Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases
Abstract
1. Introduction
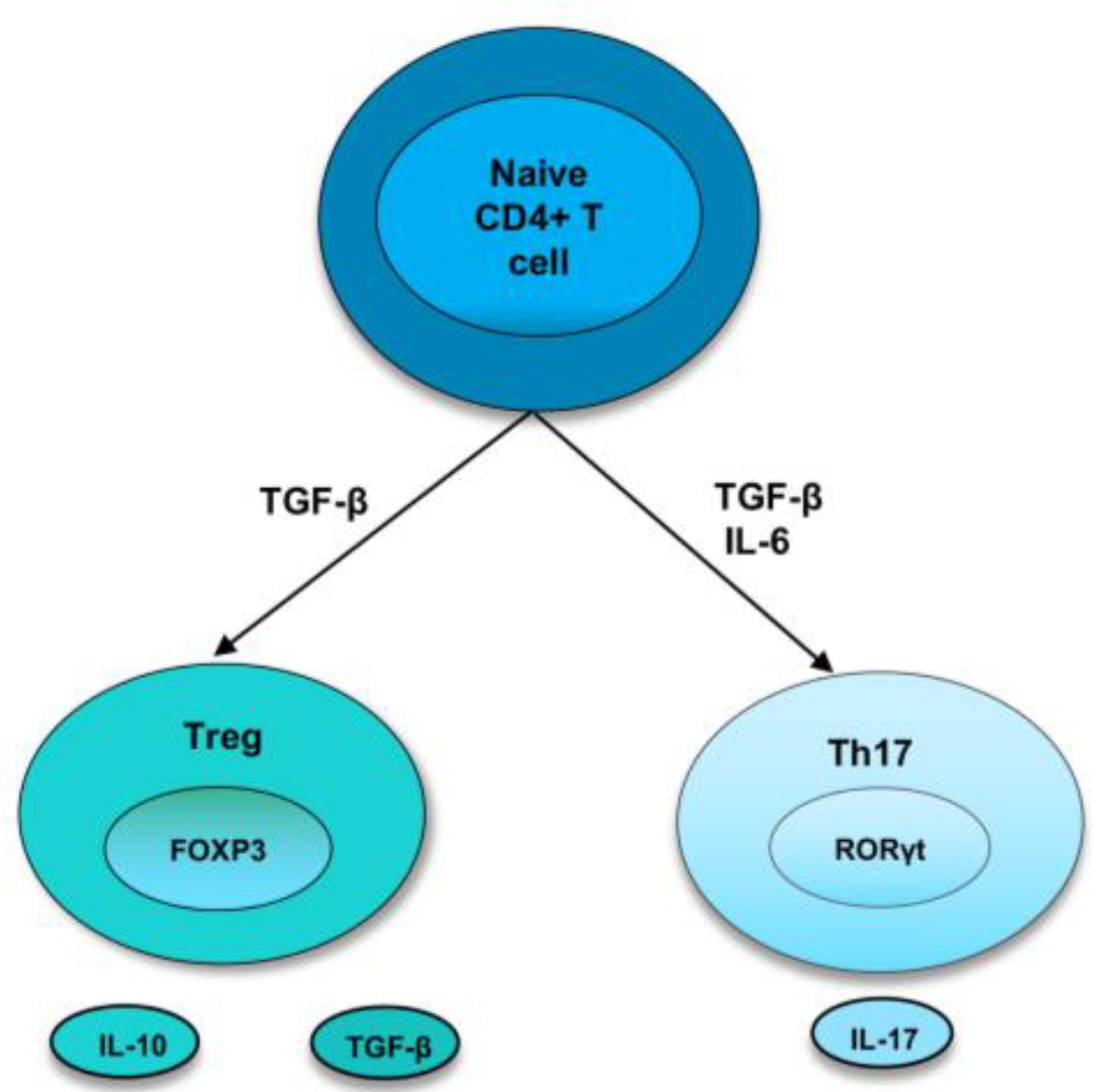
1.1. Th17 Cells
1.2. Treg Cells
2. Th17/Treg Cells in Lung Inflammatory Diseases
2.1. Chronic Obstructive Pulmonary Disease (COPD)
2.2. Acute Respiratory Distress Syndrome (ARDS)
2.3. Sarcoidosis
2.4. Asthma
2.5. Pulmonary Infectious Diseases
3. Therapeutic Strategies Targeting Th17/Treg Cells in Lung Inflammatory Diseases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shoenfeld, Y.; Isenberg, D. The mosaic of autoimmunity. Immunol. Today 1989, 10, 123–126. [Google Scholar] [CrossRef]
- Javierre, B.M.; Fernandez, A.F.; Richter, J. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010, 20, 170–179. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Infante-Duarte, C.; Horton, H.F.; Byrne, M.C.; Kamradt, T. Microbial Lipopeptides Induce the Production of IL-17 in Th Cells. J. Immunol. 2000, 165, 6107–6115. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Rouvier, E.; Luciani, M.F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [CrossRef] [PubMed]
- Andrew, J.L.; Frann, B.; Matthew, J.W.; Riyez, K.; Mary, C.; Samuel, J.G.; Kyriaki, D.-J.; Cara MMWJill, F.W.; Lynette, A.F. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007, 179, 7791–7799. [Google Scholar]
- Yao, Z.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.-M.; Painter, S.L.; Comeau, M.R.; Cohen, J.I.; Spriggs, M.K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995, 3, 811–821. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jäger, A.; Strom, T.B.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Kao, C.Y.; Yin, C.; Philip, T.; Shinichiro, W.; Fei, H.; Christy, K.; Richart, W.H.; Reen, W. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 2004, 173, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins and Host Defense. Science 1999, 286, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-Y.; Kim, H.-Y. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol. Cells 2005, 19, 180–184. [Google Scholar]
- Shin, M.S.; Lee, N.; Kang, I. Effector T-cell subsets in systemic lupus erythematosus: Update focusing on Th17 cells. Curr. Opin. Rheumatol. 2011, 23, 444–448. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Chen, Y.-M.; Wen, M.-C.; Hsieh, T.-Y.; Hung, W.-T.; Lan, J.-L. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus 2012, 21, 1385–1396. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.-J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. 2010, 107, 14292–14297. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Flavell, R.A. TGF-beta and regulatory T cell in immunity and autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; Wahl, S.M. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.P.; Rudensky, A.Y. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007, 7, 443–453. [Google Scholar] [CrossRef]
- Lourenço, J.D.; Ito, J.T.; Martins MD, A.; Tibério ID FL, C.; Lopes, F.D.T.Q.D.S. Th17/Treg Imbalance in Chronic Obstructive Pulmonary Disease: Clinical and Experimental Evidence. Front. Immunol. 2021, 12, 804919. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.; Liu, W.; Wu, K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD 2013, 10, 459–465. [Google Scholar] [CrossRef]
- Sileikiene, V.; Laurinaviciene, A.; Lesciute-Krilaviciene, D.; Jurgauskiene, L.; Malickaite, R.; Laurinavicius, A. Levels of CD4+ CD25+ T Regulatory Cells in Bronchial Mucosa and Peripheral Blood of Chronic Obstructive Pulmonary Disease Indicate Involvement of Autoimmunity Mechanisms. Adv. Respir. Med. 2019, 87, 159–166. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Chen, J.; Gu, Y.; Xu, J.; Ouyang, Y. Dendritic cells and Th17/Treg ratio play critical roles in pathogenic process of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2018, 108, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, W.; Weng, Y.; Ying, H.; Li, H.; Xia, D.; Yu, W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int. Immunopharmacol. 2012, 14, 504–512. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Yu, Z.X.; Ji, M.S.; Yan, J.; Cai, Y.; Liu, J.; Yang, H.F.; Zheng, J.X. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care 2015, 19, 82. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Zhang, M.; Fu, D.; Luo, H.; Yang, X. SPP1 exacerbates ARDS via elevating Th17/Treg and M1/M2 ratios through suppression of ubiquitination-dependent HIF-1α degradation. Cytokine 2023, 164, 156107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jiao, Y.; Jiang, W.; Zhang, X.; Zhang, L.; Jia, G. IL-33 Deficiency Attenuates Lung Inflammation by Inducing Th17 Response and Impacting the Th17/Treg Balance in LPS-Induced ARDS Mice via Dendritic Cells. J. Immunol. Res. 2022, 2022, 9543083. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Pai, M.-H.; Liu, J.-J.; Yeh, S.-L. Alanyl-glutamine resolves lipopolysaccharide-induced lung injury in mice by modulating the polarization of regulatory T cells and T helper 17 cells. J. Nutr. Biochem. 2013, 24, 1555–1563. [Google Scholar] [CrossRef]
- Qiu, H.-B.; Liu, J.; Zhang, P.-S.; Yu, Q.; Liu, L.; Yang, Y.; Guo, F.-M. Losartan inhibits conventional dendritic cell maturation and Th1 and Th17 polarization responses: Νovel mechanisms of preventive effects on lipopolysaccharide-induced acute lung injury. Int. J. Mol. Med. 2011, 29, 269–276. [Google Scholar] [CrossRef]
- Urbankowski, T.; Hoser, G.; Domagała-Kulawik, J. Th1/Th2/Th17-related cytokines in the bronchoalveolar lavage fluid of patients with sarcoidosis: Association with smoking. Pol. Arch. Med. Wewn. 2012, 122, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Grunewald, J.; Eklund, A.; Bruder, D.; Wahlström, J. Pulmonary sarcoidosis is associated with high-level inducible co-stimulator (ICOS) expression on lung regulatory T cells–possible implications for the ICOS/ICOS-ligand axis in disease course and resolution. Clin. ExImmunol. 2015, 183, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Yanxun, W.; Halimulati, A.; Yuyue, Z.; Shan, L.; Zuojun, X. The Circulating Treg/Th17 Cell Ratio Is Correlated with Relapse and Treatment Response in Pulmonary Sarcoidosis Patients after Corticosteroid Withdrawal. PLoS ONE 2016, 11, e0148207. [Google Scholar]
- Miyara, M.; Amoura, Z.; Parizot, C.; Badoual, C.; Dorgham, K.; Trad, S.; Kambouchner, M.; Valeyre, D.; Chapelon-Abric, C.; Debré, P.; et al. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med. 2006, 203, 359–370. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Richmond, B.W.; Braun, N.A.; Isom, J.; Abraham, S.; Taylor, T.R.; Drake, J.M.; Culver, D.A.; Wilkes, D.S.; Drake, W.P. Reversal of Global CD4+ Subset Dysfunction Is Associated with Spontaneous Clinical Resolution of Pulmonary Sarcoidosis. J. Immunol. 2013, 190, 5446–5453. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Chesné, J.; Braza, F.; Mahay, G.; Brouard, S.; Aronica, M.; Magnan, A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014, 190, 1094–1101. [Google Scholar] [CrossRef]
- Molet, S.; Hamid, Q.; Davoineb, F.; Nutku, E.; Tahaa, R.; Pagé, N.; Olivenstein, R.; Elias, J.; Chakir, J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001, 108, 430–438. [Google Scholar] [CrossRef]
- Al-Ramli, W.; Préfontaine, D.; Chouiali, F.; Martin, J.G.; Olivenstein, R.; Lemière, C.; Hamid, Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 2009, 123, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, A.; Pierzchala, W.; Sozañska, E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 2003, 97, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Zhou, Q.-T.; Yao, W.-Z. Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin. Med. J. 2005, 118, 953–956. [Google Scholar] [PubMed]
- Camargo, L.D.N.; dos Santos, T.M.; de Andrade, F.C.P.; Fukuzaki, S.; Lopes, F.D.T.Q.D.S.; Martins, M.D.A.; Prado, C.M.; Leick, E.A.; Righetti, R.F.; Tibério, I.D.F.L.C. Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS. Front. Pharmacol. 2020, 11, 1269. [Google Scholar] [CrossRef]
- de Brito, A.A.; Goncalves Santos, T.; Herculano, K.Z.; Estefano-Alves, C.; de Alvarenga Nascimento, C.R.; Rigonato-Oliveira, N.C.; Ligeiro de Oliveira, A.P. Photobiomodulation Therapy Restores IL-10 Secretion in a Murine Model of Chronic Asthma: Relevance to the Population of CD4. Front. Immunol. 2021, 12, 789426. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, H.; Xie, H.; Chen, L.; Li, S.; Zheng, J.; Chai, R.; Wang, Z.; Zang, Y.; He, S. Reduced CCR6+ IL-17A+ Treg Cells in Blood and CCR6-Dependent Accumulation of IL-17A+ Treg Cells in Lungs of Patients With Allergic Asthma. Front. Immunol. 2021, 12, 710750. [Google Scholar] [CrossRef]
- Tao, B.; Ruan, G.; Wang, D.; Li, Y.; Wang, Z.; Yin, G. Imbalance of Peripheral Th17 and Regulatory T Cells in Children with Allergic Rhinitis and Bronchial Asthma. Iran. J. Allergy Asthma Immunol. 2015, 14, 273–279. [Google Scholar]
- Zheng, R.; Wang, F.; Huang, Y.; Xiang, Q.; Dai, H.; Zhang, W. Elevated Th17 cell frequencies and Th17/Treg ratio are associated with airway hyperresponsiveness in asthmatic children. J. Asthma 2021, 58, 707–716. [Google Scholar] [CrossRef]
- Dai, H.; Zheng, R.; Wang, L.; Wan, J.; Tong, Y.; Zhao, W.; Zhang, W. ICS/LABA Combined With Subcutaneous Immunotherapy Modulates the Th17/Treg Imbalance in Asthmatic Children. Front. Immunol. 2022, 13, 779072. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.M.; Dias, A.S.; Lopes, L.M.; Kasahara, T.M.; Delphim, L.; Silva, J.C.C.; Bento, C.A. Leptin favors Th17/Treg cell subsets imbalance associated with allergic asthma severity. Clin. Transl. Allergy 2022, 12, e12153. [Google Scholar] [CrossRef] [PubMed]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic Mucocutaneous Candidiasis in Humans with Inborn Errors of Interleukin-17 Immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef]
- Pandiyan, P.; Conti, H.R.; Zheng, L.; Peterson, A.C.; Mathern, D.R.; Hernández-Santos, N.; Edgerton, M.; Gaffen, S.L.; Lenardo, M.J. CD4+ CD25+ Foxp3+ Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity 2011, 34, 422–434. [Google Scholar] [CrossRef]
- Rudner, X.L.; Happel, K.I.; Young, E.A.; Shellito, J.E. Interleukin-23 (IL-23)-IL-17 Cytokine Axis in Murine Pneumocystis carinii Infection. Infect. Immun. 2007, 75, 3055–3061. [Google Scholar] [CrossRef]
- McKinley, L.; Logar, A.J.; McAllister, F.; Zheng, M.; Steele, C.; Kolls, J.K. Regulatory T Cells Dampen Pulmonary Inflammation and Lung Injury in an Animal Model of Pneumocystis Pneumonia. J. Immunol. 2006, 177, 6215–6226. [Google Scholar] [CrossRef]
- Bai, H.; Gao, X.; Zhao, L.; Peng, Y.; Yang, J.; Qiao, S.; Zhao, H.; Wang, S.; Fan, Y.; Joyee, A.G.; et al. Respective IL-17A production by γδ T and Th17 cells and its implication in host defense against chlamydial lung infection. Cell Mol. Immunol. 2016, 14, 850–861. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, S.; Shekhar, S.; Peng, Y.; Qiao, S.; Zhang, C.; Shan, L.; Movassagh, H.; Gounni, A.S.; Yang, J.; et al. Semaphorin 3E Protects against Chlamydial Infection by Modulating Dendritic Cell Functions. J. Immunol. 2021, 206, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Wang, S.; Rashu, R.; Peng, Y.; Gounni, A.S.; Yang, X. Exogenous Semaphorin 3E treatment protects against chlamydial lung infection in mice. Front. Immunol. 2022, 13, 882412. [Google Scholar] [CrossRef]
- Bai, H.; Cheng, J.; Gao, X.; Joyee, A.G.; Fan, Y.; Wang, S.; Yang, X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol. 2009, 183, 5886–5895. [Google Scholar] [CrossRef]
- Sun, L.-D.; Qiao, S.; Wang, Y.; Pang, G.-J.; Zha, X.-Y.; Liu, T.-L.; Zhao, H.-L.; Liang, J.-Y.; Zheng, N.-B.; Tan, L.; et al. Vγ4+ T Cells: A Novel IL-17-Producing γδ T Subsets during the Early Phase of Chlamydial Airway Infection in Mice. Mediat. Inflamm. 2018, 2018, 6265746. [Google Scholar] [CrossRef]
- Scurlock, A.M.; Frazer, L.C.; Andrews, C.W.; O’Connell, C.M.; Foote, I.P.; Bailey, S.L.; Chandra-Kuntal, K.; Kolls, J.K.; Darville, T. Interleukin-17 Contributes to Generation of Th1 Immunity and Neutrophil Recruitment during Chlamydia muridarum Genital Tract Infection but Is Not Required for Macrophage Influx or Normal Resolution of Infection. Infect. Immun. 2011, 79, 1349–1362. [Google Scholar] [CrossRef]
- Frazer, L.C.; Scurlock, A.M.; Zurenski, M.A.; Riley, M.M.; Mintus, M.; Pociask, D.A.; Darville, T. IL-23 induces IL-22 and IL-17 production in response to Chlamydia muridarum genital tract infection, but the absence of these cytokines does not influence disease pathogenesis. Am. J. Reprod. Immunol. 2013, 70, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Champion, C.I.; Jiang, J. The role of T regulatory cells in Chlamydia trachomatis genital infection. Chlamydia 2021, 91. [Google Scholar] [CrossRef]
- Moore-Connors, J.M.; Fraser, R.; Halperin, S.A.; Wang, J. CD4+ CD25+ Foxp3+ Regulatory T Cells Promote Th17 Responses and Genital Tract Inflammation upon Intracellular Chlamydia muridarum Infection. J. Immunol. 2013, 191, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Marks, E.; Verolin, M.; Stensson, A.; Lycke, N. Differential CD28 and Inducible Costimulatory Molecule Signaling Requirements for Protective CD4+ T-Cell-Mediated Immunity against Genital Tract Chlamydia trachomatis Infection. Infect. Immun. 2007, 75, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Thomas, R.; Gao, X.; Bai, H.; Shekhar, S.; Wang, S.; Yang, J.; Zhao, W.; Yang, X. NK cells modulate T cell responses via interaction with dendritic cells in Chlamydophila pneumoniae infection. Cell Immunol. 2020, 353, 104132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Zhao, L.; Wang, X.; Wang, Y.; Yang, X.; Zhao, W. Natural killer cells regulate Th1/Treg and Th17/Treg balance in chlamydial lung infection. J. Cell Mol. Med. 2016, 20, 1339–1351. [Google Scholar] [CrossRef]
- Guo, H.; He, Z.; Li, M.; Wang, T.; Zhang, L. Imbalance of peripheral blood Th17 and Treg responses in children with refractory Mycoplasma pneumoniae pneumonia. J. Infect. Chemother. 2016, 22, 162–166. [Google Scholar] [CrossRef]
- Shi, T.; Li, N.; He, Y.; Feng, J.; Mei, Z.; Du, Y.; Jie, Z. Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb. Pathog. 2021, 156, 104867. [Google Scholar] [CrossRef]
- Qin, L.; Hu, C.-P.; Feng, J.-T.; Xia, Q. Activation of Lymphocytes Induced by Bronchial Epithelial Cells with Prolonged RSV Infection. PLoS ONE 2011, 6, e27113. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, F.-L.; Feng, X.-B.; Sun, D.-K.; Cui, Q.-Q.; Zhao, Z.-X. Changes and the clinical significance of CD4? CD25? regulatory T cells and Th17 cells in peripheral blood of infants with respiratory syncytial virus bronchiolitis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2012, 28, 426–428. [Google Scholar]
- Profita, M.; Albano, G.D.; Riccobono, L.; Di Sano, C.; Montalbano, A.M.; Gagliardo, R.; Anzalone, G.; Bonanno, A.; Pieper, M.P.; Gjomarkaj, M. Increased levels of Th17 cells are associated with non-neuronal acetylcholine in COPD patients. Immunobiology 2014, 219, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, T.; Zhang, H.; Zhang, J.; Zhong, X.; Tan, C.; Qiu, Y.; Zeng, W.; Feng, X. Erythromycin Prevents Elastin Peptide-Induced Emphysema and Modulates CD4+ T Cell Responses in Mice. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, X.; He, Z.; Zhang, J.; Bai, J.; Liu, G.; Liang, Y.; Ya, L.; Qin, X. Erythromycin Suppresses the Cigarette Smoke Extract-Exposed Dendritic Cell-Mediated Polarization of CD4+ T Cells into Th17 Cells. J. Immunol. Res. 2020, 2020, 1387952. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Z.; Zhou, H. N-Acetylcysteine Improves Inflammatory Response in COPD Patients by Regulating Th17/Treg Balance through Hypoxia Inducible Factor-1. Biomed. Res. Int. 2021, 2021, 6372128. [Google Scholar] [CrossRef]
- Maneechotesuwan, K.; Wongkajornsilp, A.; Adcock, I.; Barnes, P.J. Simvastatin Suppresses Airway IL-17 and Upregulates IL-10 in Patients with Stable COPD. Chest 2015, 148, 1164–1176. [Google Scholar] [CrossRef]
- Hellings, P.W.; Kasran, A.; Liu, Z.; Vandekerckhove, P.; Wuyts, A.; Overbergh, L.; Mathieu, C.; Ceuppens, J.L. Interleukin-17 Orchestrates the Granulocyte Influx into Airways after Allergen Inhalation in a Mouse Model of Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2003, 28, 42–50. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef]
- Nakagome, K.; Imamura, M.; Okada, H.; Kawahata, K.; Inoue, T.; Hashimoto, K.; Harada, H.; Higashi, T.; Takagi, R.; Nakano, K.; et al. Dopamine D1-Like Receptor Antagonist Attenuates Th17-Mediated Immune Response and Ovalbumin Antigen-Induced Neutrophilic Airway Inflammation. J. Immunol. 2011, 186, 5975–5982. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, W.; Zhang, H.; Yin, Y. Mammalian Target of Rapamycin Signaling Enhances Ovalbumin-Induced Neutrophilic Airway Inflammation by Promoting Th17 Cell Polarization in Murine Noneosinophilic Asthma Model. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 25–32. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.S.; Kim, S.R.; Min, K.H.; Choe, Y.H.; Moon, H.; Lee, Y.C. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J. Immunol. 2009, 183, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Kyung, S.L.; Hee, S.P.; Seoung, J.P.; Kyung, H.M.; Sun, M.J.; Yong, C.L. Involvement of IL-10 in peroxisome proliferator-activated receptor gamma-mediated anti-inflammatory response in asthma. Mol. Pharmacol. 2005, 68, 1568–1575. [Google Scholar]
- Dembele, M.; Tao, S.; Massoud, A.H.; Miah, S.M.S.; Lelias, S.; De Groot, A.S.; Mazer, B.D. Tregitopes Improve Asthma by Promoting Highly Suppressive and Antigen-Specific Tregs. Front. Immunol. 2021, 12, 634509. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fueyo, A.; Ramos, T.; Galán, A.; Jimeno, L.; Wurtzen, P.A.; Marin, A.; de Frutos, C.; Blanco, C.; Carrera, A.; Barber, D.; et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J. Allergy Clin. Immunol. 2013, 133, 130–138.e2. [Google Scholar] [CrossRef] [PubMed]
- Swamy, R.S.; Reshamwala, N.; Hunter, T.; Vissamsetti, S.; Santos, C.B.; Baroody, F.M.; Hwang, P.H.; Hoyte, E.G.; Garcia, M.A.; Nadeau, K.C. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 130, 215–224.e7. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, H.; Shan, L.; Duke-Cohan, J.S.; Halayko, A.J.; Uzonna, J.E.; Gounni, A.S. Semaphorin 3E Alleviates Hallmarks of House Dust Mite–Induced Allergic Airway Disease. Am. J. Pathol. 2017, 187, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.; Qiao, S.; Yang, X. Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 4865. https://doi.org/10.3390/ijms24054865
Thomas R, Qiao S, Yang X. Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases. International Journal of Molecular Sciences. 2023; 24(5):4865. https://doi.org/10.3390/ijms24054865
Chicago/Turabian StyleThomas, Rony, Sai Qiao, and Xi Yang. 2023. "Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases" International Journal of Molecular Sciences 24, no. 5: 4865. https://doi.org/10.3390/ijms24054865
APA StyleThomas, R., Qiao, S., & Yang, X. (2023). Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases. International Journal of Molecular Sciences, 24(5), 4865. https://doi.org/10.3390/ijms24054865






