Abstract
Coriolus versicolor (CV) is a common species from the Polyporaceae family that has been used in traditional Chinese herbal medicine for over 2000 years. Among well-described and most active compounds identified in CV are polysaccharopeptides, such as polysaccharide peptide (PSP) and Polysaccharide-K (PSK, krestin), which, in some countries, are already used as an adjuvant agent in cancer therapy. In this paper, research advances in the field of anti-cancer and anti-viral action of CV are analyzed. The results of data obtained in in vitro and in vivo studies using animal models as well as in clinical research trials have been discussed. The present update provides a brief overview regarding the immunomodulatory effects of CV. A particular focus has been given to the mechanisms of direct effects of CV on cancer cells and angiogenesis. A potential use of CV compounds in anti-viral treatment, including therapy against COVID-19 disease, has also been analyzed based on the most recent literature. Additionally, the significance of fever in viral infection and cancer has been debated, providing evidence that CV affects this phenomenon.
Keywords:
Coriolus versicolor; cancer; COVID-19; immunomodulation; angiogenesis; inflammation; fever 1. Introduction
Natural products have played a vital role in health care since ancient times. In academic medicine, there are many examples of drugs (including chemotherapeutic agents) that originated from plants and mushrooms, e.g., topotecan [1], etoposide, teniposide [2], docetaxel, and paclitaxel [3]. Interestingly, the Nobel Prize in Medicine 2015 was awarded for artemisinin—the active ingredient of the medicinal herb ‘sweet wormwood’—which is an effective anti-malarial therapy [4].
Mushrooms have long been regarded as a healthy source of nutritional value [5]. Additionally, they have emerged in recent years not only as a source of drugs, but also as adjuvants to conventional treatments. Their potential to reduce side effects related to chemotherapy or radiotherapy is of special interest. In this regard, the best investigated medicinal mushroom is Coriolus versicolor (L.) Quél. (1886), also known as Trametes versicolor (L.) Lloyd (1920), Polyporus versicolor (L.) Fries (1821), Turkey Tail, Agaricus versicolor, Boletus versicolor, Polystictus versicolor, Poria versicolor, Yun-Zhi (Chinese), and Kawaratake (Japanese) [6]. An ancient Chinese formulation of Coriolus versicolor (CV) has been used in traditional Chinese herbal medicine for over 2000 years. It is believed that CV promotes health, strength, and longevity. In traditional Chinese medicinal practice, the CV mushroom is considered useful for removing toxins, strengthening, increasing energy, improving liver and spleen function, promoting diuresis, and enhancing the immune response. It is also used to damp heat jaundice, hypochondriac pain, poor appetite, lassitude, and weakness [7].
Many studies have reported that CV has anti-oxidant, hypoglycemic, and immune-enhancing effects, and therefore, its benefits in liver disease and diabetes are expected. Indeed, in China, Japan, and the United States, CV is used as an important dietary supplement that protects the liver [8].
Among more than 270 recognized species of mushrooms with immunotherapeutic properties, 50 are described as non-toxic and have been tested in animal models, and 6 of these species have been studied in human cancers. The CV mushroom is the only one among these 6 species which has been studied in phase I, II, and III randomized clinical trials in stomach, colorectal, esophageal, and breast cancer patients [9]. In Japan and China, the extract from CV is prescribed routinely to radio- or chemotherapy-treated patients suffering from different types of cancer. It has been found that CV increases survival rates and improves activity of immune cells. Moreover, it counters the immunosuppressive effects of conventional anti-cancer therapies and reduces cancer treatment-related symptoms, such as fatigue, loss of appetite, vomiting, and pain, thereby improving the quality of life of cancer patients [10,11]. All these effects make CV extract a commonly used drug by many naturopathic physicians and integrative oncologists in the USA [12]. The number of publications on CV reflects great interest in the medical application of this mushroom. Over 400 publications concerning CV have been deposited in PubMed within the last 5 years.
The aim of this review is to research advances in the field of CV-induced effects that can be useful in a treatment of one of the most actual medical challenges of 21st century i.e., cancer and COVID-19.
2. Active Compounds in CV Extract
Since mushrooms, in contrast to animals, do not have any adaptive immune system, their chemical shield must protect them from the entire spectrum of pathogens that exist in their natural environment. The study on the composition of CV shows that it contains many compounds, such as proteins [13,14], fatty acids [15], polysaccharides [16,17], polysaccharopeptides [18,19], glucans [20,21], amino acids [15,22], vitamins [8,15], and a variety of inorganic salts [15,23]. The chemical structures of the main CV active compounds described in this review are presented in the Supplementary Materials (Figures S1–S5) [24,25,26,27].
2.1. Polysaccharopeptides
Polysaccharopeptides exert many physiological effects that are useful in the treatment of cancer, inflammation, and diabetes, such as promoting immune function and providing anti-tumor, anti-inflammation, and anti-diabetes effects [28]. Among polysaccharopeptides, polysaccharide peptide (PSP) and polysaccharide krestin (PSK) are the most studied ones for their anti-cancer and immune-enhancing properties. PSK was extracted by salting out with ammonium sulphate from the hot water extract in Japan in the 1960s and is a soluble protein-bound polysaccharide derived from the CM-101 strain of the fungus. PSP was extracted using alcohol precipitation from the hot water extract in China in the 1980s and is a polysaccharide-peptide derived from the COV-1 strain [19]. Both compounds are light or dark brown powders that are soluble and stable in water. The molecular weights of the two molecules are about 100 kDa with a respective polysaccharide-to-peptide balance of 90–10% in PSP and 60–40% in PSK. The carbohydrate moieties of each compound consist of mannose, xylose, and galactose, in addition to fructose in PSP or arabinose and rhamnose in PSK [18].
The toxicity of these compounds was tested on a variety of animals (dogs, monkeys, guinea pigs) showing no genetic and reproductive effects [29]. Importantly, neither teratogenic nor mutagenic effects of PSP have been observed in female reproductive and embryonic development in animal models [30,31].
2.2. Polysaccharides
Coriolus versicolor contains a high number of polysaccharides, including heteroglycan macromolecules. The main monosaccharide that builds these polysaccharides is glucose, followed by small amounts of mannose, rhamnose, glucuronic acid, and fructose [16].
Polysaccharides possess many physiological activities, such as promoting immune function and providing anti-tumor and anti-inflammatory effects [28,32]. They are effective in protecting the liver by increasing the activity of antioxidant enzymes and glutathione and the brain during cerebral ischemia reperfusion. Additionally, beneficial effects in diabetes and anti-bacterial activities have also been observed [16,33,34,35,36]. Numerous studies have shown that polysaccharides from CV can scavenge free reactive oxygen species (ROS) [37,38,39]. These effects may be useful in diseases, such as arteriosclerosis, Alzheimer’s disease, and cardiovascular and cerebrovascular diseases [40].
Among polysaccharides derived from CV, β-glucans are the principal components. Due to their non-starch and non-digestible natures, they can be utilized as dietary fibers by gut probiotic bacteria in the large intestine. Therefore, they are considered as potential prebiotics with anti-obesity properties [41,42,43]. Beta-glucans are believed to be one of the most well-established and potent derivatives of mushrooms that have anti-tumor, immunomodulatory, anti-viral, and anti-bacterial properties [21,44,45,46,47,48]. Notably, due to their confirmed complex mode of action, β-glucans are recognized as biological response modifiers. They induce epigenetic programming in innate immune cells to produce a more robust immune response and act as pathogen-associated molecular patterns (PAMPs), binding to specific pathogen recognition receptors. In consequence, innate and adaptive immune responses are induced [49,50].
2.3. Small Molecules
Coriolus versicolor contains pharmacologically active secondary metabolites belonging to small molecules. Wang et al. reported the isolation of four new spiroaxane sesquiterpenes, tramspiroins A-D, one new rosenonolactone 15,16-acetonide, and the known drimane sesquiterpenes isodrimenediol and funatrol D [51]. Moreover, Janjušević et al. identified 35 phenolic compounds belonging to the flavonoid (flavones, flavonols, flavanone, flavanols, biflavonoids, isoflavonoids) and hydroxy cinnamic acids, which exhibit anti-radical and acetylcholinesterase inhibitory properties. Therefore, CV extract can be eventually used as drug-like compounds or food supplements in the treatment of Alzheimer’s disease [52].
Among small molecules present in the CV extract, Yang et al. isolated a compound of about 10 kDa molecular weight named as a small peptide of Coriolus versicolor (SMCV). This compound inhibits in vitro proliferation of various human cancer cells, such as gastric cancer, leukemia, hepatoma and colon cancer, more significantly than PSP or PSK. Moreover, pre-treatment of SPCV decreases the proliferation of tumor cells in mouse and has an immunostimulating effect manifested by increase in white blood cells and IgG levels [53].
Another small molecule purified by Kuan et al. from CV is a 12-kDa non-glycosylated protein comprising 139 amino acids, including an 18-amino acids signal peptide. This protein, called YZP, has the ability to induce an increase in interleukin (IL) 10 secretion by B lymphocytes and suppress the production of pro-inflammatory cytokines by lipopolysaccharide (LPS)-activated macrophages [14].
Recently, He et al. characterized a novel 12-kDa protein named musarin. This protein shows significant growth inhibition on multiple human colorectal cancer cell lines in vitro. In the animal model, oral ingestion of musarin significantly inhibits tumor colorectal development at the similar level to gefitinib (a tyrosine kinase inhibitor used in oncology), but with a lower number of side effects [54].
3. Effect of CV on Immune Cell Properties
The analysis of the literature has shown that mushrooms contain many compounds that can activate the adaptive and innate immune system. The immunomodulating effects of CV have been studied intensively in in vitro and in vivo models. Clinical trials on this subject are also in progress [28,29,55].
3.1. Macrophages
Studies have shown that macrophages are one of the main target cells of CV extract. In vitro experiments demonstrated the direct effect of β-glucans and PSP derived from CV on the activation of macrophages, which is manifested by the increased expression of inducible nitric oxide synthase (iNOS) and production of reactive nitrogen intermediates and reactive oxygen intermediates [48,56].
The best reported immunomodulatory effect of CV is induction of cytokine productions. The water-extracted CV compounds, such as PSP and PSK, stimulate in vitro the secretion of IL-1β, IL-6, and tumor necrosis factor α (TNF-α) in different macrophage lines [57,58,59]. Moreover, the peritoneal macrophages isolated from the PSP-treated mice exhibit also increased release of prostaglandin E2 [58].
Considerable levels of research have been devoted to understanding how active compounds of CV mushroom interact with macrophages. Several studies have shown that the effect of the whole CV extract as well as PSP and PSK is mediated through Toll-like receptor (TLR) 4 signaling pathway, including the induction of nuclear factor κB (NF-κB) [57,59,60] and TRAF6 transcription, phosphorylation of c-Jun (a component of the transcription factor called activator protein 1 (AP-1)) [59,60], and increased expression CD14 glycoprotein (co-receptor especially required for LPS recognition by TLR4) [57]. It has been also shown that the whole CV extract induces production of cytokines, which is mediated by the phosphoinositide 3-kinase pathway [57].
In addition to TLR4, TLR2 and dectin-1 receptors are also involved in CV recognition by macrophages. Quayle et al. demonstrated that during stimulation of macrophages with PSK, a β-glucan fraction is recognized by the dectin-1 receptor, whereas lipid fraction towards TLR2 [47]. The ability of PSK to induce TNF-α production by macrophage as a result of TLR2 activation was also observed by Coy et al. [61]. Moreover, the dectin-1 signaling pathway, triggered by β-glucans isolated from CV, elicits TNF-α, nitric oxide and iNOS production leading to activation of macrophages toward phagocytosis [45,46]. Induction of phagocytosis in macrophages upon stimulation with β-glucans is also related to the increased expression of the scavenger receptor B1 (SR-B1) [20].
3.2. Peripheral Blood Mononuclear Cells
Numerous studies have shown that the CV extract affects all populations of peripheral blood mononuclear cells (PBMCs) as well as single cell population. One of the most documented effects of CV compounds (i.e., PSP and polysaccharides) and whole CV extract on PBMCs is the increased proliferation response. This mitogenic effect has been observed for human and rat PBMCs [62,63], human and rat lymphocytes [64,65], murine splenic lymphocytes [66], murine B lymphocytes [17] and human monocytes [67].
Numerous studies have shown that CV compounds, such as polysaccharopeptides and both aqueous and solid fractions of the CV mycelium, stimulate PBMCs to the secretion of predominantly pro-inflammatory cytokines. The CV extract-induced production of IL-1β, IL-2, IL-6, IL-12, and TNF-α was demonstrated in rat PBMCs [63], rat lymphocytes [65,68], and murine splenic lymphocytes [66]. Similarly, it was also reported that human PBMCs derived from healthy donors stimulated with polysaccharopeptides derived from CV secrete TNF-α [62], IL-1α, IL-2, IL-6, IL-8, IL-10, macrophage inflammatory protein 1 (MIP-1), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) [69,70], and interferon-γ (IFN-γ) [70,71]. Interestingly, human PBMCs isolated from breast cancer patients also exhibit an increased expression of TNF-α, IL-6, and IL-12 in response to PSP [71].
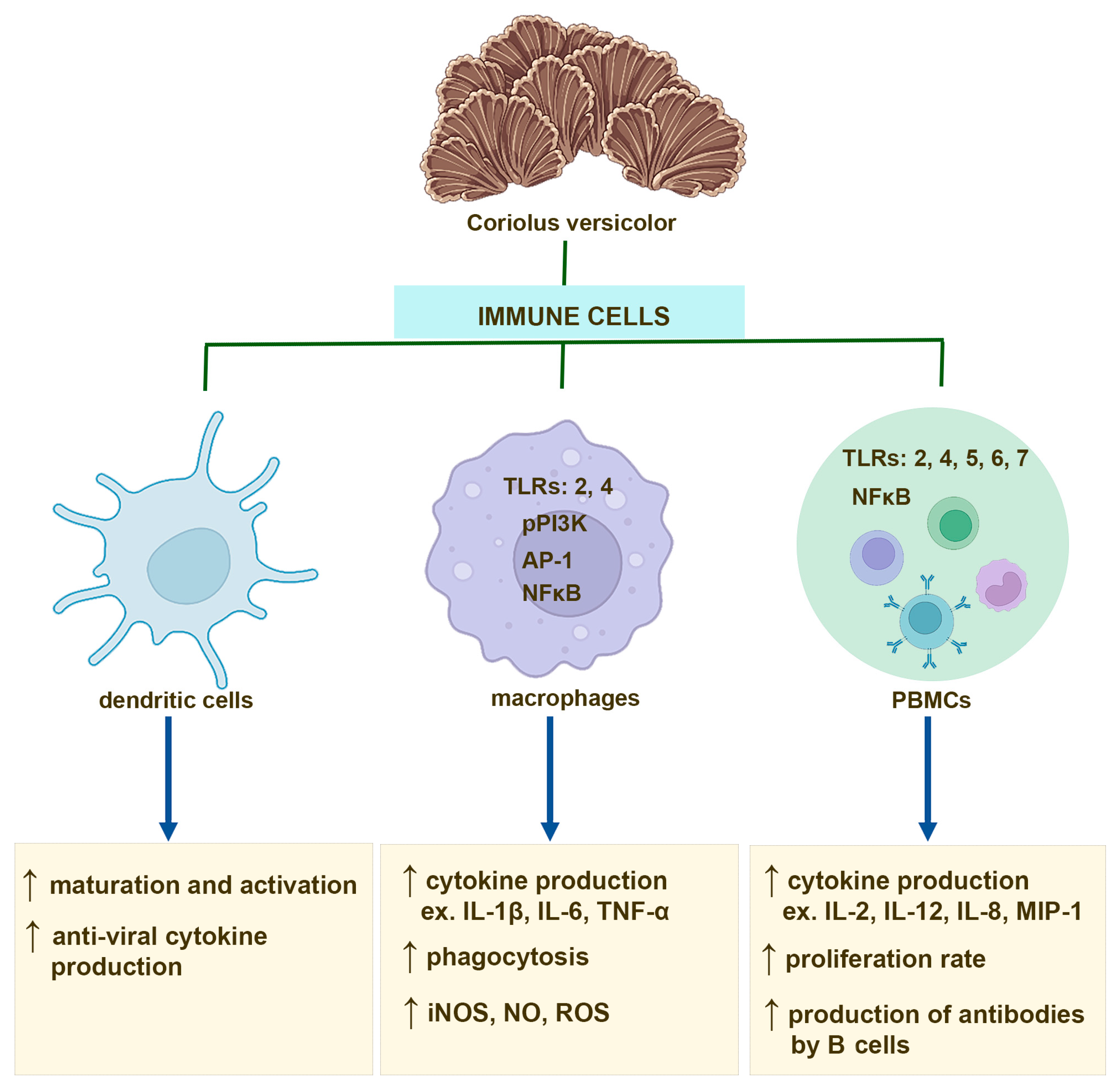
Several studies have shown that the effect of CV compounds on PBMC activation is mainly mediated through TLRs. Human PBMCs isolated from healthy donors and treated with PSP show upregulated expression of TLR4, TLR5, TLR6, and TLR7 as well as increased levels of multiple key molecules of TLR signaling pathway, such as TRAM, TRIF, and TRAF6 [69]. Interestingly, PSP also activates the TLR4-TIRAP/MAL-MyD88 signaling pathway in PBMCs derived from breast cancer patients [71]. The polysaccharides from CV exert immunoregulatory effects on B cells also via TLR4 involvement, and in consequence, inducing activation of the mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways [17]. In contrast, PSK activates NK cells and monocytes to produce cytokine through binding to TLR2 [72,73]. Moreover, it has been also reported that CV compounds, including polysaccharides and PSK, are able to enhance antibody production in B cells after binding to the B cell receptor [17,74] (Figure 1).
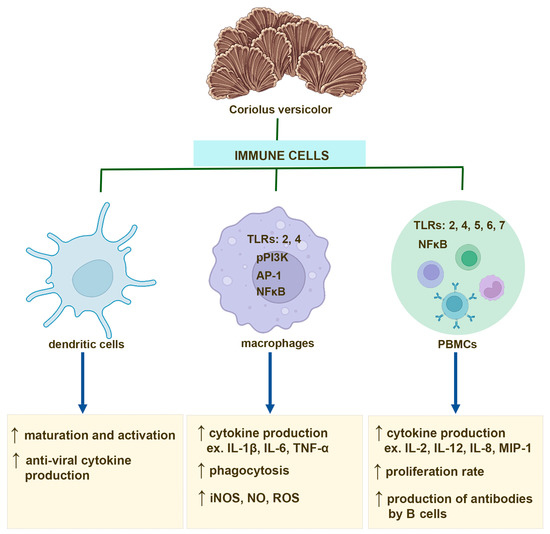
Figure 1.
Immunostimulatory effects of the compounds derived from Coriolus versicolor mushroom on dendritic cells, macrophages, and peripheral blood mononuclear cells (PBMCs), including monocytes and B and T cells.
4. Effect of CV Extract on Viral Infections
Among the bioactive properties of compounds derived from CV, their anti-viral activity against numerous viruses is well described. The high therapeutic index of CV extract was discovered against herpes simplex virus (HSV) type 1 and HSV type 2 in the experiments conducted on the kidney epithelial cells in vitro (EC50 = 77 µg/mL measured for the both HSV types) [75]. Liu et al. revealed that PSK can inhibit Epstein–Barr virus (EBV)-infected B and T cells and activate natural killer (NK) cells [76]. It has also been reported that PSP has an inhibitory effect against human immunodeficiency virus (HIV) type l reverse transcriptase and protease that are two enzymes of paramount importance to the life cycle of HIV (IC50 = 150 μg/mL and IC50 = 6.25 μg/mL measured for the interaction between HIV-1 gp120 and immobilized CD4 receptor and for the potent inhibition of recombinant HIV-1 reverse transcriptase, respectively) [77]. Rodriguez-Valentín et al. observed that PSP exerts an anti-HIV activity mediated by TLR4 and promotes the upregulation of specific anti-viral chemokines (RANTES, MIP-1) and stromal cell-derived factor 1 (SDF-1α)) known to block HIV-1 co-receptors [78]. Furthermore, oral administration of β-glucans from CV improves survival and reduces lung viral titers and weight loss in chickens and mice infected with the influenza virus [21]. CV-based vaginal gel is also available for treating women with cervical uterine high-risk human papillomavirus (HPV) infection [79,80].
Since CV compounds possess anti-viral properties against numerous viruses, it is likely that bioactive metabolites from CV might be considered as an anti-viral option against the novel coronavirus SARS-CoV-2. SARS-CoV-2 belongs to the family of coronaviruses that contains positive-sense single-stranded RNA [81]. The main viral protease, which plays an essential role in the viral life cycle, has been proposed as a key therapeutic target for drug development against coronavirus [82,83]. Since there are no effective anti-SARS-CoV-2 drugs, the natural products isolated from CV can be considered for the prevention and treatment of COVID-19.
Hetland et al. believe that CV may be utilized directly against SARS-CoV-2 infection as well as to prevent the immunological overreaction and harmful inflammation associated with COVID-19 [84]. According to Saxe, who is leading the MACH-19 (Mushrooms and Chinese Herbs for COVID-19) ongoing clinical trials approved by the Food and Drug Administration (FDA), the combination of CV with another fungus–agarikon (Fomitopsis officinalis) offers physiologically plausible immunomodulating capabilities against SARS-CoV-2 through the interaction with T lymphocyte receptors [85]. Rangsinth et al. examined 36 mushroom-derived bioactive compounds that potentially serve as the inhibitors of SARS-CoV-2 main protease. Indeed, 25 of 36 candidate compounds displayed the potential to inhibit this main viral protease. The most promising seems to be a betulinic acid derived from CV [86].
It is well established that COVID-19 is characterized by noticeably high concentrations of pro-inflammatory factors, such as IL-1, IL-2, IL-6, IL-8, TNF-α, monocyte chemoattractant protein-1 (MCP-1), G-CSF, GM-CSF, and many others [87]. Uncontrolled production of pro-inflammatory cytokines leads to cytokine storm in the lungs, which is initiated by the binding of the SARS-CoV-2 virus to the TLRs [88]. High levels of pro-inflammatory factors along with oxidative stress in patients with COVID-19 lead to fatal effects, such as acute respiratory distress syndrome (ARDS), pulmonary fibrosis, and death [87]. Zhang et al. demonstrated that anti-inflammatory therapy, including suppression of pro-inflammatory cytokine production, might have a therapeutic effect on viral diseases [89]. Numerous in vitro studies revealed the anti-inflammatory effects of both whole CV extract and its compounds, i.e., polysaccharopeptides, and proteins on PBMCs [63], B cells [14] and macrophages [14,57,90]. The anti-inflammatory properties of CV extract are associated, among others, with its ability to block the physical associations of pro-inflammatory factor, such as LPS with the specific receptors on immune cells (e.g., TLR4 or CD14 receptor) and decreasing the expression of these receptors. In consequence, a downregulation of NF-κB activity and pro-inflammatory cytokine production has been observed [57,90,91]. As an anti-inflammatory agent, CV extract has shown benefit in experimental animal models of osteoarthritis [35], inflammatory bowel disease [92], and traumatic brain injury [93].
Besides anti-inflammatory effects, the active compounds from CV, such as polysaccharides and protein-bound polysaccharides, also exhibit anti-oxidant properties by inducing the radical scavenging activity of superoxide dismutase and glutathione peroxidase, which was confirmed in vitro [39,94] and in vivo [37,95,96].
Published data indicate that active compounds of CV extract have the ability to inhibit inflammation and oxidative stress that is involved in the severe course of COVID-19. Furthermore, since natural products derived from CV show high efficiency in the treatment of many viruses, such as HIV, HPV, HSV, EBV, and influenza, the efficiency of CV compounds in the treatment of COVID-19 should be further investigated.
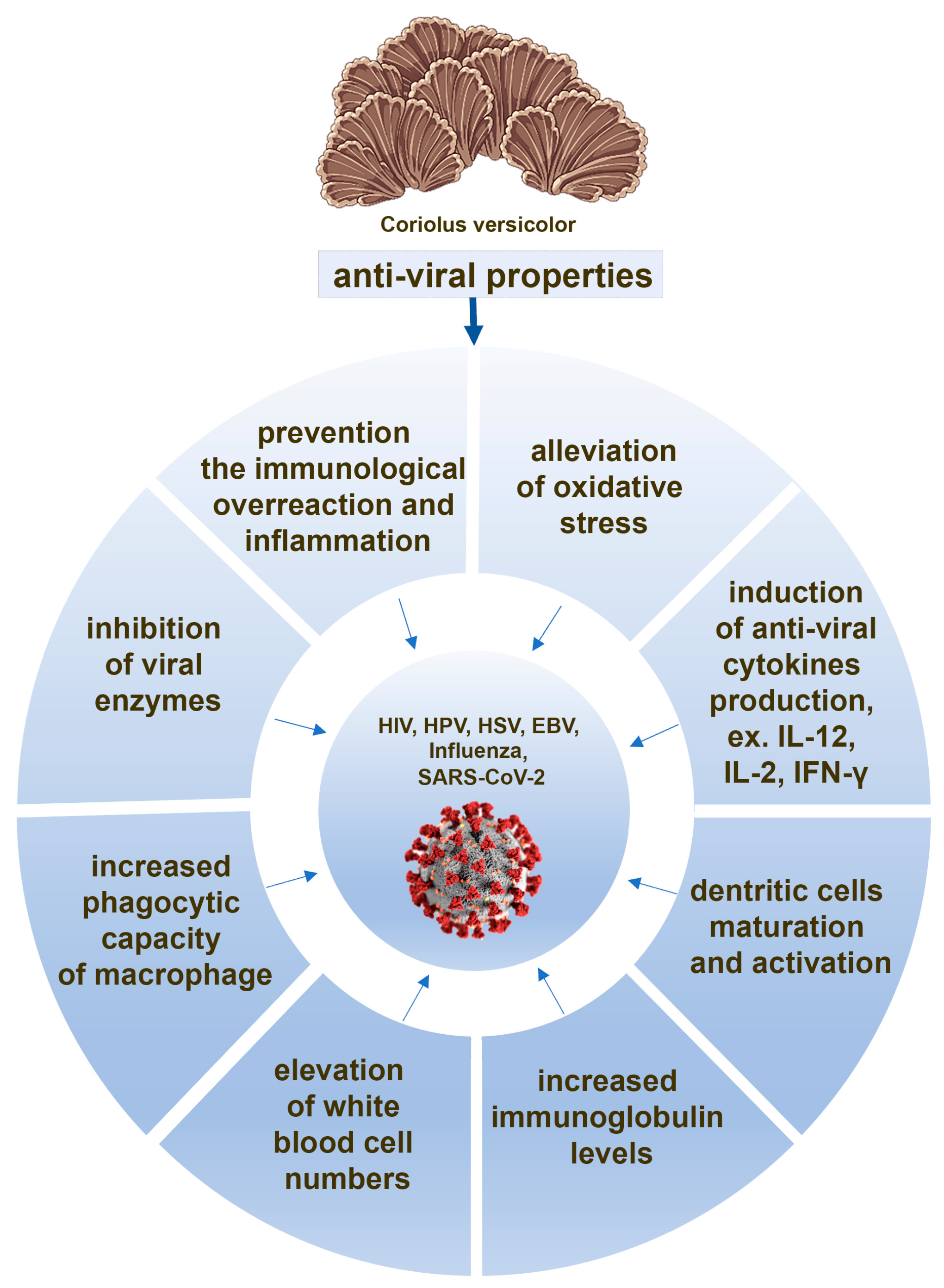
There are evidences that, in response to the both whole CV extract and its polysaccharopeptides treatment, immune cells produce cytokines with anti-viral properties, such as IL-12 [66,71,97,98], IFN-γ [66,70,99], and IL-2 [90,98]. The role of these cytokines in the treatment of COVID-19 is widely discussed, showing that IFN-γ is key for restraining SARS-CoV-2 infection [100]. IL-12 is required to maintain NK cell numbers in the early phase of SARS-CoV-2 infection [101] and IL-2 deficiency appears leading to serious effects, such as weak response for our immune system against SARS-CoV-2 [102]. All these findings indicate that the ability of CV compounds to induce anti-viral cytokine production by immune cells can be considered as a potential mechanism of its action against SARS-CoV-2 (Figure 2).
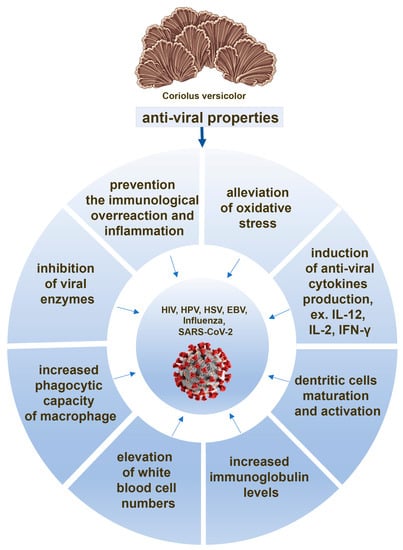
Figure 2.
The potential anti-viral actions of the compounds derived from Coriolus versicolor mushroom.
The compounds from CV, such as protein-bound polysaccharides, also induce dendritic cell maturation as well as anti-viral cytokine production by activated dendritic cells [97,103,104] (Figure 1), and they have the ability to enlarge draining lymph nodes with the higher number of activated dendritic cells [97]. This adjuvant-like activity of CV compounds may have potential therapeutic value in the preparation of a more effective COVID-19 vaccine. It is also an important issue since dendritic cells have an essential role in defending against SARS-CoV-2 infection [105,106,107] (Figure 2).
5. Effects of CV on Cancer Cells
Nowadays, chemotherapy, hormonotherapy, and targeted therapy are regarded as one of the most promising cancer systemic treatment approaches [108,109]. However, oncogene mutations, epigenetic changes, or changes within the tumor microenvironment may, among others, trigger a strong resistance of cancer cells to various modern anticancer drugs, resulting in increased tumor cells invasion and metastases [108,110]. Therefore, for these tumors, additional treatment potentiating inhibition of their proliferation and progression may increase the survival time of patients.
5.1. CV Activity in Combined Anti-Cancer Treatment
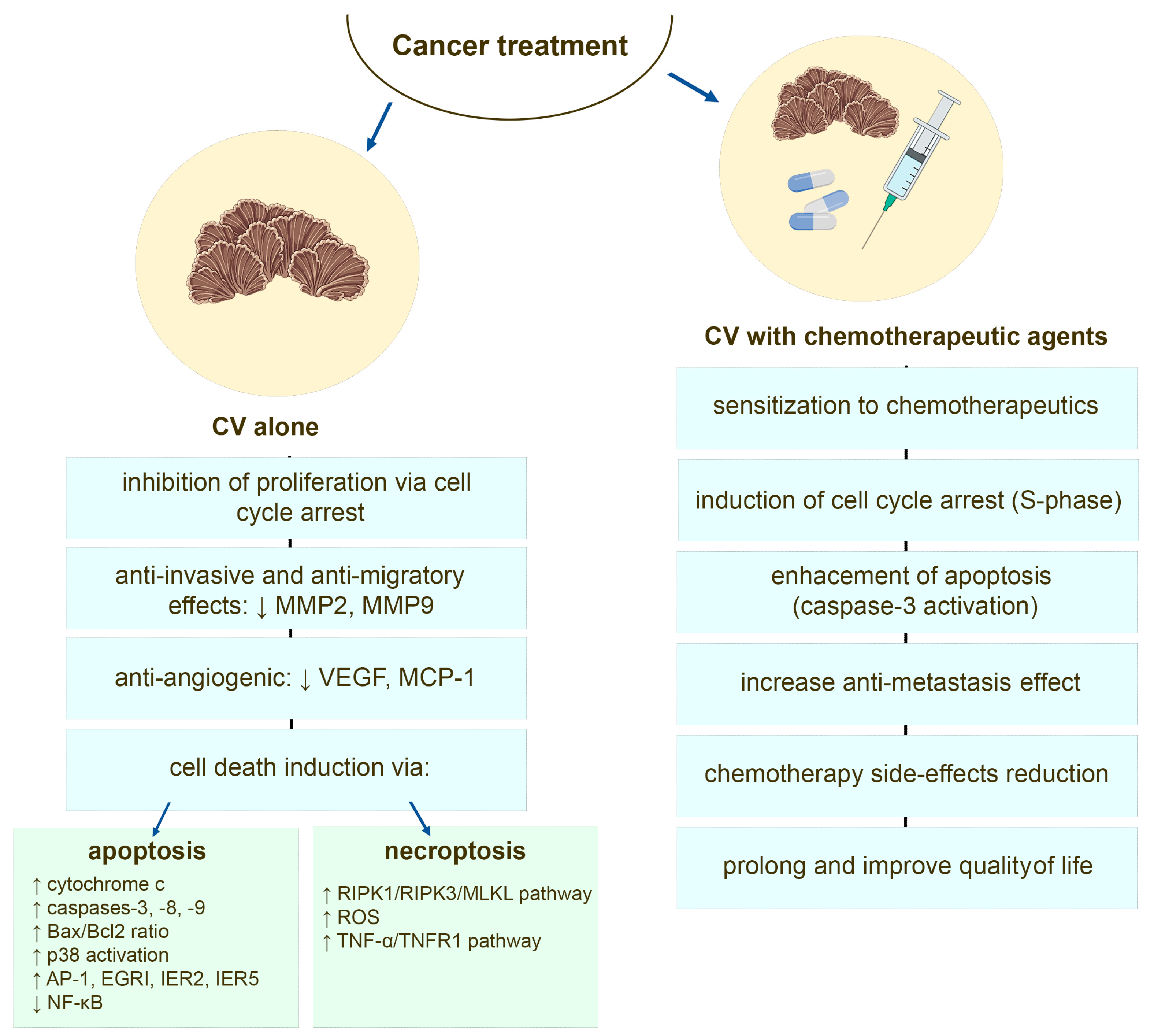
There are many studies that show that bioactive compounds of CV mushroom sensitize cancer cells towards the cytotoxic effects of chemotherapeutic agents [24,111,112,113] and additional drugs used in cancer therapy [114]. Protein-bound polysaccharides from CV enhance the apoptotic machinery induced by doxorubicin and etoposide in estrogen receptor (ER) negative human breast cancer [24] and leukemia cells, where this effect is associated with an induction of S-phase cell cycle arrest and caspase 3 activation [111,115]. The PSP pre-treatment increases also the response of human leukemia cells to camptothecin (CPT), where likewise by induction of cell cycle arrest in the DNA synthesis phase, PSP sensitizes the cancer cells to undergo apoptosis induced by CPT [116]. The combination therapy of PSK and docetaxel, examined in murine model of human prostate cancer, revealed that CV components augmented tumor regression and apoptosis of cancer cells compared to the activity of this chemotherapeutic agent alone [113]. Studies performed on other animal cancer models also described PSK-boosted effects of docetaxel-induced tumor cell apoptosis [117,118]. A recent report of the study performed using intratibial breast cancer murine model also demonstrated that CV alone is effective in decreasing tumor progression, whereas in combination with zoledronic acid (ZOL), which is used in adjuvant therapy for breast cancer [119], it shows significant anti-tumor, anti-metastasis, and anti-osteolytic effects [114] (Figure 3).
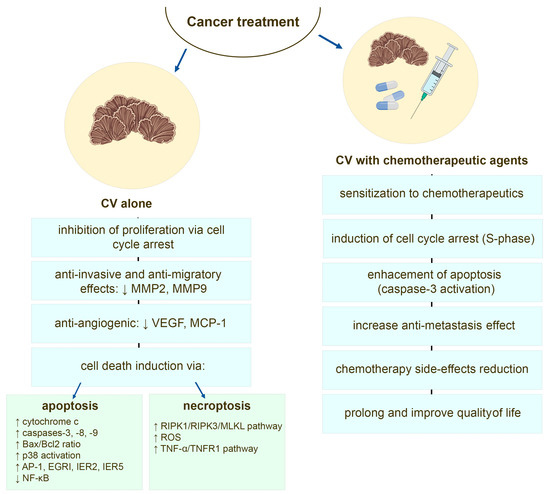
Figure 3.
The potential anti-cancer mechanisms of the compounds derived from Coriolus versicolor mushroom.
5.2. CV as Monotherapy for Cancer Treatment
There are plenty in vitro and in vivo studies demonstrating that CV compounds, among them PSP and PSK, besides cancer cells sensitization to various chemotherapeutic agents, can also induce tumoricidal effects [65,91,98,115,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134] and have inhibitory effect on tumor growth and metastasis in animal models [66,98,120,135,136]. These effects are associated with reduced proliferation mainly via cell cycle arrest [120,123,124,131,137,138] and induction of different mode of cancer cells death, such as apoptosis, necrosis, or necroptosis [120,121,122,124,125,127,128,133,134,136].
In the literature, discrepancies of CV outcomes towards cancer cells are easy to observe. This may result from the preparation and extraction methods of whole CV extract, the use of its single isolated compound as well as its concentration and cancer cell type [139] (Table 1). However, direct inhibition of tumor cell proliferation induced by CV extract and its compounds, such as PSK, PSP, and CV peptide, has been reported for leukemia [134,140], breast cancer [65,68,91], melanoma [120,128], colon cancer [129], and human esophageal carcinoma [131]. The molecular mechanism of this inhibition is still under constant investigation. The disruption of CV-treated cancer cell cycle progression and arrest at G0 phase [138], G0/G1 phase [120,124,131,137], or G1/S [111,112,123] and G2/M [123] phases have been reported. The observed anti-proliferative and cytotoxic effects of CV towards cancer cells are comparable to those induced by chemotherapeutic agents and are associated with oxidative stress that induces cancer cell death [91,127,128]. Pawlikowska et al. have found that CV-induced ROS generation trigger non-pigmented melanoma cells death [128], whereas Jędrzejewski et al. have also confirmed the ROS-dependent cytotoxic activity against breast cancer cells in the pro-inflammatory environment created by LPS [91]. The in vitro and in vivo molecular studies revealed that CV extract acting alone can induce cancer cell death through different cell death modalities. The apoptotic machinery is triggered by CV components in leukemia [115,121,125,134,136], breast cancer [98,122,124], and human esophageal carcinoma cells [131]. The components of CV extract are shown to induce mitochondria-mediated apoptosis pathway, since the release of cytochrome c, activation of caspases (-3, -8, and -9), and upregulation of the expression of Bax with a concomitant downregulation of Bcl-2 is observed [115,123,136]. The study of Ho et al. also implicates that the p53 protein might differentially act as a major upstream transcriptional apoptosis activator in different cancer cell types, among them breast cancer ones [122]. Additionally, Hirahara et al. have proven that apart from caspase-3 triggering, the activation of p38 MAPK signaling cascade is involved in the PSK-induced apoptosis [134]. The upregulation of early transcription factors such as AP-1, EGRI, IER2, and IER5, and the downregulation of NF-κB transcription pathways were also found to be involved in the PSP-mediated apoptosis [133].
Besides apoptosis, induction of necrosis as a model of cell death by CV extract has also been observed [120]. The CV-stimulated melanoma cells, which are known to be relatively resistant to drug induced apoptosis [141], were found to possess both apoptotic and necrotic tumor cell death features [120]. More recent analysis also revealed that CV-induced cell death of melanoma cells is regulated by receptor-interacting protein-1 (RIPK1) and ROS, and that this process is modified by melanin content in melanoma cells [128]. The CV extract has been also reported to induce RIPK1/RIPK3/MLKL-mediated necroptosis in non-pigmented melanoma cells and depigmented (with suppressed melanogenesis) melanoma cells, since co-treatment of the cells with necroptosis inhibitors abrogated the CV-induced cell death [126,127]. The necroptotic cell death mediated by activation of TNF-α/TNFR1 pathway was also observed in CV-stimulated ER-positive breast cancer cells [126].
The extract derived from CV, besides anti-proliferative and cytotoxic activity, also has anti-migratory and anti-invasive potentials against numerous tumor cells, including triple-negative breast cancer [98,142], estrogen receptor (ER)-positive breast cancer [91], colon cancer [129,130], pancreatic and gastric cancer [143], and melanoma [144]. The inhibition of the invasive activity of human tumor cells mediated via suppression of matrix metalloproteinase (MMP) activates, especially those of MMP-2 and MMP-9, is postulated [98,129,130,143]. Recently, Yang and co-workers revealed that CV extract and its bioactive molecules (i.e., SMCV) may reduce cancer cell invasion directly or indirectly through the suppression of TNF-α induced MMP-3 production by inactivating the p38 MAPK pathway in malignant cells [132] (Figure 3).

Table 1.
IC50 values of different cancer cell lines stimulated with the whole CV extract or its compounds.
Table 1.
IC50 values of different cancer cell lines stimulated with the whole CV extract or its compounds.
| CV Compound | Cancer Cell Line | IC50 Value (µg/mL) | References |
|---|---|---|---|
| ethanol-water whole extract | BT-20 breast cancer cells MDA-MB-231 breast cancer cells MCF-7 breast cancer cells T-47D breast cancer | >800 514.0 271.7 233.3 | Ho et al., 2005 [122] |
| aqueous ethanol whole extract | HL-60 leukemia cells | 150.6 | Ho et al., 2006 [136] |
| methanol whole extract | B16 melanoma cells | 200 | Harhaji et al., 2010 [120] |
| musarin (protein) | T84 colorectal cancer cells | 1.8 | He et al., 2021 [54] |
| α-glucans and β-glucans | LoVo colon carcinoma cells | 224.0 | Roca-Lema et al., 2019 [129] |
| aqueous ethanol whole extract | HL-60 leukemia cells B-cell lymphoma (Raji) NB-4 leukemia cells | 147.3 253.8 269.3 | Lau et al., 2004 [125] |
| small peptide of Coriolus versicolor (SPCV) | HL-60 leukemia cells LS174-T colony cancer cells SMMU-7721 hepatoma cells SCG-7901 stomach cancer cells | 30.0 142.0 138.0 323.0 | Yang et al., 1992 [53] |
| Coriolus versicolor polysaccharide (CVP) | 7703 hepatocellular carcinoma cells BCap3 breast cancer cells T-47D breast cancer cells MCF-7 breast cancer cells ZR75-30 breast cancer cells | 18.4 14.4 9.3 39.3 34.6 | Zhou et al., 2007 [145] |
| Coriolus versicolor polysaccharide (CVP) | 7703 hepatocellular carcinoma cells | 4.25 | Cai et al., 2010 [146] |
| PSK | MCF-7 breast cancer cells | 200 | Aoyagi et al., 1997 [147] |
| PSK | hepatocellular carcinoma H4-II-E cells human ovarian cancer cells | 1.5 0.33 | Kobayashi et al., 1994 [148] |
| ethanol whole extract | cervix adenocarcinoma HeLa cells colon carcinoma LS174 cells lung adenocarcinoma A549 cells | 42.4 86.1 65.6 | Knežević et al., 2018 [149] |
5.3. CV Extract Affects Cancer Angiogenesis
The importance of angiogenesis in cancer progression is well established [150]. The inhibition of this multi-stage process, defined as the new and abnormal blood vessels network development, involves endothelial cells proliferation and organization, migration as well as invasion [116,151]. Since contemporarily used anti-angiogenic drugs, based on the blockade of vascular endothelial growth factor (VEGF) signaling pathway, so far have not displayed a clinically significant benefit either as monotherapy or as a combined anticancer treatment, other methods of endothelial cell inhibition are still sought as valuable new approach to cancer therapy [152,153]. Apart from the improvement of these strategies, several other anti-angiogenic approaches are currently being investigated, such as the use of natural non-toxic phytochemicals as anti-angiogenic agents in cancer disease [154,155]. More than three decades ago, Kanoh et al. and subsequently Wada et al., using murine model of angiogenesis and a rat cornea assay as an in vivo model of fibroblast growth factor (bFGF)-induced angiogenesis, respectively, revealed that PSK from CV suppresses tumor-induced capillary vessel formation [135,156]. Further in vitro analysis showed that PSK inhibits the proliferation of endothelial cells in the presence or absence of basic bFGF as well as suppresses the bFGF-induced MAPK kinase phosphorylation [156]. The evidence of anti-angiogenic activities of PSP were also confirmed in in vivo sarcoma tumor-bearing mouse model [157], where PSP-treated tumor displayed a vasculature of few blood vessels of much less density than controls. This anti-angiogenic effect was mediated via suppression of VEGF gene expression [157]. A recent report revealed that CV compounds have the ability to decrease the release of pro-angiogenic cytokines, such as IL-6 and IL-8, in the chronic inflammatory environment, where this effect is accompanied by a decreased expression of TLR4 and phospho-IκB [91]. The CV effect on the tumor-associated macrophages (TAMs), which are the major source of angiogenic factors boosting the angiogenic switch [158] was proven in co-culture studies, where the CV-induced disruption in the crosstalk between breast cancer cells and macrophages has been reported [142]. By altering TAMs from M2 to M1 subtype, the protein-bound polysaccharides can indirectly reduce the amount of MMPs in the tumor microenvironment. The inhibitory effect on the production of angiogenesis-related factors (MCP-1 and VEGF) in macrophages was also observed [142] (Figure 3).
6. Significance of Fever for Cancer and Infectious Disease: Potential Utility of CV Extract
Fever is caused by the immune contact with PAMPs that is sensed by TLRs. The existence of a large number of TLRs enables the innate immune system to detect various PAMPs, including those of bacterial and viral origin. Stimulation of TLRs by PAMPs induces activation of signal transduction cascades. This cascade leads to translocation of NF-κB to the nucleus and activation of interferon regulatory factors 3/7 (IRF3/7) and/or activator AP-1, which cooperate to induce transcription of various cytokines including IFNs (IFNα/β) to counteract infections [159,160]. Thus, fever is not only an increase in body temperature, it is a mechanism that triggers production of many factors that are involved in immune response against many dangers, including viruses and cancer cells.
Fever is one of the main presenting symptoms of COVID-19 [161], but little public attention has been given to it as a defense mechanism. This issue was addressed in a review paper highlighting that using non-steroidal anti-inflammatory drugs (NSAIDs) to inhibit SARS-CoV-2 fever in the early stages of infection may contribute to worse outcome [162]. Since fever is a well-recognized immunostimulant, numerous reports on infections show improved survival in organisms that develop fever [161,163]. This effect is a result of fever-triggered activation of the anti-viral response [164]. Additionally, a decrease in replication of various viruses [161], including SARS-CoV-2 [165,166], has been observed in febrile temperatures. Thus, an effectively functioning immune system utilizes fever during infections. Interestingly, cancer patients reveal a history of fewer fevers during infectious diseases than people without cancer [167]. It seems unfavorable for them, since it is known that high fever is inversely related to cancer incidence [168,169]. To date, it is not known what makes it impossible to generate fever in cancer patients in response to pyrogens. Therefore, it seems necessary to find agents that can restore the ability to induce fever in cancer patients.
The analysis of CV-induced cytokines in animals showed an increase in the production of pro-inflammatory cytokines, such as IL-6, and TNF-α, typically involved in fever induction [170]. However, it has been found that CV extract alone (in a dose of 50–200 mg/kg) does not provoke fever, but induces a significant decrease in body temperature [171].
Different effects were observed in models of inflammation, when CV was administered together with endotoxin, such as LPS. Pre-injection of CV extract before LPS administration extended the duration of endotoxin fever in rats. This phenomenon was accompanied by a significant elevation of IL-6 level in plasma and pre-treatment of these rats with anti-IL-6 neutralizing antibody prevented this prolongation of endotoxin fever [170].
If an organism is exposed to endotoxin several times, a phenomenon called endotoxin tolerance develops. In this state, a decrease in the expression of pro-inflammatory cytokines is observed. In agreement, it was demonstrated that PBMCs isolated from LPS-tolerant rats produced significantly less IL-6 than the cells isolated from control animals in response to LPS stimulation in vitro. Importantly, the injection of CV extract partially prevented endotoxin tolerance development, and therefore, febrile increase in body temperature accompanied with an increased level of IL-6 was observed [172].
Pawlikowska et al. investigated whether fever-range temperatures affect CV action. It has been found that blood-derived lymphocytes cultured in fever range-hyperthermia (39.5 °C) display remarkable decrease in cell proliferation induced by protein-bound polysaccharides isolated from CV extract. This effect corresponded to the downregulation of mRNA expression of pro-inflammatory cytokines, such as IL-1β and IL-6. Furthermore, expression of these cytokines was also downregulated compared to cells cultured at 37 °C and stimulated with CV extract alone. Interestingly, in the cells which underwent combined treatment compared to ones stimulated with CV extract alone, the mRNA of TNF-α was slightly increased [68].
7. Clinical Trials
Despite PSK produced by Kureha Chemicals (Iwaki, Japan) and PSP being introduced on the market [173] in Japan in 1977 and China in 1987 [29], its clinical use in Europe and USA is still not approved by the European Medicines Agency (EMA) and FDA. Furthermore, in addition to in vitro studies and those using animal models on the role of CV administration during cancer and infectious diseases, only few clinical trials were performed or are ongoing.
Torkelson et al. observed that in breast cancer patients treated with radiotherapy, the administration of CV extract increases NK cytotoxic function and lymphocyte counts [12]. In the other clinical trials, the breast cancer patients who have taken PSP and Danshen (another herbal derivative from Salvia miltiorrhiza) respond to the treatment with the increase in T-helper lymphocytes (CD4+) and B lymphocytes number [174]. An elevation in leukocyte and neutrophil counts, as well as serum IgG and IgM levels, were also observed in non-small cell lung cancer patients treated with PSP [175]. In addition, Bao et al. noticed lower lymphopenia during radiotherapy of patients with nasopharyngeal carcinoma after the administration of combination of PSP-Danshen [176].
Clinical trials conducted in gastric, lung, or colorectal cancer patients have shown that simultaneous PSP/PSK treatment along with chemotherapy boosts their immune function, including NK cell activity [13,177,178]. Both molecules have been reported to possess a beneficial effect on extending the survival rate in cancer patients [13]. Moreover, trials involving patients with advanced hepatocellular carcinoma with malfunction of the liver confirmed the positive effect of daily consumption of CV capsules on their quality of life [179].
Polysaccharides from CV are also promising in the treatment of hepatitis B as well as HPV [29,180]. Serrano et al. demonstrated that Papilocare (Procare Health, Valencia, Spain), which is a CV-based vaginal gel has given a better clinical benefit than the conventional treatment in clinical practice for high-risk HPV patients in terms of its efficacy to treat HPV-related cervical lesions and to clear all HPV strains after a single 6-month period of use [181]. Another clinical trial, conducted by Scuto et al., showed that CV supplementation minimizes consequences associated with neurodegeneration, neuroinflammation, and oxidative stress of cochleovestibular system pathologies, including Meniere’s disease [182]. Anti-oxidative properties of CV were also noticed in clinical trials involving patients with breast cancer [95,96]. In addition, polysaccharopeptides from CV have been clinically tested as a prebiotic on the gut microbiota of healthy volunteers [42] and in patients with inflammatory bowel diseases (CV powder as an ingredient of Mycodigest supplement [183]).
Furthermore, in a clinical trial database approved by FDA [183], there are two ongoing clinical trials related to COVID-19. One of them concerns testing mushroom-based products as a drug for COVID-19. The influence of 14-days consumption of CV or Fomitopsis officinalis (Fo) capsules on recovery patients with COVID-19-positive test with mild-to-moderate symptoms, who do not require hospitalization will be assessed. Taking into account data on the immunomodulating and immunostimulating properties of mushroom extract, the scientists from the University of California, decided to use CV or Fo capsule as an adjunct to COVID-19 vaccination. The randomized, double-blind clinical trial to evaluate the effect of dietary supplementation of CV or Fo capsule on titration of antibody and on mild side-effects after vaccination is planned.
Apart from clinical trials conducted on cancer or infectious patients, the beneficial immunostimulating effect of CV extract was also confirmed in healthy volunteers in a randomized, double-blind clinical trial conducted by Wong and co-workers [184]. The elevation of PBMC gene expression of IL-2 receptor, increase in absolute counts of T helper cells and ratio of T helper/T suppressor and cytotoxic cells as well as enhancement of ex vivo production of IFN-γ by activated PBMCs have been observed. Importantly, CV consumption had no adverse effects on liver or renal functions, and therefore, it can be considered beneficial for the immunological defense of healthy subjects [184].
8. Conclusions
Since the current most common challenges in medicine, among which COVID-19 and cancer, are still fatal, there is an urgent need for finding new remedies. Medicinal mushrooms have been always an important source for the discovery of new therapeutics for human diseases. An ancient Chinese formulation of CV has been used in traditional Chinese herbal medicine for over 2000 years. In recent years, scientific research into its health-promoting properties has intensified.
This mushroom shows a wide spectrum of benefits, which may be useful in combating modern medical challenges. Herein, we have presented data determining the proof of strong anti-viral, anti-inflammatory, anti-oxidative, and immunostimulating properties of CV. Simultaneously, other reports confirmed the impressive anti-cancer response of CV extract and its compounds directed towards wide range of cancer types and revealed the molecular background of this process. By induction of different cell death modalities, such as apoptosis or necroptosis, CV extract appears to be an effective adjuvant therapy. Moreover, analysis of other reports revealed that CV also affects fever, the innate immunity mechanism beneficial for both cancer and viral infections recovery.
This review has some limitations resulting mainly from the procedures of CV preparation performed by a variety of research groups. The authors used different doses of either whole CV extract or its single compounds. Furthermore, they often used different extraction methods, which can result in inconsistent compositions of an extract, even if the same material was used. Moreover, many papers do not provide detailed information regarding the composition of the CV extract, which makes it impossible to clearly compare the results obtained by different authors. Furthermore, research showing a direct effect of CV extract in the treatment of SARS-CoV-2 are needed to clearly confirm its potential as an effective agent against COVID-19 disease.
Despite the discrepancies mentioned above, Coriolus versicolor belongs to standard oncologic treatment in the mainstream modern Japanese cancer system. The Western countries’ oncologists have only recently begun to turn their attention to immune potentiating therapies. Therefore, in order to proceed with clinical trials in the United States and Europe, the immunological and anti-cancer mechanisms must be well-established to justify proceeding with the prospective human clinical trials. In this review, a wide spectrum of research data showing the potential of CV in the treatment of COVID-19 and cancer diseases was presented. The dissemination of this knowledge is important to plan randomized clinical trials confirming all these beneficial effects in patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24054864/s1.
Author Contributions
Conceptualization, T.J., M.P. and S.W.; Writing-original draft, T.J., M.P., J.S. and S.W.; researched and compiled the data from the literature, T.J., M.P., J.S. and S.W.; writing—review and editing, T.J., M.P. and S.W.; visualization, T.J., M.P. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Bjorkholm, M. Etoposide and Teniposide in the Treatment of Acute Leukemia. Med. Oncol. Tumor Pharmacother. 1990, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Alken, S.; Kelly, C.M. Cancer Management and Research Dovepress Benefit Risk Assessment and Update on the Use of Docetaxel in the Management of Breast Cancer. Cancer Manag. Res. 2013, 5, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Z.; Miller, L.H. The Discovery of Artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal Mushrooms: Valuable Biological Resources of High Exploitation Potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Kirk, P. Species Fungorum for CoL+. In Catalogue of Life Checklist; Bánki, O., Roskov, Y., Döring, M., Ower, G., Vandepitte, L., Hobern, D., Remsen, D., Schalk, P., DeWalt, R., Keping, M., et al., Eds.; Kew Gardens: London, UK, 2020. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, S.; Li, M.; Ma, Y.; Zheng, Y.; Zhang, D.; Wu, L. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus Versicolor. Foods 2022, 11, 2126. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from Mushroom: Health Attributes and Food Industry Applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef]
- Standish, L.J.; Wenner, C.A.; Sweet, E.S.; Bridge, C.; Nelson, A.; Martzen, M.; Novack, J.; Torkelson, C. Trametes versicolor mushroom immune therapy in breast cancer. J. Soc. Integr. Oncol. 2008, 6, 122–128. [Google Scholar]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological Mechanism and Clinical Effect of Protein-Bound Polysaccharide K (KRESTIN®): Review of Development and Future Perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal Mushroom Modulators of Molecular Targets as Cancer Therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes Versicolor in Women with Breast Cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef] [PubMed]
- Eliza, W.L.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus Versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.C.; Wu, Y.J.; Hung, C.L.; Sheu, F. Trametes Versicolor Protein YZP Activates Regulatory B Lymphocytes-Gene Identification through De Novo Assembly and Function Analysis in a Murine Acute Colitis Model. PLoS ONE 2013, 8, e72422. [Google Scholar] [CrossRef]
- Kıvrak, İ.; Kıvrak, Ş.; Karababa, E. Assessment of Bioactive Compounds and Antioxidant Activity of Turkey Tail Medicinal Mushroom Trametes Versicolor,(Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, Y.; Zhang, Q.; Zhang, H.; Yang, B.; Wang, Z.; Zhu, W.; Li, B.; Wang, Q.; Kuang, H. Screening and Comparison of Antioxidant Activities of Polysaccharides from Coriolus Versicolor. Int. J. Biol. Macromol. 2014, 69, 12–19. [Google Scholar] [CrossRef]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus Versicolor Mushroom Polysaccharides Exert Immunoregulatory Effects on Mouse B Cells via Membrane Ig and TLR-4 to Activate the MAPK and NF-ΚB Signaling Pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus Versicolor: Physiological Activity, Uses, and Production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef]
- Fisher, M.; Yang, L. Anticancer Effects and Mechanisms of Polysaccharide-K (PSK): Implications of Cancer Immunotherapy. Anticancer Res. 2002, 22, 1737–1754. [Google Scholar]
- Kim, T.; Kim, Y.J.; Sohn, E.H. Effects of Beta-Glucan from Coriolus Versicolor on Scavenger Receptor B1 Expression and Their Involvement of Dectin-1 and Casein Kinase 2. Korean J. Plant Res. 2012, 25, 664–669. [Google Scholar] [CrossRef][Green Version]
- Shi, S.; Yin, L.; Shen, X.; Dai, Y.; Wang, J.; Yin, D.; Zhang, D.; Pan, X. β-Glucans from Trametes Versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection. Viruses 2022, 14, 237. [Google Scholar] [CrossRef]
- Miletić, D.; Turło, J.; Podsadni, P.; Sknepnek, A.; Szczepańska, A.; Lević, S.; Nedović, V.; Nikšić, M. Turkey Tail Medicinal Mushroom, Trametes Versicolor (Agaricomycetes), Crude Exopolysaccharides with Antioxidative Activity. Int. J. Med. Mushrooms 2020, 22, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Shibamoto, T.; Horiuchi, M.; Umano, K.; Kondo, K.; Otsuka, Y. Antioxidant/Anti-Inflammatory Activities and Chemical Composition of Extracts from the Mushroom Trametes Versicolor. Int. J. Food Sci. Nutr. 2013, 2, 85. [Google Scholar] [CrossRef]
- Wan, J.M.; Sit, W.H.; Louie, J.C. Polysaccharopeptide Enhances the Anticancer Activity of Doxorubicin and Etoposide on Human Breast Cancer Cells ZR-75-30. Int. J. Oncol. 2008, 32, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 7 February 2023).
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus Versicolor Polysaccharopeptide as an Immunotherapeutic in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 361–381. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Jiang, Y.; Liu, Y.; Luo, H.; Hao, C.; Zeng, P.; Zhang, L. Preclinical and Clinical Studies of Coriolus Versicolor Polysaccharopeptide as an Immunotherapeutic in China. Discov. Med. 2017, 23, 207–219. [Google Scholar]
- Hor, S.Y.; Ahmad, M.; Farsi, E.; Lim, C.P.; Asmawi, M.Z.; Yam, M.F. Acute and Subchronic Oral Toxicity of Coriolus Versicolor Standardized Water Extract in Sprague-Dawley Rats. J. Ethnopharmacol. 2011, 137, 1067–1076. [Google Scholar] [CrossRef]
- Ng, T.B.; Chan, W.Y. Polysaccharopeptide from the Mushroom Coriolus Versicolor Possesses Analgesic Activity but Does Not Produce Adverse Effects on Female Reproductive or Embryonic Development in Mice. Gen. Pharmacol. 1997, 29, 269–273. [Google Scholar] [CrossRef]
- Hsu, W.K.; Hsu, T.H.; Lin, F.Y.; Cheng, Y.K.; Yang, J.P.W. Separation, Purification, and α-Glucosidase Inhibition of Polysaccharides from Coriolus Versicolor LH1 Mycelia. Carbohydr. Polym. 2013, 92, 297–306. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Biomedical Effects of Mushrooms with Emphasis on Pure Compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Hong, M.; Tan, H.Y.; Pan, G.; Feng, Y. Hepatoprotective Effects of a Functional Formula of Three Chinese Medicinal Herbs: Experimental Evidence and Network Pharmacology-Based Identification of Mechanism of Action and Potential Bioactive Components. Molecules 2018, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Z.; Cui, R.; Chu, H. Polysaccharopeptide from Trametes Versicolor Blocks Inflammatory Osteoarthritis Pain-Morphine Tolerance Effects via Activating Cannabinoid Type 2 Receptor. Int. J. Biol. Macromol. 2019, 126, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Li, Y.; Zhao, Y.; Xiong, F.; Liu, Y.; Xue, H.; Yang, Z.; Ni, S.; Sahil, A.; et al. Coriolus Versicolor Alleviates Diabetic Cardiomyopathy by Inhibiting Cardiac Fibrosis and NLRP3 Inflammasome Activation. Phytother. Res. 2019, 33, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, X.; Zhang, L.; Yang, L. A Study on the Antioxidant Effect of Coriolus Versicolor Polysaccharide in Rat Brain Tissues. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Jiang, Y.; Ji, H.; Zhao, L.; Xiao, W.; Wang, Z.; Ding, G. The Synergistic Beneficial Effects of Ginkgo Flavonoid and Coriolus Versicolor Polysaccharide for Memory Improvements in a Mouse Model of Dementia. Evid. Based Complement. Alternat. Med. 2015, 2015, 128394. [Google Scholar] [CrossRef]
- Pang, Z.J.; Chen, Y.; Zhou, M. Polysaccharide Krestin Enhances Manganese Superoxide Dismutase Activity and MRNA Expression in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2000, 28, 331–341. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom Polysaccharides as Potential Prebiotics with Their Antitumor and Immunomodulating Properties: A Review. Bioact. Carbohydr. Diet. Fibre. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Pallav, K.; Dowd, S.E.; Villafuerte, J.; Yang, X.; Kabbani, T.; Hansen, J.; Dennis, M.; Leffler, D.A.; Kelly, C.P. Effects of Polysaccharopeptide from Trametes Versicolor and Amoxicillin on the Gut Microbiome of Healthy Volunteers: A Randomized Clinical Trial. Gut Microbes 2014, 5, 458–467. [Google Scholar] [CrossRef]
- Li, X.; Chen, P.; Zhang, P.; Chang, Y.; Cui, M.; Duan, J. Protein-Bound β-Glucan from Coriolus Versicolor Has Potential for Use Against Obesity. Mol. Nutr. Food Res. 2019, 63, 1801231. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Hago, A.M.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, Characterization, and Antitumor Activity of a Novel Glucan from the Fruiting Bodies of Coriolus Versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Kang, S.C.; Sohn, E.H. Phagocytic Effects of β-Glucans from the Mushroom Coriolus Versicolor Are Related to Dectin-1, NOS, TNF-α Signaling in Macrophages. Biomol. Ther. 2011, 19, 438–444. [Google Scholar] [CrossRef][Green Version]
- Kang, S.C.; Koo, H.J.; Park, S.; Lim, J.D.; Kim, Y.J.; Kim, T.; Namkoong, S.; Jang, K.H.; Pyo, S.; Jang, S.A.; et al. Effects of β-Glucans from Coriolus Versicolor on Macrophage Phagocytosis Are Related to the Akt and CK2/Ikaros. Int. J. Biol. Macromol. 2013, 57, 9–16. [Google Scholar] [CrossRef]
- Quayle, K.; Coy, C.; Standish, L.; Lu, H. The TLR2 Agonist in Polysaccharide-K Is a Structurally Distinct Lipid Which Acts Synergistically with the Protein-Bound β-Glucan. J. Nat. Med. 2015, 69, 198–208. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Huang, K.Y.; Jiang, Y.L.; Yang, G.L.; Wang, C.F.; Li, Y. β-Glucans from Coriolus Versicolor Protect Mice against S. Typhimurium Challenge by Activation of Macrophages. Int. J. Biol. Macromol. 2016, 86, 352–361. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Ikewaki, N.; Iwasaki, M.; Kurosawa, G.; Rao, K.S.; Lakey-Beitia, J.; Preethy, S.; Abraham, S.J.K. β-Glucans: Wide-Spectrum Immune-Balancing Food-Supplement-Based Enteric (β-WIFE) Vaccine Adjuvant Approach to COVID-19. Hum. Vaccin. Immunother. 2021, 17, 2808. [Google Scholar] [CrossRef]
- Wang, S.R.; Zhang, L.; Chen, H.P.; Li, Z.H.; Dong, Z.J.; Wei, K.; Liu, J.K. Four New Spiroaxane Sesquiterpenes and One New Rosenonolactone Derivative from Cultures of Basidiomycete Trametes Versicolor. Fitoterapia 2015, 105, 127–131. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes Versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Yang, M.M.; Chen, Z.; Kwok, J.S. The Anti-Tumor Effect of a Small Polypeptide from Coriolus Versicolor (SPCV). Am. J. Chin. Med. 1992, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Liu, S.; Newburg, D.S. Musarin, a Novel Protein with Tyrosine Kinase Inhibitory Activity from Trametes Versicolor, Inhibits Colorectal Cancer Stem Cell Growth. Biomed. Pharmacother. 2021, 144, 112339. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Lau, C.B.S.; Leung, P.C. Medicinal Plants and Mushrooms with Immunomodulatory and Anticancer Properties—A Review on Hong Kong’s Experience. Molecules 2021, 26, 2173. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.K.; Ng, T.B.; Sze, S.F.; Tsui, K.W. Activation of Peritoneal Macrophages by Polysaccharopeptide from the Mushroom, Coriolus Versicolor. Immunopharmacology 1993, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Wrotek, S.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A. Dual Effect of the Extract from the Fungus Coriolus Versicolor on Lipopolysaccharide-Induced Cytokine Production in RAW 264.7 Macrophages Depending on the Lipopolysaccharide Concentration. J. Inflamm. Res. 2022, 15, 3599. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Yeung, J.H.K. Polysaccharide Peptides from COV-1 Strain of Coriolus Versicolor Induce Hyperalgesia via Inflammatory Mediator Release in the Mouse. Life Sci. 2006, 78, 2463–2470. [Google Scholar] [CrossRef]
- Price, L.A.; Wenner, C.A.; Sloper, D.T.; Slaton, J.W.; Novack, J.P. Role for Toll-like Receptor 4 in TNF-Alpha Secretion by Murine Macrophages in Response to Polysaccharide Krestin, a Trametes Versicolor Mushroom Extract. Fitoterapia 2010, 81, 914–919. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A Study on Immunomodulatory Mechanism of Polysaccharopeptide Mediated by TLR4 Signaling Pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef]
- Coy, C.; Standish, L.J.; Bender, G.; Lu, H. Significant Correlation between TLR2 Agonist Activity and TNF-α Induction in J774.A1 Macrophage Cells by Different Medicinal Mushroom Products. Int. J. Med. Mushrooms 2015, 17, 713–722. [Google Scholar] [CrossRef]
- Lee, C.L.; Yang, X.; Wan, J.M.F. The Culture Duration Affects the Immunomodulatory and Anticancer Effect of Polysaccharopeptide Derived from Coriolus Versicolor. Enzyme Microb. Technol. 2006, 38, 14–21. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Kozak, W. Protein-Bound Polysaccharides from Coriolus Versicolor Attenuate LPS-Induced Synthesis of pro-Inflammatory Cytokines and Stimulate PBMCs Proliferation. Immunol. Lett. 2016, 178, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Jiang, P.; Sit, W.H.; Yang, X.; Wan, J.M.F. Regulatory Properties of Polysaccharopeptide Derived from Coriolus Versicolor and Its Combined Effect with Ciclosporin on the Homeostasis of Human Lymphocytes. J. Pharm. Pharmacol. 2010, 62, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska, M.; Piotrowski, J.; Jędrzejewski, T.; Kozak, W. Polysaccharide Peptides from Coriolus Versicolor Exert Differential Immunomodulatory Effects on Blood Lymphocytes and Breast Cancer Cell Line MCF-7 in Vitro. Immunol. Lett. 2016, 174, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Lau, C.B.S.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.L.; Chow, M.S.S. Differential Effect of Coriolus Versicolor (Yunzhi) Extract on Cytokine Production by Murine Lymphocytes in Vitro. Int. Immunopharmacol. 2004, 4, 1549–1557. [Google Scholar] [CrossRef]
- Sekhon, B.K.; Sze, D.M.Y.; Chan, W.K.; Fan, K.; Li, G.Q.; Moore, D.E.; Roubin, R.H. PSP Activates Monocytes in Resting Human Peripheral Blood Mononuclear Cells: Immunomodulatory Implications for Cancer Treatment. Food Chem. 2013, 138, 2201–2209. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Jędrzejewski, T.; Piotrowski, J.; Kozak, W. Fever-Range Hyperthermia Inhibits Cells Immune Response to Protein-Bound Polysaccharides Derived from Coriolus Versicolor Extract. Mol. Immunol. 2016, 80, 50–57. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Lai, S.; Xu, C.; Lu, F.; Xiao, X.; Bao, Y. Immunomodulatory Effects of Polysaccharopeptide (PSP) in Human PBMC through Regulation of TRAF6/TLR Immunosignal-Transduction Pathways. Immunopharmacol. Immunotoxicol. 2010, 32, 576–584. [Google Scholar] [CrossRef]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The Mycelium of the Trametes Versicolor (Turkey Tail) Mushroom and Its Fermented Substrate Each Show Potent and Complementary Immune Activating Properties in Vitro. BMC Complement. Altern. Med. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Tan, Y.; Yu, S.; Bao, Y.X. A Study on the Immunomodulation of Polysaccharopeptide through the TLR4-TIRAP/MAL-MyD88 Signaling Pathway in PBMCs from Breast Cancer Patients. Immunopharmacol. Immunotoxicol. 2013, 35, 497–504. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 Agonist PSK Activates Human NK Cells and Enhances the Antitumor Effect of HER2-Targeted Monoclonal Antibody Therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef]
- Yang, Y.; Inatsuka, C.; Gad, E.; Disis, M.L.; Standish, L.J.; Pugh, N.; Pasco, D.S.; Lu, H. Protein-Bound Polysaccharide-K Induces IL-1β via TLR2 and NLRP3 Inflammasome Activation. Innate Immun. 2014, 20, 857–866. [Google Scholar] [CrossRef]
- Maruyama, S.; Akasaka, T.; Yamada, K.; Tachibana, H. Protein-Bound Polysaccharide-K (PSK) Directly Enhanced IgM Production in the Human B Cell Line BALL-1. Biomed. Pharmacother. 2009, 63, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral Activity of Basidiomycete Mycelia against Influenza Type A (Serotype H1N1) and Herpes Simplex Virus Type 2 in Cell Culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Arbiser, J.L.; Holmgren, A.; Klein, G.; Klein, E. PSK and Trx80 Inhibit B-Cell Growth in EBV-Infected Cord Blood Mononuclear Cells through T Cells Activated by the Monocyte Products IL-15 and IL-12. Blood 2005, 105, 1606–1613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collins, R.A.; Ng, T.B. Polysaccharopeptide from Coriolus Versicolor Has Potential for Use against Human Immunodeficiency Virus Type 1 Infection. Life Sci. 1997, 60, PL383–PL387. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valentín, M.; López, S.; Rivera, M.; Ríos-Olivares, E.; Cubano, L.; Boukli, N.M. Naturally Derived Anti-HIV Polysaccharide Peptide (PSP) Triggers a Toll-Like Receptor 4-Dependent Antiviral Immune Response. J. Immunol. Res. 2018, 2018, 8741698. [Google Scholar] [CrossRef]
- Criscuolo, A.A.; Sesti, F.; Piccione, E.; Mancino, P.; Belloni, E.; Gullo, C.; Ciotti, M. Therapeutic Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Women with Cervical Uterine High-Risk HPV Infection: A Retrospective Observational Study. Adv. Ther. 2021, 38, 1202–1211. [Google Scholar] [CrossRef]
- Palacios, S.; Losa, F.; Dexeus, D.; Cortés, J. Beneficial Effects of a Coriolus Versicolor-Based Vaginal Gel on Cervical Epithelization, Vaginal Microbiota and Vaginal Health: A Pilot Study in Asymptomatic Women. BMC Womens Health. 2017, 17, 21. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-Based Design of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M pro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Bernardshaw, S.V.; Grinde, B. Can Medicinal Mushrooms Have Prophylactic or Therapeutic Effect against COVID-19 and Its Pneumonic Superinfection and Complicating Inflammation? Scand. J. Immunol. 2021, 93, e12937. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. Trials Test Mushrooms and Herbs as Anti–COVID-19 Agents. JAMA 2021, 326, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-Derived Bioactive Compounds Potentially Serve as the Inhibitors of SARS-CoV-2 Main Protease: An in Silico Approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef]
- Rao, K.S.; Suryaprakash, V.; Senthilkumar, R.; Preethy, S.; Katoh, S.; Ikewaki, N.; Abraham, S.J.K. Role of Immune Dysregulation in Increased Mortality Among a Specific Subset of COVID-19 Patients and Immune-Enhancement Strategies for Combatting Through Nutritional Supplements. Front. Immunol. 2020, 11, 1548. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The Use of Anti-Inflammatory Drugs in the Treatment of People with Severe Coronavirus Disease 2019 (COVID-19): The Experience of Clinical Immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Asai, Y.; Takaori, K.; Yamamoto, T.; Ogawa, T. Protein-Bound Polysaccharide Isolated from Basidiomycetes Inhibits Endotoxin-Induced Activation by Blocking Lipopolysaccharide-Binding Protein and CD14 Functions. FEMS Immunol. Med. Microbiol. 2005, 43, 91–98. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus Versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Fusco, R.; Genovese, T.; Cordaro, M.; D’Amico, R.; Trovato Salinaro, A.; Ontario, M.L.; Modafferi, S.; Cuzzocrea, S.; Di Paola, R.; et al. Coriolus Versicolor Downregulates TLR4/NF-κB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants 2022, 11, 406. [Google Scholar] [CrossRef]
- D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef]
- Jun, L.; Mei, Z.; Yuan, C. Reversal of Inhibition of Reactive Oxygen Species on Respiratory Burst of Macrophages by Polysaccharide from Coriolus Versicolor. Int. J. Immunopharmacol. 1993, 15, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Oxidative Stress Relief for Cancer-Bearing Hosts by the Protein-Bound Polysaccharide of Coriolus Versicolor QUEL with SOD Mimicking Activity. Cancer Biother. 1994, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kariya, K.; Nakamura, K.; Nomoto, K.; Matama, S.; Saigenji, K. Mimicking of Superoxide Dismutase Activity by Protein-Bound Polysaccharide of Coriolus Versicolor QUEL, and Oxidative Stress Relief for Cancer Patients. Mol. Biother. 1992, 4, 40–46. [Google Scholar] [PubMed]
- Engel, A.L.; Sun, G.C.; Gad, E.; Rastetter, L.R.; Strobe, K.; Yang, Y.; Dang, Y.; Disis, M.L.; Lu, H. Protein-Bound Polysaccharide Activates Dendritic Cells and Enhances OVA-Specific T Cell Response as Vaccine Adjuvant. Immunobiology 2013, 218, 1468–1476. [Google Scholar] [CrossRef]
- Luo, K.W.; Yue, G.G.L.; Ko, C.H.; Lee, J.K.M.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. In Vivo and In Vitro Anti-Tumor and Anti-Metastasis Effects of Coriolus Versicolor Aqueous Extract on Mouse Mammary 4T1 Carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef]
- Pramudya, M.; Wahyuningsih, S.P.A. Immunomodulatory Potential of Polysaccharides from Coriolus Versicolor against Intracellular Bacteria Neisseria Gonorrhoeae. Vet. World 2019, 12, 735. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Kinning, K.T.; Sullivan, K.D.; Araya, P.; Smith, K.P.; Granrath, R.E.; Shaw, J.R.; Baxter, R.; Jordan, K.R.; Russell, S.; et al. Specialized Interferon Action in COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2116730119. [Google Scholar] [CrossRef]
- Tjan, L.H.; Furukawa, K.; Nagano, T.; Kiriu, T.; Nishimura, M.; Arii, J.; Hino, Y.; Iwata, S.; Nishimura, Y.; Mori, Y. Early Differences in Cytokine Production by Severity of Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 1145–1149. [Google Scholar] [CrossRef]
- Yazan, A. Interleukin-2 Level for Normal People and COVID-19 Infection: Is It Our Concern Is COVID-19 Infection or Interleukin-2 Level Before the Infection? Eurasian J. Med. Oncol. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Kanazawa, M.; Mori, Y.; Yoshihara, K.; Iwadate, M.; Suzuki, S.; Endoh, Y.; Ohki, S.; Takita, K.I.; Sekikawa, K.; Takenoshita, S.I. Effect of PSK on the Maturation of Dendritic Cells Derived from Human Peripheral Blood Monocytes. Immunol. Lett. 2004, 91, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Meng, Y.; Wang, Z.; Shan, F.; Wang, Q.; Zhang, N. Maturation of Morphology, Phenotype and Functions of Murine Bone Marrow-Derived Dendritic Cells(DCs) Induced by Polysaccharide Kureha(PSK). Hum. Vaccin. Immunother. 2012, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Yang, J.; Deng, H.; Chen, D.; Yang, X.P.; Tang, Z.H. Depletion and Dysfunction of Dendritic Cells: Understanding SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 843342. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S.; Capitelli, L.; Bocchino, M. A Bird’s Eye View on the Role of Dendritic Cells in SARS-CoV-2 Infection: Perspectives for Immune-Based Vaccines. Allergy 2022, 77, 100–110. [Google Scholar] [CrossRef]
- Jonny, J.; Putranto, T.A.; Sitepu, E.C.; Irfon, R. Dendritic Cell Vaccine as a Potential Strategy to End the COVID-19 Pandemic. Why Should It Be Ex Vivo? Expert Rev. Vaccines 2022, 21, 1111–1120. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Hasan Abir, M.; Faijanur, M.; Siddiquee, R.; Ahmed, H.; Rahman, N.; Nainu, F.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B.; Batist, G.; Wu, J.H.; Witcher, M. Mechanisms and Insights into Drug Resistance in Cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Hernández, A.d.R. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Sit, W.H.; Wan, J.M.F. Induction of S Phase Cell Arrest and Caspase Activation by Polysaccharide Peptide Isolated from Coriolus Versicolor Enhanced the Cell Cycle Dependent Activity and Apoptotic Cell Death of Doxorubicin and Etoposide, but Not Cytarabine in HL-60 Cells. Oncol. Rep. 2005, 14, 145–155. [Google Scholar] [CrossRef]
- Wan, J.M.; Sit, W.H.; Yang, X.; Jiang, P.; Wong, L.L. Polysaccharopeptides Derived from Coriolus Versicolor Potentiate the S-Phase Specific Cytotoxicity of Camptothecin (CPT) on Human Leukemia HL-60 Cells. Chin. Med. 2010, 5, 16. [Google Scholar] [CrossRef]
- Wenner, C.A.; Martzen, M.R.; Lu, H.; Verneris, M.R.; Wang, H.; Slaton, J.W. Polysaccharide-K Augments Docetaxel-Induced Tumor Suppression and Antitumor Immune Response in an Immunocompetent Murine Model of Human Prostate Cancer. Int. J. Oncol. 2012, 40, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Yue, G.G.L.; Gao, S.; Luo, K.W.; Siu, W.S.; Shum, W.T.; Shiu, H.T.; Lee, J.K.M.; Li, G.; Leung, P.C.; et al. Evaluation of the Combined Use of Metronomic Zoledronic Acid and Coriolus Versicolor in Intratibial Breast Cancer Mouse Model. J. Ethnopharmacol. 2017, 204, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sit, W.H.; Chan, D.K.O.; Wan, J.M.F. The Cell Death Process of the Anticancer Agent Polysaccharide-Peptide (PSP) in Human Promyelocytic Leukemic HL-60 Cells. Oncol. Rep. 2005, 13, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, W.; Wang, Y.; Bikow, J.; Lu, B.; Keating, A.; Verma, S.; Parker, T.G.; Han, R.; Wen, X.Y. Rosuvastatin, Identified From a Zebrafish Chemical Genetic Screen for Antiangiogenic Compounds, Suppresses the Growth of Prostate Cancer. Eur. Urol. 2010, 58, 418–426. [Google Scholar] [CrossRef]
- Kinoshita, J.; Fushida, S.; Harada, S.; Makino, I.; Nakamura, K.; Oyama, K.; Fujita, H.; Ninomiya, I.; Fujimura, T.; Kayahara, M.; et al. PSK Enhances the Efficacy of Docetaxel in Human Gastric Cancer Cells through Inhibition of Nuclear Factor-ΚB Activation and Survivin Expression. Int. J. Oncol. 2010, 36, 593–600. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shoda, M.; Iijma, H.; Nagai, S.; Wada, J.; Suzuki, H.; Chikazawa, N.; Tasaka, T.; Kameda, C.; Tanaka, H.; et al. A Protein-Bound Polysaccharide, PSK, Enhances Tumor Suppression Induced by Docetaxel in a Gastric Cancer Xenograft Model. Anticancer Res. 2009, 29, 843–850. [Google Scholar]
- Cai, X.J.; Wang, Z.; Cao, J.W.; Ni, J.J.; Xu, Y.Y.; Yao, J.; Xu, H.; Liu, F.; Yang, G.-Y. Anti-Angiogenic and Anti-Tumor Effects of Metronomic Use of Novel Liposomal Zoledronic Acid Depletes Tumor-Associated Macrophages in Triple Negative Breast Cancer. Oncotarget 2017, 8, 84248–84257. [Google Scholar] [CrossRef]
- Harhaji, L.; Mijatović, S.; Maksimović-Ivanić, D.; Stojanović, I.; Momčilović, M.; Maksimović, V.; Tufegdžić, S.; Marjanović, Ž.; Mostarica-Stojković, M.; Vučinić, Ž.; et al. Anti-Tumor Effect of Coriolus Versicolor Methanol Extract against Mouse B16 Melanoma Cells: In Vitro and in Vivo Study. Food Chem. Toxicol. 2008, 46, 1825–1833. [Google Scholar] [CrossRef]
- Hirahara, N.; Fujioka, M.; Edamatsu, T.; Fujieda, A.; Serine, F.; Wada, T.; Tanaka, T. Protein-Bound Polysaccharide-K (PSK) Induces Apoptosis and Inhibits Proliferation of Promyelomonocytic Leukemia HL-60 Cells. Anticancer Res. 2011, 31, 2733–2738. [Google Scholar]
- Ho, C.Y.; Kim, C.-F.; Leung, K.N.; Kwok-Pui, F.; Tse, T.-F.; Chan, H.; Bik, C.; Lau, S.; Ho, Y.; Fung, K.-P.; et al. Differential Anti-Tumor Activity of Coriolus Versicolor (Yunzhi) Extract through P53-and/or Bcl-2-Dependent Apoptotic Pathway in Human Breast Cancer Cells Cheong. Cancer Biol. Ther. 2005, 4, 638–644. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, P.; Park, S.; Wu, J.M. Induction of Cell Cycle Changes and Modulation of Apoptogenic/Anti-Apoptotic and Extracellular Signaling Regulatory Protein Expression by Water Extracts of I’m-YunityTM (PSP). BMC Complement. Altern. Med. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Berruguilla, E.; Romero, I.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A. The Immunomodulator PSK Induces in Vitro Cytotoxic Activity in Tumour Cell Lines via Arrest of Cell Cycle and Induction of Apoptosis. BMC Cancer 2008, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.B.S.; Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.L.; Chow, M.S.S. Cytotoxic Activities of Coriolus Versicolor (Yunzhi) Extract on Human Leukemia and Lymphoma Cells by Induction of Apoptosis. Life Sci. 2004, 75, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, M.; Jędrzejewski, T.; Slominski, A.T.; Brożyna, A.A.; Wrotek, S. Pigmentation Levels Affect Melanoma Responses to Coriolus Versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk. Int. J. Mol. Sci. 2021, 22, 5735. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Jędrzejewski, T.; Brożyna, A.A.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Induce RIPK1/RIPK3/MLKL-Mediated Necroptosis in ER-Positive Breast Cancer and Amelanotic Melanoma Cells. Cell. Physiol. Biochem. 2020, 54, 591–604. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Piotrowski, J.; Jędrzejewski, T.; Kozak, W.; Slominski, A.T.; Brożyna, A.A. Coriolus Versicolor-Derived Protein-Bound Polysaccharides Trigger the Caspase-Independent Cell Death Pathway in Amelanotic but Not Melanotic Melanoma Cells. Phytother. Res. 2020, 34, 173–183. [Google Scholar] [CrossRef]
- Roca-Lema, D.; Martinez-Iglesias, O.; Fernández De Ana Portela, C.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-Proliferative and Anti-Invasive Effect of Polysaccharide-Rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef]
- Uwafuji, S.; Goi, T.; Naruse, T.; Kurebayashi, H.; Nakazawa, T.; Hirono, Y.; Yamaguchi, A. Protein-Bound Polysaccharide K Reduced the Invasive Ability of Colon Cancer Cell Lines. Anticancer Res. 2013, 33, 4841–4846. [Google Scholar]
- Wang, D.F.; Lou, N.; Li, X.D. Effect of Coriolus Versicolor Polysaccharide-B on the Biological Characteristics of Human Esophageal Carcinoma Cell Line Eca109. Cancer Biol. Med. 2012, 9, 164–167. [Google Scholar] [CrossRef]
- Yang, C.L.H.; Chik, S.C.C.; Lau, A.S.Y.; Chan, G.C.F. Coriolus Versicolor and Its Bioactive Molecule Are Potential Immunomodulators against Cancer Cell Metastasis via Inactivation of MAPK Pathway. J. Ethnopharmacol. 2023, 301, 115790. [Google Scholar] [CrossRef]
- Zeng, F.; Hon, C.C.; Sit, W.H.; Chow, K.Y.C.; Hui, R.K.H.; Law, I.K.M.; Ng, V.W.L.; Yang, X.T.; Leung, F.C.C.; Wan, J.M.F. Molecular Characterization of Coriolus Versicolor PSP-Induced Apoptosis in Human Promyelotic Leukemic HL-60 Cells Using CDNA Microarray. Int. J. Oncol. 2005, 27, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, N.; Edamatsu, T.; Fujieda, A.; Fujioka, M.; Wada, T.; Tajima, Y. Protein-Bound Polysaccharide-K Induces Apoptosis via Mitochondria and P38 Mitogen-Activated Protein Kinase-Dependent Pathways in HL-60 Promyelomonocytic Leukemia Cells. Oncol. Rep. 2013, 30, 99–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanoh, T.; Matsunaga, K.; Saito, K.; Fujii, T. Suppression of in Vivo Tumor-Induced Angiogenesis by the Protein-Bound Polysaccharide PSK. In Vivo 1994, 8, 247–250. [Google Scholar] [PubMed]
- Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.; Lau, C.B.S. Coriolus Versicolor (Yunzhi) Extract Attenuates Growth of Human Leukemia Xenografts and Induces Apoptosis through the Mitochondrial Pathway. Oncol. Rep. 2006, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Kunicki, J.; Darzynkiewicz, Z.; Wu, J.M. Effects of Extracts of Coriolus Versicolor (I’m-YunityTM) on Cell-Cycle Progression and Expression of Interleukins-1β,-6, and -8 in Promyelocytic HL-60 Leukemic Cells and Mitogenically Stimulated and Nonstimulated Human Lymphocytes. J. Altern. Complement. Med. 2004, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical Antileukemia Activity of Tramesan: A Newly Identified Bioactive Fungal Metabolite. Oxid. Med. Cell Longev. 2017, 2017, 5061639. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.H.; Rashedi, I.; Kschrameating, A. Immunomodulatory Properties of Coriolus Versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.M.P.; Kwan, C.Y. In Vitro Inhibition of Proliferation of HL-60 Cells by Tetrandrine and Coriolus Versicolor Peptide Derived from Chinese Medicinal Herbs. Life Sci. 1997, 60, PL135–PL140. [Google Scholar] [CrossRef]
- Soengas, M.S.; Lowe, S.W. Apoptosis and Melanoma Chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Fungus Disrupt the Crosstalk between Breast Cancer Cells and Macrophages through Inhibition of Angiogenic Cytokines Production and Shifting Tumour-Associated Macrophages from the M2 to M1 Subtype. Cell Physiol. Biochem. 2020, 54, 615–628. [Google Scholar] [CrossRef]
- Zhang, H.; Morisaki, T.; Matsunaga, H.; Sato, N.; Uchiyama, A.; Hashizume, K.-T.; Nagumo, F.; Tadano, J.; Katano, M. Protein-Bound Polysaccharide PSK Inhibits Tumor Invasiveness by down-Regulation of TGF-Β1 and MMPs. Clin. Exp. Metastasis 2000, 18, 343–352. [Google Scholar] [PubMed]
- Matsunaga, K.; Ohhara, M.; Oguchi, Y.; Iijima, H.; Kobayashi, H. Antimetastatic Effect of PSK, a Protein-Bound Polysaccharide, against the B16-BL6 Mouse Melanoma. Invasion Metastasis 1996, 16, 27–38. [Google Scholar] [PubMed]
- Zhou, X.; Jiang, H.; Lin, J.; Tang, K. Cytotoxic activities of Coriolus versicolor (Yunzhi) extracts on human liver cancer and breast cancer cell line. Afr. J. Biotechnol. 2007, 6, 1740–1743. [Google Scholar] [CrossRef]
- Cai, X.; Pi, Y.; Zhou, X.; Tian, L.; Qiao, S.; Lin, Y. Hepatoma Cell Growth Inhibition by Inducing Apoptosis with Polysaccharide Isolated from Turkey Tail Medicinal Mushroom, Trametes versicolor (L.: Fr.) Lloyd (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2010, 12, 257–263. [Google Scholar] [CrossRef]
- Aoyagi, H.; Iino, Y.; Takeo, T.; Horii, Y.; Morishita, Y.; Horiuchi, R. Effects of OK-432 (picibanil) on the estrogen receptors of MCF-7 cells and potentiation of antiproliferative effects of tamoxifen in combination with OK-432. Oncology. 1997, 54, 414–423. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Enhancement of anti-cancer activity of cisdiaminedichloroplatinum by the protein-bound polysaccharide of Coriolus versicolor QUEL (PS-K) in vitro. Cancer Biother. 1994, 9, 351–358. [Google Scholar] [CrossRef]
- Knežević, A.; Stajić, M.; Sofrenić, I.; Stanojković, T.; Milovanović, I.; Tešević, V.; Vukojević, J. Antioxidative, antifungal, cytotoxic and antineurodegenerative activity of selected Trametes species from Serbia. PLoS ONE 2018, 13, e0203064. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 18, 1182–1186. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Taylor, A.P.; Rodriguez, M.; Adams, K.; Goldenberg, D.M.; Blumenthal, R.D. Altered Tumor Vessel Maturation and Proliferation in Placenta Growth Factor-Producing Tumors: Potential Relationship to Post-Therapy Tumor Angiogenesis and Recurrence. Int. J. Cancer 2003, 105, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M.Á. Antiangiogenic Phytochemicals Constituent of Diet as Promising Candidates for Chemoprevention of Cancer. Antioxidants 2022, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Vafopoulou, P.; Kourti, M. Anti-Angiogenic Drugs in Cancer Therapeutics: A Review of the Latest Preclinical and Clinical Studies of Anti-Angiogenic Agents with Anticancer Potential. J. Cancer Metastasis Treat. 2022, 8, 1–26. [Google Scholar] [CrossRef]
- Wada, T.; Yoko, W.; Kenji, B.; Mariko, K.; Yoshiharu, O.; Kenichi, M.; Takao, A.; Kikuo, N. Suppression Mechanism of Angiogenesis by PSK. Ann. Cancer Res. Ther. 2002, 10, 93–106. [Google Scholar] [CrossRef]
- Ho, J.C.K.; Konerding, M.A.; Gaumann, A.; Groth, M.; Liu, W.K. Fungal Polysaccharopeptide Inhibits Tumor Angiogenesis and Tumor Growth in Mice. Life Sci. 2004, 75, 1343–1356. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of Tumor Associated Macrophages in Tumor Angiogenesis and Lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- Mabrey, F.L.; Morrell, E.D.; Wurfel, M.M. TLRs in COVID-19: How They Drive Immunopathology and the Rationale for Modulation. Innate Immun. 2021, 27, 503–513. [Google Scholar] [CrossRef]
- Iwanaszko, M.; Kimmel, M. NF-ΚB and IRF Pathways: Cross-Regulation on Target Genes Promoter Level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical Characteristics of 3062 COVID-19 Patients: A Meta-Analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef]
- Wrotek, S.; Legrand, E.K.; Dzialuk, A.; Alcock, J. Let Fever Do Its Job: The Meaning of Fever in the Pandemic Era. Evol. Med. Public Health 2021, 9, 26–35. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Lusamba Kalonji, N.; Deng, X.; Momma, H.; Shimotai, Y.; Nagatomi, R. Effects of High Temperature on Pandemic and Seasonal Human Influenza Viral Replication and Infection-Induced Damage in Primary Human Tracheal Epithelial Cell Cultures. Heliyon 2019, 5, e01149. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Herder, V.; Dee, K.; Wojtus, J.K.; Goldfarb, D.; Rozario, C.; Gu, Q.; Jarrett, R.F.; Epifano, I.; Stevenson, A.; McFarlane, S.; et al. Elevated Temperature Inhibits SARS-CoV-2 Replication in Respiratory Epithelium Independently of the Induction of IFN-Mediated Innate Immune Defences. PLoS Biol. 2020, 19, e3001065. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Z.; Ou, J.; Zhang, H.; Zhang, Q.; Dong, M.; Zhang, G. Temperature Dependence of the SARS-CoV-2 Affinity to Human ACE2 Determines COVID-19 Progression and Clinical Outcome. Comput. Struct. Biotechnol. J. 2021, 19, 161–167. [Google Scholar] [CrossRef]
- Wrotek, S.; Kamecki, K.; Kwiatkowski, S.; Kozak, W. Cancer Patients Report a History of Fewer Fevers during Infections than Healthy Controls. J. Pre-Clin. Clin. Res. 2009, 3, 31–35. [Google Scholar]
- Wrotek, S.; Brycht, Ł.; Wrotek, W.; Kozak, W. Fever as a Factor Contributing to Long-Term Survival in a Patient with Metastatic Melanoma: A Case Report. Complement. Ther. Med. 2018, 38, 7–10. [Google Scholar] [CrossRef]
- Radha, G.; Lopus, M. The Spontaneous Remission of Cancer: Current Insights and Therapeutic Significance. Transl. Oncol. 2021, 14, 101166. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Kowalczewska, M.; Wrotek, S.; Kozak, W. Polysaccharide Peptide from Coriolus Versicolor Induces Interleukin 6-Related Extension of Endotoxin Fever in Rats. Int. J. Hyperthermia. 2015, 31, 626–634. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Wrotek, S.; Kozak, W. Polysaccharide Peptide Induces a Tumor Necrosis Factor-α-Dependent Drop of Body Temperature in Rats. J. Therm. Biol. 2014, 44, 1–4. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Piotrowski, J.; Pawlikowska, M.; Wrotek, S.; Kozak, W. Extract from Coriolus Versicolor Fungus Partially Prevents Endotoxin Tolerance Development by Maintaining Febrile Response and Increasing IL-6 Generation. J. Therm. Biol. 2019, 83, 69–79. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Pereira, S.R.; Xavier, A.M.R.B. Biotechnological Applications of Trametes Versicolor and Their Enzymes. Curr. Biotechnol. 2016, 6, 78–88. [Google Scholar] [CrossRef]
- Wong, C.K.; Bao, Y.X.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Lam, C.W.K. Immunomodulatory Activities of Yunzhi and Danshen in Post-Treatment Breast Cancer Patients. Am. J. Chinese Med. 2005, 33, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.W.; Lam, C.L.; Yan, C.; Mak, J.C.; Ooi, G.C.; Ho, J.C.; Lam, B.; Man, R.; Sham, J.S.; Lam, W.K. Coriolus Versicolor Polysaccharide Peptide Slows Progression of Advanced Non-Small Cell Lung Cancer. Respir. Med. 2003, 97, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.X.; Wong, C.K.; Leung, S.F.; Chan, A.T.C.; Li, P.W.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Yin, Y.B.; Lam, C.W.K. Clinical Studies of Immunomodulatory Activities of Yunzhi-Danshen in Patients with Nasopharyngeal Carcinoma. J. Altern. Complement. Med. 2006, 12, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B. A Review of Research on the Protein-Bound Polysaccharide (Polysaccharopeptide, PSP) from the Mushroom Coriolus Versicolor (Basidiomycetes: Polyporaceae). Gen. Pharmacol. 1998, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, S.; Ogawa, T.; Makita, F.; Tanahashi, Y.; Ohya, T.; Tomizawa, N.; Satoh, Y.; Kobayashi, I.; Izumi, M.; Takeyoshi, I.; et al. Beneficial Effects of Protein-Bound Polysaccharide K plus Tegafur/Uracil in Patients with Stage II or III Colorectal Cancer: Analysis of Immunological Parameters. Oncol. Rep. 2006, 15, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.W.; Choo, S.P. Coriolus Versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Donatini, B. Control of Oral Human Papillomavirus (HPV) by Medicinal Mushrooms, Trametes Versicolor and Ganoderma Lucidum: A Preliminary Clinical Trial. Int. J. Med. Mushrooms 2014, 16, 497–498. [Google Scholar] [CrossRef]
- Serrano, L.; López, A.C.; González, S.P.; Palacios, S.; Dexeus, D.; Centeno-Mediavilla, C.; Coronado, P.; de La Fuente, J.; López, J.A.; Vanrell, C.; et al. Efficacy of a Coriolus Versicolor–Based Vaginal Gel in Women With Human Papillomavirus–Dependent Cervical Lesions: The PALOMA Study. J. Low. Genit. Tract. Dis. 2021, 25, 130–136. [Google Scholar] [CrossRef]
- Scuto, M.; di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Salinaro, A.T.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus Versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2020, 21, 284. [Google Scholar] [CrossRef]
- Home-ClinicalTrials. Available online: https://www.clinicaltrials.gov/ (accessed on 12 December 2022).
- Wong, C.K.; Tse, P.S.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Lam, C.W.K. Immunomodulatory Effects of Yun Zhi and Danshen Capsules in Health Subjects-a Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Int. Immunopharmacol. 2004, 4, 201–211. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).