lateral sclerosis. Transl. Neurodegener. 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Bald, E.M.; Nance, C.S.; Schultz, J.L. Melatonin may slow disease progression in amyotrophic lateral sclerosis: Findings from the Pooled Resource Open-Access ALS Clinic Trials database. Muscle Nerve 2021, 63, 572–576. [Google Scholar] [CrossRef]
- Weishaupt, J.H.; Bartels, C.; Polking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Huther, G.; et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Glasauer, A.; Hoover, P.; Yang, S.; Blatt, H.; Mullen, A.R.; Getsios, S.; Gottardi, C.J.; DeBerardinis, R.J.; Lavker, R.M.; et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal 2013, 6, ra8. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.L.; Ghode, P.; Ong, D.S.T. Redox regulation of cell state and fate. Redox Biol. 2019, 25, 101056. [Google Scholar] [CrossRef]
- Liang, R.; Ghaffari, S. Stem cells, redox signaling, and stem cell aging. Antioxid. Redox Signal. 2014, 20, 1902–1916. [Google Scholar] [CrossRef]
- Shen, X.; Tang, C.; Wei, C.; Zhu, Y.; Xu, R. Melatonin Induces Autophagy in Amyotrophic Lateral Sclerosis Mice via Upregulation of SIRT1. Mol. Neurobiol. 2022, 59, 4747–4760. [Google Scholar] [CrossRef]
- Kadir, R.; Harel, T.; Markus, B.; Perez, Y.; Bakhrat, A.; Cohen, I.; Volodarsky, M.; Feintsein-Linial, M.; Chervinski, E.; Zlotogora, J.; et al. ALFY-Controlled DVL3 Autophagy Regulates Wnt Signaling, Determining Human Brain Size. PLoS Genet. 2016, 12, e1005919. [Google Scholar] [CrossRef]
- Wu, X.; Fleming, A.; Ricketts, T.; Pavel, M.; Virgin, H.; Menzies, F.M.; Rubinsztein, D.C. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat. Commun. 2016, 7, 10533. [Google Scholar] [CrossRef] [PubMed]
- Casares-Crespo, L.; Calatayud-Baselga, I.; Garcia-Corzo, L.; Mira, H. On the Role of Basal Autophagy in Adult Neural Stem Cells and Neurogenesis. Front. Cell. Neurosci. 2018, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Bachstetter, A.D.; Cole, M.J.; Fister, M.; Hudson, C.; Bickford, P.C. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur. J. Neurosci. 2007, 26, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef]
- Rubio-Gonzalez, A.; Bermejo-Millo, J.C.; de Luxan-Delgado, B.; Potes, Y.; Perez-Martinez, Z.; Boga, J.A.; Vega-Naredo, I.; Caballero, B.; Solano, J.J.; Coto-Montes, A.; et al. Melatonin Prevents the Harmful Effects of Obesity on the Brain, Including at the Behavioral Level. Mol. Neurobiol. 2018, 55, 5830–5846. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Wong, Y.K.; Chen, X.; Yang, C.; Xu, C.; Sun, J.; Wang, J. The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer’s disease. Int. J. Biol. Sci. 2022, 18, 983–994. [Google Scholar] [CrossRef]
- Patel, R.; Parmar, N.; Pramanik Palit, S.; Rathwa, N.; Ramachandran, A.V.; Begum, R. Diabetes mellitus and melatonin: Where are we? Biochimie 2022, 202, 2–14. [Google Scholar] [CrossRef]
- Drel, V.R.; Mashtalir, N.; Ilnytska, O.; Shin, J.; Li, F.; Lyzogubov, V.V.; Obrosova, I.G. The leptin-deficient (ob/ob) mouse: A new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes 2006, 55, 3335–3343. [Google Scholar] [CrossRef]
- de Luxan-Delgado, B.; Potes, Y.; Rubio-Gonzalez, A.; Caballero, B.; Solano, J.J.; Fernandez-Fernandez, M.; Bermudez, M.; Rodrigues Moreira Guimaraes, M.; Vega-Naredo, I.; Boga, J.A.; et al. Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 2016, 61, 108–123. [Google Scholar] [CrossRef]
- de Luxan-Delgado, B.; Caballero, B.; Potes, Y.; Rubio-Gonzalez, A.; Rodriguez, I.; Gutierrez-Rodriguez, J.; Solano, J.J.; Coto-Montes, A. Melatonin administration decreases adipogenesis in the liver of ob/ob mice through autophagy modulation. J. Pineal Res. 2014, 56, 126–133. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, S.; Li, F.; Wang, B.; Wang, J.; Cao, L.; Xia, T.; Yao, Q.; Chen, H.; et al. Melatonin Enhances Proliferation and Modulates Differentiation of Neural Stem Cells Via Autophagy in Hyperglycemia. Stem Cells 2019, 37, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, Y.; Yuan, Q.; Pan, Y.; Wang, L.; Liu, Q.; Wang, F.; Wang, J.; Hao, A. Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J. Pineal Res. 2015, 59, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Wongchitrat, P.; Lansubsakul, N.; Kamsrijai, U.; Sae-Ung, K.; Mukda, S.; Govitrapong, P. Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochem. Int. 2016, 100, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Bujalance, C.; Del Marco, A.; Jose Ramos-Rodriguez, J.; Infante-Garcia, C.; Bella Gomez-Santos, S.; Herrera, M.; Garcia-Alloza, M. Cell proliferation and neurogenesis alterations in Alzheimer’s disease and diabetes mellitus mixed murine models. J. Neurochem. 2020, 154, 673–692. [Google Scholar] [CrossRef]
- Mu, R.; Wu, X.; Yuan, D.; Zhao, J.; Tang, S.; Hong, H.; Long, Y. Activation of TGR5 Ameliorates Streptozotocin-Induced Cognitive Impairment by Modulating Apoptosis, Neurogenesis, and Neuronal Firing. Oxidative Med. Cell. Longev. 2022, 2022, 3716609. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, W.B.; Yang, P.; Dong, D.; Sun, W.; Yang, P. High Glucose Inhibits Neural Stem Cell Differentiation Through Oxidative Stress and Endoplasmic Reticulum Stress. Stem Cells Dev. 2018, 27, 745–755. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potes, Y.; Cachán-Vega, C.; Antuña, E.; García-González, C.; Menéndez-Coto, N.; Boga, J.A.; Gutiérrez-Rodríguez, J.; Bermúdez, M.; Sierra, V.; Vega-Naredo, I.; et al. Benefits of the Neurogenic Potential of Melatonin for Treating Neurological and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 4803. https://doi.org/10.3390/ijms24054803
Potes Y, Cachán-Vega C, Antuña E, García-González C, Menéndez-Coto N, Boga JA, Gutiérrez-Rodríguez J, Bermúdez M, Sierra V, Vega-Naredo I, et al. Benefits of the Neurogenic Potential of Melatonin for Treating Neurological and Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2023; 24(5):4803. https://doi.org/10.3390/ijms24054803
Chicago/Turabian StylePotes, Yaiza, Cristina Cachán-Vega, Eduardo Antuña, Claudia García-González, Nerea Menéndez-Coto, Jose Antonio Boga, José Gutiérrez-Rodríguez, Manuel Bermúdez, Verónica Sierra, Ignacio Vega-Naredo, and et al. 2023. "Benefits of the Neurogenic Potential of Melatonin for Treating Neurological and Neuropsychiatric Disorders" International Journal of Molecular Sciences 24, no. 5: 4803. https://doi.org/10.3390/ijms24054803
APA StylePotes, Y., Cachán-Vega, C., Antuña, E., García-González, C., Menéndez-Coto, N., Boga, J. A., Gutiérrez-Rodríguez, J., Bermúdez, M., Sierra, V., Vega-Naredo, I., Coto-Montes, A., & Caballero, B. (2023). Benefits of the Neurogenic Potential of Melatonin for Treating Neurological and Neuropsychiatric Disorders. International Journal of Molecular Sciences, 24(5), 4803. https://doi.org/10.3390/ijms24054803







