CCR5∆32 and SDF1 3′A: Gene Variants, Expression and Influence on Biological Markers for the Clinical Progression to AIDS among HIV-1 Virus Controllers in a Mixed Population of the Amazon Region of Brazil
Abstract
1. Introduction
2. Results
2.1. Epidemiological Variables, Gene Variants and Gene Expression
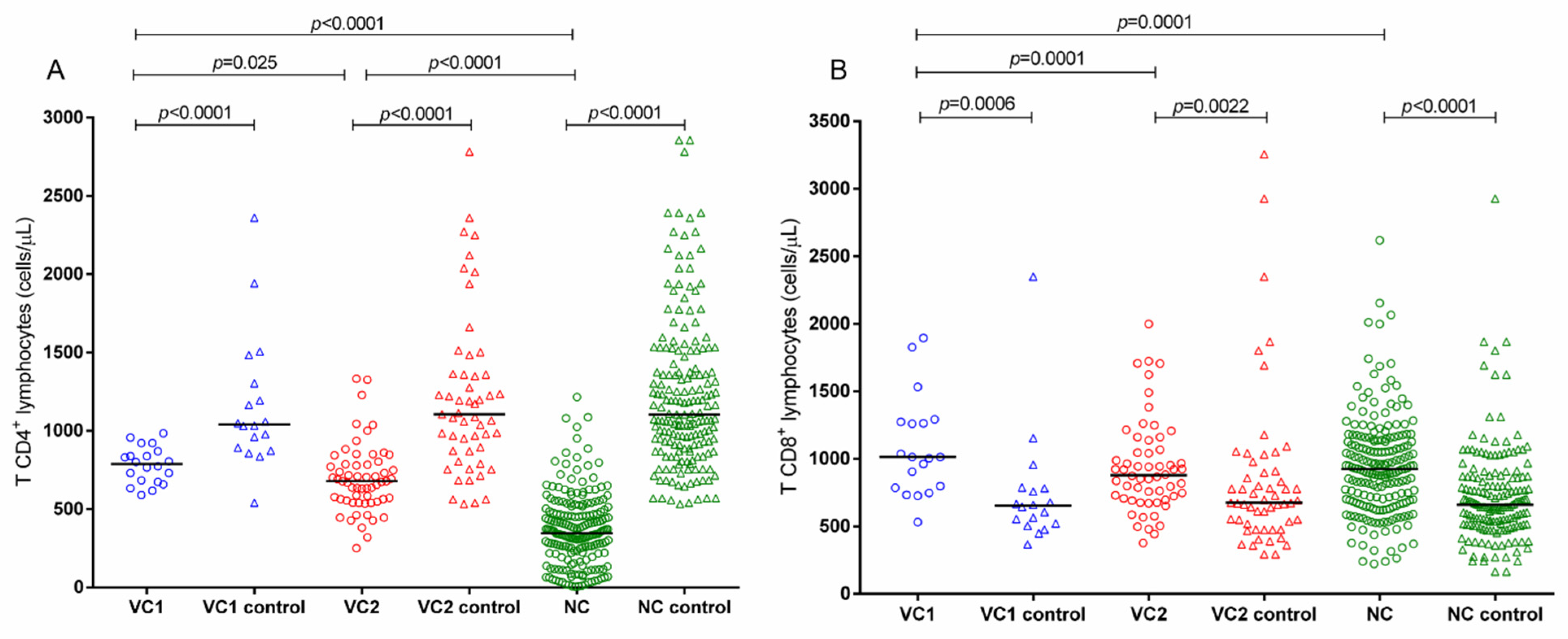
2.2. Associations between CD4+ T and CD8+ T Lymphocytes and HIV-1 Plasma Viral Load with Infection Progression and Genetic Variants
3. Discussion
4. Materials and Methods
4.1. Type of Study and Selection Criteria
4.2. Data Collection
4.3. Quantification of the Plasma Viral Load of HIV-1 and CD4+/CD8+ TLs
4.4. Identification of CCR5Δ32 and SDF1-3′A Polymorphisms
4.5. RNA Extraction
4.6. Reverse Transcription for Complementary DNA (cDNA) Synthesis
4.7. Gene Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 12 February 2022).
- Boletim Epidemioloógico HIV/AIDS—2020, Brasil. Available online: http://www.aids.gov.br/pt-br/pub/2020/boletim-epidemiologico-hivaids-2020 (accessed on 20 February 2022).
- Guerra, A.B.; Siravenha, L.Q.; Laurentino, R.V.; Feitosa, R.N.M.; Azevedo, V.N.; Vallinoto, A.C.R.; Ishak, R.; Machado, L.F.A. Seroprevalence of HIV, HTLV, CMV, HBV and rubella virus infections in pregnant adolescents who received care in the city of Belém, Pará, Northern Brazil. BMC Pregnancy Childbirth 2018, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Vallinoto, A.C.; Aguiar, S.; Sá, K.G.; Freitas, F.B.; Ferreira, G.; Lima, S.S.; Hermes, R.B.; Machado, L.F.; Cayres-Vallinoto, I.; Ishak, M.; et al. Prevalence and risk behaviour for human immunodeficiency virus 1 infection in Marajó Island, Northern Brazil. Ann. Hum. Biol. 2016, 43, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, A.B.; Silva, F.Q.; Santos, F.J.A.; Cardoso, Y.M.N.; Di Miceli, J.F.F.; Resque, R.L.; Silva-Oliveira, G.C.; Martins, L.C.; Pinheiro, L.M.L.; Machado, L.F.A.; et al. Prevalence and risk factors for HIV-1 infection in people who use illicit drugs in northern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Carrington, M.; Nelson, G.; O’Brien, S.J. Considering genetic profiles in functional studies of immune responsiveness to HIV-1. Immunol. Lett. 2001, 79, 131–140. [Google Scholar] [CrossRef]
- Grabar, S.; Selinger-Leneman, H.; Abgrall, S.; Pialoux, G.; Weiss, L.; Costagliola, D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 2009, 23, 1163–1169. [Google Scholar] [CrossRef]
- Olson, A.D.; Meyer, L.; Prins, M.; Thiebaut, R.; Gurdasani, D.; Guiguet, M.; Chaix, M.L.; Amornkul, P.; Babiker, A.; Sandhu, M.S.; et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS ONE 2014, 9, e86719. [Google Scholar] [CrossRef]
- Ruiz-Mateos, E.; Poveda, E.; Lederman, M.M. Antiretroviral Treatment for HIV Elite Controllers? Pathog. Immun. 2020, 5, 121–133. [Google Scholar] [CrossRef]
- Plaçais, L.; Boufassa, F.; Lécuroux, C.; Gardiennet, E.; Avettand-Fenoel, V.; Saez-Cirion, A.; Lambotte, O.; Noël, N.; ANRS CO21 study group. Antiretroviral therapy for HIV controllers: Reasons for initiation and outcomes in the French ANRS-CO21 CODEX cohort. EClinicalMedicine 2021, 37, 100963. [Google Scholar] [CrossRef]
- Cruz, N.V.; Amorim, R.; Oliveira, F.E.; Speranza, F.A.; Costa, L.J. Mutations in the nef and vif genes associated with progression to AIDS in elite controller and slow-progressor patients. J. Med. Virol. 2013, 85, 563–574. [Google Scholar] [CrossRef]
- Miura, T.; Brockman, M.A.; Brumme, C.J.; Brumme, Z.L.; Carlson, J.M.; Pereyra, F.; Trocha, A.; Addo, M.M.; Block, B.L.; Rothchild, A.C.; et al. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: Lack of gross genetic defects or common amino acid changes. J. Virol. 2008, 82, 8422–8430. [Google Scholar] [CrossRef]
- Pushker, R.; Jacqué, J.M.; Shields, D.C. Meta-analysis to test the association of HIV-1 nef amino acid differences and deletions with disease progression. J. Virol. 2010, 84, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Casado, C.; Marrero-Hernández, S.; Márquez-Arce, D.; Pernas, M.; Marfil, S.; Borràs-Grañana, F.; Olivares, I.; Cabrera-Rodríguez, R.; Valera, M.S.; de Armas-Rillo, L.; et al. Viral Characteristics Associated with the Clinical Nonprogressor Phenotype Are Inherited by Viruses from a Cluster of HIV-1 Elite Controllers. mBio 2018, 9, e02338-17. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; O’Connell, K.; Yang, H.C.; Han, Y.; Xu, J.; Jilek, B.; Williams, T.M.; Ray, S.C.; Siliciano, R.F.; Blankson, J.N. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J. Virol. 2008, 82, 7395–7410. [Google Scholar] [CrossRef] [PubMed]
- D’Ettorre, G.; Paiardini, M.; Zaffiri, L.; Andreotti, M.; Ceccarelli, G.; Rizza, C.; Indinnimeo, M.; Vella, S.; Mastroianni, C.M.; Silvestri, G.; et al. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr. HIV Res. 2011, 9, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, M.; Raposo, R.A.; Deng, X.; Li, M.; Liegler, T.; Sinclair, E.; Salama, M.S.; Ghanem, H.-D.; Hoh, R.; Wong, J.K.; et al. Expression profile of host restriction factors in HIV-1 elite controllers. Retrovirology 2013, 10, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, A.; Koga, M.; Mizutani, T.; Parbie, P.K.; Prawisuda, D.; Yusa, N.; Sedohara, A.; Kikuchi, T.; Ikeuchi, K.; Adachi, E.; et al. Unique Gut Microbiome in HIV Patients on Antiretroviral Therapy (ART) Suggests Association with Chronic Inflammation. Microbiol. Spectr. 2021, 9, e0070821. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Conley, A.J.; Brewah, Y.A.; Jones, G.M.; Leath, S.; Boots, L.J.; Davey, V.; Pantaleo, G.; Demarest, J.F.; Carter, C.; et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1995, 1, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Goulder, P.J.; Phillips, R.E.; Colbert, R.A.; McAdam, S.; Ogg, G.; Nowak, M.A.; Giangrande, P.; Luzzi, G.; Morgan, B.; Edwards, A.; et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 1997, 3, 212–217. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kunstman, J.; Kuroda, M.J.; Schmitz, J.E.; Santra, S.; Peyerl, F.W.; Krivulka, G.R.; Beaudry, K.; Lifton, M.A.; Gorgone, D.A.; et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 2002, 415, 335–339. [Google Scholar] [CrossRef]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 7, 2709–2714. [Google Scholar] [CrossRef]
- Carrington, M.; O’Brien, S.J. The influence of HLA genotype on AIDS. Annu. Rev. Med. 2003, 54, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Kiepiela, P.; Ngumbela, K.; Thobakgale, C.; Ramduth, D.; Honeyborne, I.; Moodley, E.; Reddy, S.; de Pierres, C.; Mncube, Z.; Mkhwanazi, N.; et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 2007, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Lassen, K.G.; Arts, E.J. HIV-1 replicative fitness in elite controllers. Curr. Opin. HIV AIDS 2011, 6, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Le, A.Q.; Shahid, A.; Brumme, Z.L. HIV-1 mutational escape from host immunity. In Encyclopedia of AIDS; Hope, T., Stevenson, M., Richman, D., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- May, M.E.; Pohlmeyer, C.W.; Kwaa, A.K.; Mankowski, M.C.; Bailey, J.R.; Blankson, J.N. Combined Effects of HLA-B*57/5801 Elite Suppressor CD8+ T Cells and NK Cells on HIV-1 Replication. Front. Cell. Infect. Microbiol. 2020, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Limou, S.; Le Clerc, S.; Coulonges, C.; Carpentier, W.; Dina, C.; Delaneau, O.; Labib, T.; Taing, L.; Sladek, R.; Deveau, C.; et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 2009, 199, 419–426. [Google Scholar] [CrossRef]
- Ballana, E.; Ruiz-de Andres, A.; Mothe, B.; Ramirez de Arellano, E.; Aguilar, F.; Badia, R.; Grau, E.; Clotet, B.; del Val, M.; Brander, C.; et al. Differential prevalence of the HLA-C—35 CC genotype among viremic long term non-progressor and elite controller HIV+ individuals. Immunobiology 2012, 217, 889–894. [Google Scholar] [CrossRef]
- Dragic, T.; Trkola, A.; Lin, S.W.; Nagashima, K.A.; Kajumo, F.; Zhao, L.; Olson, W.C.; Wu, L.; Mackay, C.R.; Allaway, G.P.; et al. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J. Virol. 1998, 72, 279–285. [Google Scholar] [CrossRef]
- Carrington, M.; Kissner, T.; Gerrard, B.; Ivanov, S.; O’Brien, S.J.; Dean, M. Novel alleles of the chemokine-receptor gene CCR5. Am. J. Hum. Genet. 1997, 61, 1261–1267. [Google Scholar] [CrossRef]
- Li, H.; Xie, H.Y.; Zhou, L.; Wang, W.L.; Liang, T.B.; Zhang, M.; Zheng, S.S. Polymorphisms of CCL3L1/CCR5 genes and recurrence of hepatitis B in liver transplant recipients. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 593–598. [Google Scholar] [CrossRef]
- Li, J.Z.; Arnold, K.B.; Lo, J.; Dugast, A.S.; Plants, J.; Ribaudo, H.J.; Cesa, K.; Heisey, A.; Kuritzkes, D.R.; Lauffenburger, D.A.; et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect. Dis 2015, 2, ofu117. [Google Scholar] [CrossRef]
- De Faria Junior, G.M.; Ayo, C.M.; de Oliveira, A.P.; Lopes, A.G.; Frederico, F.B.; Silveira-Carvalho, A.P.; Previato, M.; Barbosa, A.P.; Murata, F.H.A.; de Almeida Junior, G.C.; et al. CCR5 chemokine receptor gene polymorphisms in ocular toxoplasmosis. Acta Trop. 2018, 178, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Koor, G.W.; Paximadis, M.; Picton, A.C.P.; Karatas, F.; Loubser, S.A.; He, W.; Ahuja, S.K.; Chaisson, R.E.; Martinson, N.; Ebrahim, O.; et al. Cis-regulatory genetic variants in the CCR5 gene and natural HIV-1 control in black South Africans. Clin. Immunol. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zaunders, J.; Dyer, W.B.; Churchill, M.; Munier, C.M.L.; Cunningham, P.H.; Suzuki, K.; McBride, K.; Hey-Nguyen, W.; Koelsch, K.; Wang, B.; et al. Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5 Δ32 heterozygous and HLA-B57 genotype. J. Virus Erad. 2019, 5, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Paxton, W.A.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; MacDonald, M.E.; Stuhlmann, H.; Koup, R.A.; Landau, N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef] [PubMed]
- McLaren, P.J.; Coulonges, C.; Ripke, S.; van den Berg, L.; Buchbinder, S.; Carrington, M.; Cossarizza, A.; Dalmau, J.; Deeks, S.G.; Delaneau, O.; et al. Association study of common genetic variants and HIV-1 acquisition in 6300 infected cases and 7200 controls. PLoS Pathog. 2013, 9, e1003515. [Google Scholar]
- Matti, C.; Legler, D.F. CCR5 deficiency/CCR5Δ32: Resistant to HIV infection at the cost of curtailed CD4+ T cell memory responses. EMBO J. 2020, 39, e105854. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Winkler, C.; Modi, W.; Smith, M.W.; Nelson, G.W.; Wu, X.; Carrington, M.; Dean, M.; Honjo, T.; Tashiro, K.; Yabe, D.; et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC). Science 1998, 279, 389–393. [Google Scholar] [CrossRef]
- Garcia-Moruja, C.; Rueda, P.; Torres, C.; Alcamí, J.; Luque, F.; Caruz, A. Molecular phenotype of CXCL12beta 3′UTR G801A polymorphism (rs1801157) associated to HIV-1 disease progression. Curr. HIV Res. 2009, 7, 384–389. [Google Scholar] [CrossRef]
- Armani-Tourret, M.; Zhou, Z.; Gasser, R.; Staropoli, I.; Cantaloube-Ferrieu, V.; Benureau, Y.; Garcia-Perez, J.; Pérez-Olmeda, M.; Lorin, V.; Puissant-Lubrano, B.; et al. Mechanisms of HIV-1 evasion to the antiviral activity of chemokine CXCL12 indicate potential links with pathogenesis. PLoS Pathog. 2021, 17, e1009526. [Google Scholar] [CrossRef]
- Hartana, C.A.; Rassadkina, Y.; Gao, C.; Martin-Gayo, E.; Walker, B.D.; Lichterfeld, M.; Yu, X.G. Long noncoding RNA MIR4435-2HG enhances metabolic function of myeloid dendritic cells from HIV-1 elite controllers. J. Clin. Investig. 2021, 131, e146136. [Google Scholar] [CrossRef] [PubMed]
- Koofhethile, C.K.; Ndhlovu, Z.M.; Thobakgale-Tshabalala, C.; Prado, J.G.; Ismail, N.; Mncube, Z.; Mkhize, L.; van der Stok, M.; Yende, N.; Walker, B.D.; et al. CD8+ T Cell Breadth and Ex Vivo Virus Inhibition Capacity Distinguish between Viremic Controllers with and without Protective HLA Class I Alleles. J. Virol. 2016, 90, 6818–6831. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, F.; Addo, M.M.; Kaufmann, D.E.; Liu, Y.; Miura, T.; Rathod, A.; Baker, B.; Trocha, A.; Rosenberg, R.; Mackey, E.; et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 2008, 197, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.T.M.; Gomes, É.R.; Dos Santos, M.B.; Lima, S.S.; Queiroz, M.A.F.; Machado, L.F.A.; Cayres-Vallinoto, I.M.V.; Vallinoto, A.C.R.; de O Guimarães Ishak, M.; Ishak, R. Immunological and virological characterization of HIV-1 viremia controllers in the North Region of Brazil. BMC Infect. Dis. 2017, 17, 381. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.T.M.; da Silva Graça Amoras, E.; Gomes, É.R.; Queiroz, M.A.F.; Júnior, E.C.S.; de Vasconcelos Massafra, J.M.; da Silva Lemos, P.; Júnior, J.L.V.; Ishak, R.; Vallinoto, A.C.R. Immune escape mutations in HIV-1 controllers in the Brazilian Amazon region. BMC Infect. Dis. 2020, 20, 546. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.S.; Keating, S.M.; Abdel-Mohsen, M.; Gibb, S.L.; Heitman, J.W.; Inglis, H.C.; Martin, J.N.; Zhang, J.; Kaidarova, Z.; Deng, X.; et al. Cytokines Elevated in HIV Elite Controllers Reduce HIV Replication In Vitro and Modulate HIV Restriction Factor Expression. J. Virol. 2017, 91, e02051-16. [Google Scholar] [CrossRef]
- International HIV Controllers Study; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.W.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar] [CrossRef]
- Tiemessen, C.T. The many faces of HIV elite control. EBioMedicine 2021, 66, 103305. [Google Scholar] [CrossRef]
- Sajadi, M.M.; Constantine, N.T.; Mann, D.L.; Charurat, M.; Dadzan, E.; Kadlecik, P.; Redfield, R.R. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J. Acquir. Immune Defic. Syndr. 2009, 50, 403–408. [Google Scholar] [CrossRef]
- Lambotte, O. HIV controllers: How these patients control viral replication? Med. Sci. 2012, 28, 172–178. [Google Scholar]
- Kiros, Y.K.; Elinav, H.; Gebreyesus, A.; Gebremeskel, H.; Azar, J.; Chemtob, D.; Abreha, H.; Elbirt, D.; Shahar, E.; Chowers, M.; et al. Identification and characterization of HIV positive Ethiopian elite controllers in both Africa and Israel. HIV Med. 2019, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Jung, N.; Trapp, S.; Flossdorf, P.; Meyer-Olson, D.; Schulze Zur Wiesch, J.; Stephan, C.; Mauss, S.; Weiss, V.; von Bergwelt-Baildon, M.; et al. Cytokine and Chemokine Signature in Elite Versus Viremic Controllers Infected with HIV. AIDS Res. Hum. Retrovir. 2016, 32, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Song, K.; Sauer, M.M.; Nason, M.C.; Giret, M.T.; Carvalho, K.I.; Costa, P.R.; Roederer, M.; Kallás, E.G. Early immunologic and virologic predictors of clinical HIV-1 disease progression. AIDS 2013, 27, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.E.B.; Guerreiro, J.F. The indigenous contribution to the formation of the population of the Brazilian Amazon region. Rev. Bras. Genet. 1995, 18, 311–315. [Google Scholar]
- Pimenta, J.R.; Zuccherato, L.W.; Debes, A.A.; Maselli, L.; Soares, R.P.; Moura-Neto, R.S.; Rocha, J.; Bydlowski, S.P.; Pena, S.D. Color and genomic ancestry in Brazilians: A study with forensic microsatellites. Hum. Hered. 2006, 62, 190–195. [Google Scholar] [CrossRef]
- Pena, S.D.; Di Pietro, G.; Fuchshuber-Moraes, M.; Genro, J.P.; Hutz, M.H.; Kehdy, F.S.; Kohlrausch, F.; Magno, L.A.; Montenegro, R.C.; Moraes, M.O.; et al. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS ONE 2011, 6, e17063. [Google Scholar] [CrossRef] [PubMed]
- Salzano, F.M.; Sans, M. Interethnic admixture and the evolution of Latin American populations. Genet. Mol. Biol. 2014, 37 (Suppl. 1), 151–170. [Google Scholar] [CrossRef]
- Carvalhaes, F.A.P.L.; Carvalho, M.I.M.; Guerreiro, J.F. The Mutation delta CCR5 in human populations of the Amazon. Genet. Mol. Biol. 1999, 22, 613. [Google Scholar]
- Grimaldi, R.; Shindo, N.; Acosta, A.X.; Dourado, I.; Brites, C.; de Melo Carvalho, O.; Brito, I.; Bou-Habib, D.C.; Galvão-Castro, B. Prevalence of the CCR5Delta32 mutation in Brazilian populations and cell susceptibility to HIV-1 infection. Hum. Genet. 2002, 111, 102–104. [Google Scholar] [CrossRef]
- Vargas, A.E.; Marrero, A.R.; Salzano, F.M.; Bortolini, J.A.B.; Chies, J.A.B. Frequency of CCR5D32 in Brazilian populations. Braz. J. Med. Biol. Res. 2006, 39, 321–325. [Google Scholar] [CrossRef]
- Boldt, A.B.; Culpi, L.; Tsuneto, L.T.; Souza, I.R.; Kun, J.F.; Petzl-Erler, M.L. Analysis of the CCR5 gene coding region diversity in five South American populations reveals two new non-synonymous alleles in Amerindians and high CCR5*D32 frequency in Euro-Brazilians. Genet. Mol. Biol. 2009, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Munerato, P.; Azevedo, M.L.; Sucupira, M.C.; Pardini, R.; Pinto, G.H.; Catroxo, M.; Souza, I.E.; Diaz, R.S. Frequency of polymorphisms of genes coding for HIV-1 co-receptors CCR5 and CCR2 in a Brazilian population. Braz. J. Infect. Dis. 2003, 7, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, F.A.P.L.; Cardoso, G.L.; Vallinoto, A.C.R.; Machado, L.F.A.; Ishak, M.O.G.; Ishak, R.; Guerreiro, J.F. Frequencies of CCR5∆32, CCR2-64I and SDF1-3′A mutations in human immunodeficiency virus (HIV) seropositive subjects and seronegative individuals from the state of Pará in Brazilian Amazonia. Genet. Mol. Biol. 2005, 28, 665–669. [Google Scholar] [CrossRef]
- Donyavi, T.; Bokharaei-Salim, F.; Nahand, J.S.; Garshasbi, S.; Esghaei, M.; Sadeghi, M.; Jamshidi, S.; Khanaliha, K. Evaluation of CCR5-Δ32 mutation among individuals with high risk behaviors, neonates born to HIV-1 infected mothers, HIV-1 infected individuals, and healthy people in an Iranian population. J. Med. Virol. 2020, 92, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.M.; Saragosti, S.; Lapoumeroulie, C.; Cognaux, J.; Forceille, C.; et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996, 382, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Leboute, A.P.; de Carvalho, M.W.; Simões, A.L. Absence of the deltaccr5 mutation in indigenous populations of the Brazilian Amazon. Hum. Genet. 1999, 105, 442–443. [Google Scholar]
- Lewandowska, M.; Franciszkiewicz, K.; Prokop, J.; Ofori, H.; Jagodzinski, P.P. Distribution of two HIV-1-resistant polymorphisms (SDF1-3′A and CCR2-64I alleles) in the Polish population. J. Hum. Genet. 2002, 47, 585–589. [Google Scholar] [CrossRef]
- Su, Q.; Lu, Z.; Tang, Y.; Wei, S.; Zhou, Z.; Jiang, F. Distribution of CCR5-{delta}32, CCR2-64I, and SDF1-3′A in Guangxi Zhuang population. J. Int. Assoc. Physicians AIDS Care 2010, 9, 145–159. [Google Scholar]
- Clapham, P.R. HIV and chemokines: Ligands sharing cell-surface receptors. Trends Cell. Biol. 1997, 7, 264–268. [Google Scholar] [CrossRef]
- Kuritzkes, D.R. HIV pathogenesis and viral markers HIV/AIDS. Clin. Manag. 2000, 2, 1–27. [Google Scholar]
- Gornalusse, G.G.; Mummidi, S.; Gaitan, A.A.; Jimenez, F.; Ramsuran, V.; Picton, A.; Rogers, K.; Manoharan, M.S.; Avadhanam, N.; Murthy, K.K.; et al. Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc. Natl. Acad. Sci. USA 2015, 112, E4762–E4771. [Google Scholar] [CrossRef] [PubMed]
- Mehlotra, R.K. CCR5 Promoter Polymorphism -2459G > A: Forgotten or Ignored? Cells 2019, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Lied, A.; Kulkarni, V.; Rucevic, M.; Martin, M.P.; Walker-Sperling, V.; Anderson, S.K.; Ewy, R.; Singh, S.; Nguyen, H.; et al. CCR5AS lncRNA variation differentially regulates CCR5, influencing HIV disease outcome. Nat. Immunol. 2019, 20, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Agosto-Mojica, A.; Aalinkeel, R.; Reynolds, J.L.; Nair, B.B.; Sykes, D.E.; Martinez, J.; Adams, J.; Singh, N.; Bernstein, Z.; et al. Role of chemokine and cytokine polymorphisms in the progression of HIV-1 disease. Biochem. Biophys. Res. Commun. 2010, 396, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, F.H.; Passaes, C.P.; Bello, G.; Teixeira, S.L.; Vorsatz, C.; Babic, D.; Sharkey, M.; Grinsztejn, B.; Veloso, V.; Stevenson, M.; et al. HIV controllers with different viral load cutoff levels have distinct virologic and immunologic profiles. J. Acquir. Immune Defic. Syndr. 2015, 68, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Carrington, M.; Winkler, C.; Huttley, G.A.; Smith, M.W.; Allikmets, R.; Goedert, J.J.; Buchbinder, S.P.; Vittinghoff, E.; Gomperts, E.; et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 1996, 273, 1856–1862. [Google Scholar]
- Huang, Y.; Paxton, W.A.; Wolinsky, S.M.; Neumann, A.U.; Zhang, L.; He, T.; Kang, S.; Ceradini, D.; Jin, Z.; Yazdanbakhsh, K.; et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996, 2, 1240–1243. [Google Scholar] [CrossRef]
- Bratt, G.; Leandersson, A.C.; Albert, J.; Sandström, E.; Wahren, B. MT-2 tropism and CCR-5 genotype strongly influence disease progression in HIV-1-infected individuals. AIDS 1998, 12, 729–736. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Rosenberg, P.S.; Goedert, J.J.; Ashton, L.J.; Benfield, T.L.; Buchbinder, S.P.; Coutinho, R.A.; Eugen-Olsen, J.; Gallart, T.; Katzenstein, T.L.; et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1-3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann. Intern. Med. 2001, 135, 782–795. [Google Scholar] [CrossRef]
- Hladik, F.; Liu, H.; Speelmon, E.; Livingston-Rosanoff, D.; Wilson, S.; Sakchalathorn, P.; Hwangbo, Y.; Greene, B.; Zhu, T.; McElrath, M.J. Combined effect of CCR5-Delta32 heterozygosity and the CCR5 promoter polymorphism -2459 A/G on CCR5 expression and resistance to human immunodeficiency virus type 1 transmission. J. Virol. 2005, 79, 11677–11684. [Google Scholar] [CrossRef]
- Koot, M.; Keet, I.P.; Vos, A.H.; de Goede, R.E.; Roos, M.T.; Coutinho, R.A.; Miedema, F.; Schellekens, P.T.; Tersmette, M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 1993, 118, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sucupira, M.C.; Sanabani, S.; Cortes, R.M.; Giret, M.T.; Tomiyama, H.; Sauer, M.M.; Sabino, E.C.; Janini, L.M.; Kallas, E.G.; Diaz, R.S. Faster HIV-1 disease progression among Brazilian individuals recently infected with CXCR4-utilizing strains. PLoS ONE 2012, 7, e30292. [Google Scholar] [CrossRef] [PubMed]
- Rosás-Umbert, M.; Llano, A.; Bellido, R.; Olvera, A.; Ruiz-Riol, M.; Rocafort, M.; Fernández, M.A.; Cobarsi, P.; Crespo, M.; Dorrell, L.; et al. Mechanisms of Abrupt Loss of Virus Control in a Cohort of Previous HIV Controllers. J. Virol. 2019, 93, e01436-18. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Martínez, C.; García, F.; Plana, M.; Palou, E.; Lejeune, M.; Aróstegui, J.I.; De Lazzari, E.; Rodriguez, C.; Barrasa, A.; et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3′A genotype, and expression of CXCR4 on T lymphocytes: Their impact on resistance to human immunodeficiency virus type 1 infection and its progression. J. Infect. Dis. 2002, 186, 922–931. [Google Scholar] [CrossRef]
- Reiche, E.M.; Watanabe, M.A.; Bonametti, A.M.; Morimoto, H.K.; Morimoto, A.A.; Wiechmann, S.L.; Matsuo, T.; Miranda, H.C.; Reiche, F.V.; Oliveira, K.B. Stromal cell-derived factor 1 (SDF1) genetic polymorphism in a sample of healthy individuals, seronegative individuals exposed to human immunodeficiency virus type 1 (HIV-1) and patients infected with HIV-1 from the Brazilian population. Int. J. Immunogenet. 2006, 33, 127–133. [Google Scholar] [CrossRef]
- Protocolo Clínico e Diretrizes Terapêuticas Para Manejo da Infecção Pelo HIV em Adultos—Brasil. Available online: http://www.aids.gov.br/pt-br/pub/2013/protocolo-clinico-e-diretrizes-terapeuticas-para-manejo-da-infeccao-pelo-hiv-em-adultos (accessed on 12 March 2022).
- Dezzutti, C.S.; Guenthner, P.C.; Green, T.A.; Cohen, O.J.; Spira, T.J.; Lal, R.B. Stromal-derived factor-1 chemokine gene variant is associated with the delay of HIV-1 disease progression in two longitudinal cohorts. AIDS 2000, 14, 894–896. [Google Scholar] [CrossRef]
- Wei, M.; Rong, C.; Zhao, J.; Liu, X.; Yang, F.; Zeng, J. Role of SDF-1 3′A polymorphism in HIV-1 disease progression: A systematic review and meta-analysis. Gene 2018, 677, 182–188. [Google Scholar] [CrossRef]
- Van Rij, R.P.; Broersen, S.; Goudsmit, J.; Coutinho, R.A.; Schuitemaker, H. The role of a stromal cell-derived factor-1 chemokine gene variant in the clinical course of HIV-1 infection. AIDS 1998, 12, F85–F90. [Google Scholar] [CrossRef]
- Brambilla, A.; Villa, C.; Rizzardi, G.; Veglia, F.; Ghezzi, S.; Lazzarin, A.; Cusini, M.; Muratori, S.; Santagostino, E.; Gringeri, A.; et al. Shorter survival of SDF1-3′A/3′A homozygotes linked to CD4+ T cell decrease in advanced human immunodeficiency virus type 1 infection. J. Infect. Dis. 2000, 182, 311–315. [Google Scholar] [CrossRef][Green Version]
- Daar, E.S.; Lynn, H.S.; Donfield, S.M.; Lail, A.; O′Brien, S.J.; Huang, W.; Winkler, C.A.; Hemophilia Growth and Development Study. Stromal cell-derived factor-1 genotype, coreceptor tropism, and HIV type 1 disease progression. J. Infect. Dis. 2005, 192, 1597–1605. [Google Scholar] [CrossRef]
- Gianesin, K.; Freguja, R.; Carmona, F.; Zanchetta, M.; Del Bianco, P.; Malacrida, S.; Montagna, M.; Rampon, O.; Giaquinto, C.; De Rossi, A. The Role of Genetic Variants of Stromal Cell-Derived Factor 1 in Pediatric HIV-1 Infection and Disease Progression. PLoS ONE 2012, 7, e44460. [Google Scholar] [CrossRef]
- Mehlotra, R.K.; Hall, N.B.; Bruse, S.E.; John, B.; Zikursh, M.J.B.; Stein, C.M.; Siba, P.M.; Zimmerman, P.A. CCR2, CCR5, and CXCL12 variation and HIV/AIDS in Papua New Guinea. Infect. Genet. Evol. 2015, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tiensiwakul, P. Stromal cell-derived factor (SDF) 1-3′A polymorphism may play a role in resistance to HIV-1 infection in seronegative high-risk Thais. Intervirology 2004, 47, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Passam, A.M.; Zafiropoulos, A.; Miyakis, S.; Zagoreos, I.; Stavrianeas, N.G.; Krambovitis, E.; Spandidos, D.A. CCR2-64I and CXCL12 3′A alleles confer a favorable prognosis to AIDS patients undergoing HAART therapy. J. Clin. Virol. 2005, 34, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yeregui, E.; Viladés, C.; Domingo, P.; Ceausu, A.; Pacheco, Y.M.; Veloso, S.; Inciarte, A.; Vidal-González, J.; Peraire, M.; Perpiñán, C. High circulating SDF-1and MCP-1 levels and genetic variations in CXCL12, CCL2 and CCR5: Prognostic signature of immune recovery status in treated HIV-positive patients. EBioMedicine 2020, 62, 103077. [Google Scholar] [CrossRef]
- Ikegawa, M.; Yuan, J.; Matsumoto, K.; Herrmann, S.; Iwamoto, A.; Nakamura, T.; Matsushita, S.; Kimura, T.; Honjo, T.; Tashiro, K. Elevated plasma stromal cell-derived factor 1 protein level in the progression of HIV type 1 infection/AIDS. AIDS Res. Hum. Retrovir. 2001, 17, 587–595. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, R.B.; Singh, K.; Bhasin, R.; Anand Shukla, A.; Chauhan, S.S.; Luthra, K. Distribution of CCR5delta32, CCR2-64I and SDF1-3′A and plasma levels of SDF-1 in HIV-1 seronegative North Indians. J. Clin. Virol. 2007, 38, 198–203. [Google Scholar] [CrossRef]
- Gonzalez, E.; Dhanda, R.; Bamshad, M.; Mummidi, S.; Geevarghese, R.; Catano, G.; Anderson, S.A.; Walter, E.A.; Stephan, K.T.; Hammer, M.F.; et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1alpha: Impact on the epidemiology of the HIV-1 pandemic. Proc. Natl. Acad. Sci. USA 2001, 98, 5199–5204. [Google Scholar] [CrossRef]
- Martin, M.P.; Carrington, M. Immunogenetics of HIV disease. Immunol. Rev. 2013, 254, 245–264. [Google Scholar] [CrossRef]
- McLaren, P.J.; Coulonges, C.; Bartha, I.; Lenz, T.L.; Deutsch, A.J.; Bashirova, A.; Buchbinder, S.; Carrington, M.N.; Cossarizza, A.; Dalmau, J.; et al. Polymorphisms of large effect explain the majority of the host genetic contribution to variation of HIV-1 virus load. Proc. Natl. Acad. Sci. USA 2015, 112, 14658–14663. [Google Scholar] [CrossRef]
- Mehlotra, R.K.; Dazard, J.E.; John, B.; Zimmerman, P.A.; Weinberg, A.; Jurevic, R.J. Copy Number Variation within Human β-Defensin Gene Cluster Influences Progression to AIDS in the Multicenter AIDS Cohort Study. J. AIDS Clin. Res. 2012, 3, 1000184. [Google Scholar] [CrossRef] [PubMed]
- Willie, B.; Hall, N.B.; Stein, C.M.; Jurevic, R.J.; Weinberg, A.; Mehlotra, R.K.; Zimmerman, P.A. Association of Toll-like receptor polymorphisms with HIV status in North Americans. Genes. Immun. 2014, 15, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Boritz, E.A.; Darko, S.; Swaszek, L.; Wolf, G.; Wells, D.; Wu, X.; Henry, A.R.; Laboune, F.; Hu, J.; Ambrozak, D. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell 2016, 166, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Picado, J.; Deeks, S.G. Persistent HIV-1 replication during antiretroviral therapy. Curr. Opin. HIV AIDS 2016, 11, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.W.; Margolick, J.B.; Phair, J.P.; Rinaldo, C.R.; Detels, R.; Jacobson, L.P.; Muñoz, A. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA 2007, 297, 2349–2350. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; McCune, J.M.; Roland, M.; Page-Shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Lampiris, H.; et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 2008, 197, 126–133. [Google Scholar] [CrossRef]
- Okulicz, J.F.; Marconi, V.C.; Landrum, M.L.; Wegner, S.; Weintrob, A.; Ganesan, A.; Hale, B.; Crum-Cianflone, N.; Delmar, J.; Barthel, V.; et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J. Infect. Dis. 2009, 200, 1714–1723. [Google Scholar] [CrossRef]
- Bello, G.; Velasco-de-Castro, C.A.; Bongertz, V.; Rodrigues, C.A.; Giacoia-Gripp, C.B.; Pilotto, J.H.; Grinsztejn, B.; Veloso, V.G.; Morgado, M.G. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J. Med. Virol. 2009, 81, 1681–1690. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Rastegar, D.A.; O’Connell, K.A.; Dinoso, J.B.; Wilke, C.O.; Blankson, J.N. T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin. Infect. Dis. 2009, 49, 1763–1766. [Google Scholar] [CrossRef]
- Margolick, J.B.; Muñoz, A.; Donnenberg, A.D.; Park, L.P.; Galai, N.; Giorgi, J.V.; O’Gorman, M.R.; Ferbas, J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat. Med. 1995, 1, 674–680. [Google Scholar] [CrossRef]
- Lécuroux, C.; Sáez-Cirión, A.; Girault, I.; Versmisse, P.; Boufassa, F.; Avettand-Fenoël, V.; Rouzioux, C.; Meyer, L.; Pancino, G.; Lambotte, O.; et al. Both HLA-B*57 and plasma HIV RNA levels contribute to the HIV-specific CD8+ T cell response in HIV controllers. J. Virol. 2014, 88, 176–187. [Google Scholar] [CrossRef]
- Martins, M.A.; Tully, D.C.; Cruz, M.A.; Power, K.A.; Veloso de Santana, M.G.; Bean, D.J.; Ogilvie, C.B.; Gadgil, R.; Lima, N.S.; Magnani, D.M.; et al. Vaccine-Induced Simian Immunodeficiency Virus-Specific CD8+ T-Cell Responses Focused on a Single Nef Epitope Select for Escape Variants Shortly after Infection. J. Virol. 2015, 89, 10802–10820. [Google Scholar] [CrossRef] [PubMed]
- Tansiri, Y.; Rowland-Jones, S.L.; Ananworanich, J.; Hansasuta, P. Clinical outcome of HIV Viraemic controllers and Noncontrollers with normal CD4 counts is exclusively determined by antigen-specific CD8+ T-cell-mediated HIV suppression. PLoS ONE 2015, 10, e0118871. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Deleage, C.; Darko, S.; Ransier, A.; Truong, D.P.; Agarwal, D.; Japp, A.S.; Wu, V.H.; Kuri-Cervantes, L.; Abdel-Mohsen, M.; et al. Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8+ T cells. Sci. Transl. Med. 2019, 11, eaax4077. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, M.S.; Soares, L.S.; Galvão-Lima, L.J.; Zambuzi, F.A.; Cacemiro, M.C.; Brauer, V.S.; Frantz, F.G. HIV infection: Focus on the innate immune cells. Immunol. Res. 2016, 64, 1118–1132. [Google Scholar] [CrossRef]
- Kløverpris, H.N.; Kazer, S.W.; Mjösberg, J.; Mabuka, J.M.; Wellmann, A.; Ndhlovu, Z.; Yadon, M.C.; Nhamoyebonde, S.; Muenchhoff, M.; Simoni, Y.; et al. Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 2016, 44, 391–405. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol: Chloroform. CSH Protoc. 2006, 2006, pdb.prot4455. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| VC1 (n = 2) | VC2 (n = 8) | NC. (n = 28) | p | ||||
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 1 | (50%) | 3 | (37.5%) | 16 | (57%) | 0.6571 |
| Female | 1 | (50%) | 5 | (62.6%) | 12 | (43%) | |
| Age (median; Q1 and Q3) | 39.5 | (38–41) | 31 | (28–36) | 35 | (28–39) | 0.5695 |
| Number of school years | |||||||
| Illiterate | 0 | (0) | 1 | (12.5%) | 0 | (0) | 0.9098 |
| Primary Education | 1 | (50%) | 3 | (37.5%) | 15 | (53%) | |
| Secondary Education | 1 | (50%) | 3 | (37.5%) | 10 | (36%) | |
| Higher Education | 0 | (0) | 1 | (12.5%) | 3 | (11%) | |
| Sexual behavior | |||||||
| Heterosexual | 2 | (100%) | 6 | (75%) | 18 | (64%) | 0.5251 |
| Homosexual man | 0 | (0) | 0 | (0) | 5 | (18%) | |
| Bisexual | 0 | (0) | 2 | (25%) | 5 | (18%) | |
| Other risk behavior | |||||||
| IVDU | 0 | (0) | 0 | (0) | 2 | (7%) | 0.7305 |
| NIDU | 1 | (50%) | 5 | (62%) | 18 | (64%) | 0.9340 |
| Sexual intercourse without a condom | 1 | (50%) | 2 | (25%) | 13 | (46%) | 0.5759 |
| Anal sex | 0 | (0) | 4 | (50%) | 17 | (60%) | 0.2080 |
| Sex with sex worker | 1 | (50%) | 0 | (0) | 5 | (18%) | 0.2178 |
| HIV+ partner | 2 | (100%) | 3 | (37%) | 14 | (50%) | 0.2430 |
| Genotype and Allele Profile | VC1 (n = 2) n (%) | VC2 (n = 8) n (%) | NC (n = 28) n (%) | Control Group (n = 300) n (%) | p |
|---|---|---|---|---|---|
| CCR5Δ32 | |||||
| CCR5/CCR5 | 2 (100) | 8 (100) | 24 (85.7) | 280 (93.33) | 0.7223 |
| CCR5/Δ32 | 0 | 0 | 4 (14.3) | 19 (6.34) | |
| Δ32/Δ32 | 0 | 0 | 0 | 1 (0.33) | |
| * CCR5 | 4 (100) | 16 (100) | 52 (92.86) | 579 (96.5) | 0.3836 |
| * Δ32 | 0 | 0 | 4 (7.14) | 21 (3.5) | |
| SDF1-3′A | |||||
| G/G | 1 (50) | 7 (87.5) | 12 (42.86) | 180 (60.0) | 0.0779 |
| G/A | 0 | 1 (12.5) | 15 (53.57) | 107 (35.67) | |
| A/A | 1 (50) | 0 | 1 (3.57) | 13 (4.33) | |
| * G | 2 (50) | 15 (93.75) | 39 (69.64) | 467 (77.83) | 0.0910 |
| * A | 2 (50) | 1 (6.25) | 17 (30.36) | 133 (22.17) |
| Alleles | Wild-Type (n = 18) | ∆32 (n = 4) | 3′A (n = 18) | p | |||
|---|---|---|---|---|---|---|---|
| Total quantifications | 153 | - | 35 | - | 142 | - | |
| Viral Load | n | % | n | % | n | % | <0.0001 * |
| <50 | 18 | 11.8 | 2 | 5.7 | 6 | 4.2 | |
| 50|—1000 | 33 | 21.6 | 11 | 31.4 | 16 | 11.3 | |
| 1000|—10,000 | 59 | 38.5 | 15 | 42. | 45 | 31.7 | |
| 10,000|—100,000 | 36 | 23.5 | 4 | 11.4 | 68 | 47.9 | |
| ≥100,000 | 7 | 4.6 | 3 | 8.6 | 7 | 4.9 | |
| Groups According to Disease Progression | Males Age (Range) | Females Age (Range) | Length of Infection (Years) | CD4 + T Lymphocytes | HIV-1 Viral Load | Remarks |
|---|---|---|---|---|---|---|
| VC1 VIREMIA CONTROLLERS1 | 1 (52) | 1 (45) | >6 | >500 cells/mm3 | <50 copies/mL | No episodes of viral load increase; CD4+ T > 500 cells/mm3 in 90% of measurements; stable for more than 6 years; no ART intervention |
| VC2 VIREMIA CONTROLLERS 2 | 3 (34–47) | 5 (35–53) | >6 | >500 cells/mm3 | ≤log104 (≤10,000 copies/mL) | Episodes of increased HIV-1 viral load; decrease in CD4+ TL in ~40% of counts; natural remission to regular levels without ART intervention |
| NC NON-VIREMIA CONTROLLERS | 16 (28–68) | 12 (24–68) | >6 | <500 cells/mm3 | >log104 (>10,000 copies/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, É.R.G.; Queiroz, M.A.F.; Lima, S.S.; Machado, L.F.A.; Cayres-Vallinoto, I.M.V.; Vallinoto, A.C.R.; Figueiredo, F.A.d.P.L.; Guerreiro, J.F.; Guimarães Ishak, M.d.O.; Ishak, R. CCR5∆32 and SDF1 3′A: Gene Variants, Expression and Influence on Biological Markers for the Clinical Progression to AIDS among HIV-1 Virus Controllers in a Mixed Population of the Amazon Region of Brazil. Int. J. Mol. Sci. 2023, 24, 4958. https://doi.org/10.3390/ijms24054958
Lima ÉRG, Queiroz MAF, Lima SS, Machado LFA, Cayres-Vallinoto IMV, Vallinoto ACR, Figueiredo FAdPL, Guerreiro JF, Guimarães Ishak MdO, Ishak R. CCR5∆32 and SDF1 3′A: Gene Variants, Expression and Influence on Biological Markers for the Clinical Progression to AIDS among HIV-1 Virus Controllers in a Mixed Population of the Amazon Region of Brazil. International Journal of Molecular Sciences. 2023; 24(5):4958. https://doi.org/10.3390/ijms24054958
Chicago/Turabian StyleLima, Érica Ribeiro Gomes, Maria Alice Freitas Queiroz, Sandra Souza Lima, Luiz Fernando Almeida Machado, Izaura Maria Vieira Cayres-Vallinoto, Antonio Carlos Rosário Vallinoto, Fernanda Andreza de Pinho Lott Figueiredo, João Farias Guerreiro, Marluísa de Oliveira Guimarães Ishak, and Ricardo Ishak. 2023. "CCR5∆32 and SDF1 3′A: Gene Variants, Expression and Influence on Biological Markers for the Clinical Progression to AIDS among HIV-1 Virus Controllers in a Mixed Population of the Amazon Region of Brazil" International Journal of Molecular Sciences 24, no. 5: 4958. https://doi.org/10.3390/ijms24054958
APA StyleLima, É. R. G., Queiroz, M. A. F., Lima, S. S., Machado, L. F. A., Cayres-Vallinoto, I. M. V., Vallinoto, A. C. R., Figueiredo, F. A. d. P. L., Guerreiro, J. F., Guimarães Ishak, M. d. O., & Ishak, R. (2023). CCR5∆32 and SDF1 3′A: Gene Variants, Expression and Influence on Biological Markers for the Clinical Progression to AIDS among HIV-1 Virus Controllers in a Mixed Population of the Amazon Region of Brazil. International Journal of Molecular Sciences, 24(5), 4958. https://doi.org/10.3390/ijms24054958







