Heterochronic Parabiosis Causes Dacryoadenitis in Young Lacrimal Glands
Abstract
:1. Introduction
2. Results
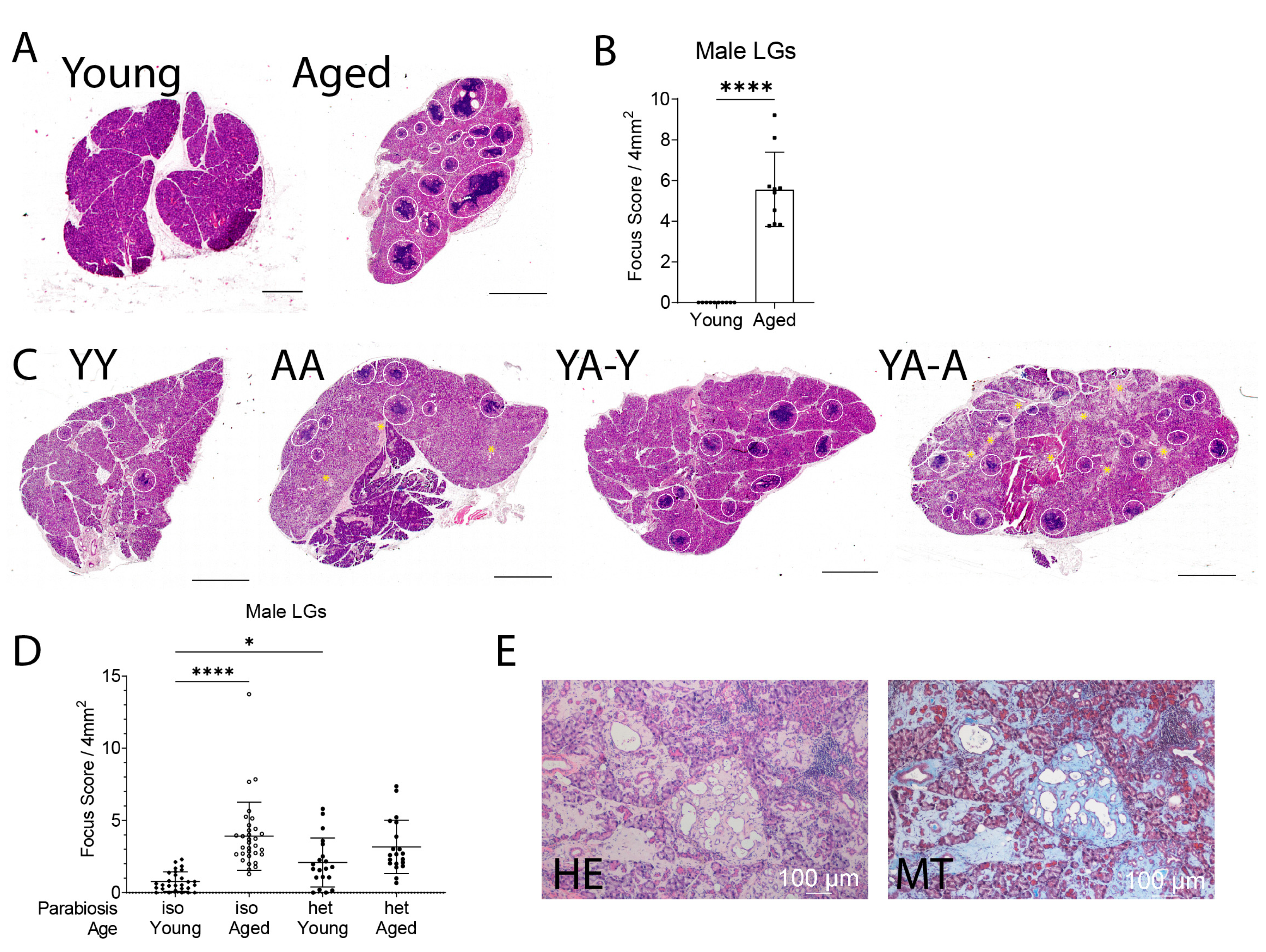
2.1. Lacrimal Gland Pathology Was Worsened in Heterochronic Young Mice
2.2. Significant Increase in Inflammatory and B-Cell-Related mRNA in Aged Lacrimal Glands Regardless of Parabiosis
2.3. Increased T and B Cell Populations in Heterochronic Young Lacrimal Glands
2.4. Heterochronic Parabiosis in Female Lacrimal Glands Does Not Improve Lymphocytic Infiltration or Inflammatory Marker Expression
2.5. Aged Male Mice Have Worse Lacrimal Gland Infiltration and a Greater Frequency of Fibrosis than Aged Female Mice
3. Discussion
3.1. Lacrimal Gland Phenotype
3.2. Inflammatory Marker Expression
3.3. Immune Cell Identification
3.4. Sex Differences in Parabiotic Lacrimal Glands
4. Materials and Methods
4.1. Animals
4.2. Calculation of Lymphocytic Infiltration
4.3. Masson’s Trichrome Stain
4.4. RNA Isolation and Real-Time PCR
4.5. Flow Cytometry
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baudouin, C.; Irkec, M.; Messmer, E.M.; Benitez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am.J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, E.M.; Alves, M.; Rios, J.D.; Dartt, D.A. The aging lacrimal gland: Changes in structure and function. Ocul. Surf. 2008, 6, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, R.G.; de Paiva, C.S.; Alves, M.R. Age-related Autoimmune Changes in Lacrimal Glands. Immune Netw. 2019, 19, e3. [Google Scholar] [CrossRef]
- El-Fadaly, A.B.; El-Shaarawy, E.A.; Rizk, A.A.; Nasralla, M.M.; Shuaib, D.M. Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Ann. Anat. 2014, 196, 336–351. [Google Scholar] [CrossRef]
- Coursey, T.G.; Bian, F.; Zaheer, M.; Pflugfelder, S.C.; Volpe, E.A.; de Paiva, C.S. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2017, 10, 743–756. [Google Scholar] [CrossRef] [Green Version]
- de Souza, R.G.; Yu, Z.; Hernandez, H.; Trujillo-Vargas, C.M.; Lee, A.; Mauk, K.E.; Cai, J.; Alves, M.R.; de Paiva, C.S. Modulation of Oxidative Stress and Inflammation in the Aged Lacrimal Gland. Am. J. Pathol. 2021, 191, 294–308. [Google Scholar] [CrossRef]
- Bian, F.; Xiao, Y.; Barbosa, F.L.; de Souza, R.G.; Hernandez, H.; Yu, Z.; Pflugfelder, S.C.; de Paiva, C.S. Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells. Mucosal Immunol. 2019, 12, 897–908. [Google Scholar] [CrossRef]
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.F.; Tzioufas, A.G.; Vitali, C.; et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjogren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Vargas, C.M.; Mauk, K.E.; Hernandez, H.; de Souza, R.G.; Yu, Z.; Galletti, J.G.; Dietrich, J.; Paulsen, F.; de Paiva, C.S. Immune phenotype of the CD4(+) T cells in the aged lymphoid organs and lacrimal glands. GeroScience 2022, 44, 2105–2128. [Google Scholar] [CrossRef] [PubMed]
- Galletti, J.G.; Scholand, K.K.; Trujillo-Vargas, C.M.; Yu, Z.; Mauduit, O.; Delcroix, V.; Makarenkova, H.P.; de Paiva, C.S. Ectopic lymphoid structures in the aged lacrimal glands. Clin. Immunol. 2023, 248, 109251. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.S.; deRoos, P.; Honey, K.; Beers, C.; Rudensky, A.Y. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J. Immunol. 2002, 168, 2618–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.P.; Villadangos, J.A.; Dranoff, G.; Small, C.; Gu, L.; Haley, K.J.; Riese, R.; Ploegh, H.L.; Chapman, H.A. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 1999, 10, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Masternak, K.; Muhlethaler-Mottet, A.; Villard, J.; Zufferey, M.; Steimle, V.; Reith, W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000, 14, 1156–1166. [Google Scholar] [CrossRef]
- Muhlethaler-Mottet, A.; Otten, L.A.; Steimle, V.; Mach, B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997, 16, 2851–2860. [Google Scholar] [CrossRef]
- Coursey, T.G.; Henriksson, J.T.; Barbosa, F.L.; de Paiva, C.S.; Pflugfelder, S.C. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chauhan, S.K.; Saban, D.R.; Sadrai, Z.; Okanobo, A.; Dana, R. Interferon-gamma-secreting NK cells promote induction of dry eye disease. J. Leukoc. Biol. 2011, 89, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, W.; de Paiva, C.S.; Corrales, R.M.; Volpe, E.A.; McClellan, A.J.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6279–6285. [Google Scholar] [CrossRef]
- Martin, F.; Oliver, A.M.; Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001, 14, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Ansel, K.M.; Ngo, V.N.; Hyman, P.L.; Luther, S.A.; Forster, R.; Sedgwick, J.D.; Browning, J.L.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef]
- Brandes, M.; Legler, D.F.; Spoerri, B.; Schaerli, P.; Moser, B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 2000, 12, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Bert, P. Expériences et considérations sur la greffe animale. J. L’anatomie Physiol. 1864, 1, 69–87. [Google Scholar]
- Kamran, P.; Sereti, K.I.; Zhao, P.; Ali, S.R.; Weissman, I.L.; Ardehali, R. Parabiosis in mice: A detailed protocol. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [Green Version]
- Eggel, A.; Wyss-Coray, T. A revival of parabiosis in biomedical research. Swiss Med. Wkly. 2014, 144, w13914. [Google Scholar] [CrossRef]
- Conboy, M.J.; Conboy, I.M.; Rando, T.A. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Hamrah, P.; Zheng, L.; Mantopoulos, D.; Turhan, A.; Andrian, U.H.v. Physiologic Homeostasis and Turnover of Corneal Bone Marrow-Derived Cells: Lessons from the Parabiosis Model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1114. [Google Scholar]
- Wieghofer, P.; Hagemeyer, N.; Sankowski, R.; Schlecht, A.; Staszewski, O.; Amann, L.; Gruber, M.; Koch, J.; Hausmann, A.; Zhang, P.; et al. Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J. 2021, 40, e105123. [Google Scholar] [CrossRef]
- Heuss, N.D.; Pierson, M.J.; Roehrich, H.; McPherson, S.W.; Gram, A.L.; Li, L.; Gregerson, D.S. Optic nerve as a source of activated retinal microglia post-injury. Acta Neuropathol. Commun. 2018, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Rong, L. Exploration of Molecular Mechanism of the Retina Aging. Ph.D. Thesis, The Hong Kong Polytechnic University, Hong Kong, China, 2021. [Google Scholar]
- Schein, O.D.; Hochberg, M.C.; Munoz, B.; Tielsch, J.M.; Bandeen-Roche, K.; Provost, T.; Anhalt, G.J.; West, S. Dry eye and dry mouth in the elderly: A population-based assessment. Arch. Intern. Med. 1999, 159, 1359–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Kinoshita, S.; Yokoi, N.; Ogawa, Y.; Shibuya, M.; Nakashima, H.; Hisamura, R.; Imada, T.; Imagawa, T.; Uehara, M.; et al. Lacrimal hypofunction as a new mechanism of dry eye in visual display terminal users. PLoS ONE 2010, 5, e11119. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.E.; Adeghate, E.; Lawrence, P.A.; Pallot, D.J.; Garner, A.; Singh, J. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J. Auton. Nerv. Syst. 1998, 69, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Wakamatsu, T.H.; Dogru, M.; Ogawa, Y.; Igarashi, A.; Ibrahim, O.M.; Inaba, T.; Shimizu, T.; Noda, S.; Obata, H.; et al. Age-related dysfunction of the lacrimal gland and oxidative stress: Evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am. J. Pathol. 2012, 180, 1879–1896. [Google Scholar] [CrossRef]
- Bromberg, B.B.; Welch, M.H. Lacrimal protein secretion: Comparison of young and old rats. Exp. Eye Res. 1985, 40, 313–320. [Google Scholar] [CrossRef]
- Draper, C.E. Effects of age on morphology, protein synthesis and secretagogue-evoked secretory responses in the rat lacrimal gland. Mol. Cell. Biochem. 2003, 248, 7–16. [Google Scholar] [CrossRef]
- Draper, C.E.; Adeghate, E.A.; Singh, J.; Pallot, D.J. Evidence to Suggest Morphological and Physiological Alterations of Lacrimal Gland Acini with Ageing. Exp. Eye Res. 1999, 68, 265–276. [Google Scholar] [CrossRef]
- Obata, H.; Yamamoto, S.; Horiuchi, H.; Machinami, R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology 1995, 102, 678–686. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Wang, S.; Ye, Y.; Ren, J.; Chen, R.; Li, W.; Li, J.; Zhao, L.; Zhao, Q.; Sun, G.; et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell 2022, 29, 990–1005. [Google Scholar] [CrossRef]
- Jeon, O.H.; Mehdipour, M.; Gil, T.H.; Kang, M.; Aguirre, N.W.; Robinson, Z.R.; Kato, C.; Etienne, J.; Lee, H.G.; Alimirah, F.; et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat. Metab. 2022, 4, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Palovics, R.; Keller, A.; Schaum, N.; Tan, W.; Fehlmann, T.; Borja, M.; Kern, F.; Bonanno, L.; Calcuttawala, K.; Webber, J.; et al. Molecular hallmarks of heterochronic parabiosis at single-cell resolution. Nature 2022, 603, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Rebo, J.; Mehdipour, M.; Gathwala, R.; Causey, K.; Liu, Y.; Conboy, M.J.; Conboy, I.M. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat. Commun. 2016, 7, 13363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ximerakis, M.; Holton, K.M.; Giadone, R.M.; Ozek, C.; Saxena, M.; Santiago, S.; Adiconis, X.; Dionne, D.; Nguyen, L.; Shah, K.M.; et al. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types. bioRxiv 2022. [Google Scholar] [CrossRef]
- Smith, L.K.; He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Yankova, T.; Dubiley, T.; Shytikov, D.; Pishel, I. Three Month Heterochronic Parabiosis Has a Deleterious Effect on the Lifespan of Young Animals, Without a Positive Effect for Old Animals. Rejuvenation Res. 2022, 25, 191–199. [Google Scholar] [CrossRef]
- Mitnitski, A.; Howlett, S.E.; Rockwood, K. Heterogeneity of Human Aging and Its Assessment. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.D.; Moodie, E.M.; Forget, M.F.; Desmarais, P.; Keezer, M.R.; Wolfson, C. Health Heterogeneity in Older Adults: Exploration in the Canadian Longitudinal Study on Aging. J. Am. Geriatr. Soc. 2021, 69, 678–687. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.Q.; Pflugfelder, S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef]
- Volpe, E.A.; Henriksson, J.T.; Wang, C.; Barbosa, F.L.; Zaheer, M.; Zhang, X.; Pflugfelder, S.C.; de Paiva, C.S. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget 2016, 7, 64605–66461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; de Paiva, C.S.; Su, Z.; Volpe, E.A.; Li, D.Q.; Pflugfelder, S.C. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp. Eye Res. 2014, 118, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelegrino, F.S.; Volpe, E.A.; Gandhi, N.B.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res. Ther. 2012, 14, R234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coursey, T.G.; Bohat, R.; Barbosa, F.L.; Pflugfelder, S.C.; de Paiva, C.S. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J. Immunol. 2014, 193, 5264–5272. [Google Scholar] [CrossRef] [Green Version]
- Plüger, E.B.E.; Boes, M.; Alfonso, C.; Schröter, C.J.; Kalbacher, H.; Ploegh, H.L.; Driessen, C. Specific role for cathepsin S in the generation of antigenic peptidesin vivo. Eur. J. Immunol. 2002, 32, 467–476. [Google Scholar] [CrossRef]
- Yang, H.; Kala, M.; Scott, B.G.; Goluszko, E.; Chapman, H.A.; Christadoss, P. Cathepsin S is required for murine autoimmune myasthenia gravis pathogenesis. J. Immunol. 2005, 174, 1729–1737. [Google Scholar] [CrossRef] [Green Version]
- Rupanagudi, K.V.; Kulkarni, O.P.; Lichtnekert, J.; Darisipudi, M.N.; Mulay, S.R.; Schott, B.; Gruner, S.; Haap, W.; Hartmann, G.; Anders, H.J. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann. Rheum. Dis. 2015, 74, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Hsing, L.C.; Kirk, E.A.; McMillen, T.S.; Hsiao, S.H.; Caldwell, M.; Houston, B.; Rudensky, A.Y.; LeBoeuf, R.C. Roles for cathepsins S, L, and B in insulitis and diabetes in the NOD mouse. J. Autoimmun. 2010, 34, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Madrigal, S.; Shah, M.; Frousiakis, S.E.; Renduchintala, K.; Zhu, J.; Bricel, S.; Silka, K.; et al. Tear cathepsin S as a candidate biomarker for Sjogren’s syndrome. Arthritis Rheumatol. 2014, 66, 1872–1881. [Google Scholar] [CrossRef] [Green Version]
- Klinngam, W.; Janga, S.R.; Lee, C.; Ju, Y.; Yarber, F.; Shah, M.; Guo, H.; Wang, D.; MacKay, J.A.; Edman, M.C.; et al. Inhibition of Cathepsin S Reduces Lacrimal Gland Inflammation and Increases Tear Flow in a Mouse Model of Sjogren’s Syndrome. Sci. Rep. 2019, 9, 9559. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Li, J.; Govindarajan, G.; Hamm-Alvarez, S.F.; Alam, J.; Li, D.Q.; de Paiva, C.S. Cathepsin S is a novel target for age-related dry eye. Exp. Eye Res. 2022, 214, 108895. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; McGlauchlen, K.; Vogel, L.A. Aged B lymphocytes retain their ability to express surface markers but are dysfunctional in their proliferative capability during early activation events. Immun. Ageing 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, I.; Goldeck, D.; Larbi, A.; Pawelec, G. Aging affects the proportions of T and B cells in a group of elderly men in a developing country--a pilot study from Pakistan. Age 2013, 35, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Aydar, Y.; Balogh, P.; Tew, J.G.; Szakal, A.K. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J. Immunol. 2003, 171, 5975–5987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Bella, S.; Bierti, L.; Presicce, P.; Arienti, R.; Valenti, M.; Saresella, M.; Vergani, C.; Villa, M.L. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 2007, 122, 220–228. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, X.; Lu, X.; Zhong, X.; Lubahn, D.; Reneker, L.W. Accelerated Aging in the Lacrimal Glands of Estrogen-deficient Aromatase (Aro) and Estrogen Receptor (ER) Knockout Mice. Investig. Ophthalmol. Vis. Sci. 2021, 62, 707. [Google Scholar]
- Tellefsen, S.; Morthen, M.K.; Richards, S.M.; Lieberman, S.M.; Rahimi Darabad, R.; Kam, W.R.; Sullivan, D.A. Sex Effects on Gene Expression in Lacrimal Glands of Mouse Models of Sjogren Syndrome. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5599–5614. [Google Scholar] [CrossRef] [Green Version]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]






| Parameters | Sex Comparison | Male | Female | Sex Effect | p-Value |
|---|---|---|---|---|---|
| Lacrimal gland evaluation | Isochronic young focus score | 0.76 | 0.57 | M = F | p > 0.99 |
| Isochronic aged focus score | 3.91 | 3.33 | M = F | p = 0.92 | |
| Heterochronic young focus score | 2.1 | 0.68 | M > F | p = 0.06 | |
| Heterochronic aged focus score | 3.16 | 2.71 | M = F | p = 0.97 | |
| Occurrence of fibrosis in aged lacrimal gland | ↑↑↑ | ╳ | M >> F | p < 0.001 | |
| Gene expression analysis in lacrimal gland | Tnf in heterochronic aged mice | 1.22 fold | 2.34 fold | F > M | p = 0.043 |
| Ctss in isochronic aged mice | 2.26 fold | 4.33 fold | F > M | p = 0.046 | |
| Ctss in heterochronic aged mice | 2.15 fold | 4.02 fold | F > M | p = 0.024 | |
| Cxcl13 in isochronic young mice | 8.24 fold | 0.61 fold | M > F | p = 0.0003 | |
| Cxcl9 in isochronic aged mice | 1.18 fold | 3.90 fold | F > M | p = 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholand, K.K.; Mack, A.F.; Guzman, G.U.; Maniskas, M.E.; Sampige, R.; Govindarajan, G.; McCullough, L.D.; de Paiva, C.S. Heterochronic Parabiosis Causes Dacryoadenitis in Young Lacrimal Glands. Int. J. Mol. Sci. 2023, 24, 4897. https://doi.org/10.3390/ijms24054897
Scholand KK, Mack AF, Guzman GU, Maniskas ME, Sampige R, Govindarajan G, McCullough LD, de Paiva CS. Heterochronic Parabiosis Causes Dacryoadenitis in Young Lacrimal Glands. International Journal of Molecular Sciences. 2023; 24(5):4897. https://doi.org/10.3390/ijms24054897
Chicago/Turabian StyleScholand, Kaitlin K., Alexis F. Mack, Gary U. Guzman, Michael E. Maniskas, Ritu Sampige, Gowthaman Govindarajan, Louise D. McCullough, and Cintia S. de Paiva. 2023. "Heterochronic Parabiosis Causes Dacryoadenitis in Young Lacrimal Glands" International Journal of Molecular Sciences 24, no. 5: 4897. https://doi.org/10.3390/ijms24054897
APA StyleScholand, K. K., Mack, A. F., Guzman, G. U., Maniskas, M. E., Sampige, R., Govindarajan, G., McCullough, L. D., & de Paiva, C. S. (2023). Heterochronic Parabiosis Causes Dacryoadenitis in Young Lacrimal Glands. International Journal of Molecular Sciences, 24(5), 4897. https://doi.org/10.3390/ijms24054897







