Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets
Abstract
:1. Introduction
2. Epidemiology of OSF
3. Risk Factors and Pathogenesis of OSF
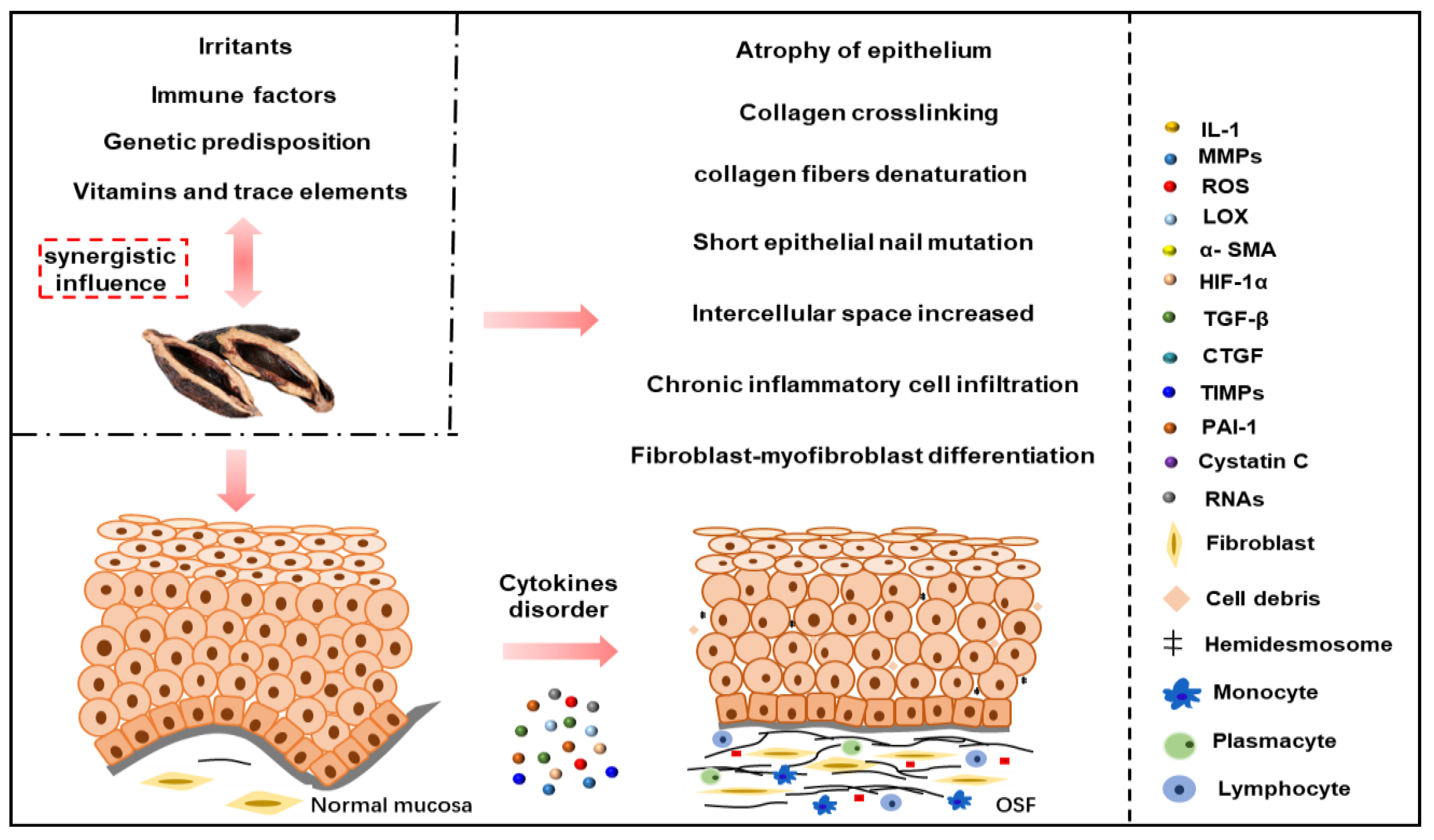
3.1. Stimulating Factors
3.2. Vitamins and Trace Elements
3.3. Immune Factors
3.4. Genetic Factors
3.5. Betel Quid Chewing
3.6. Specific RNAs to OSF
4. Malignant Transformation of OSF
5. Mechanism of OSF Malignant Transformation into OSCC
6. Diagnosis
6.1. Clinical Diagnosis
6.2. Histological Diagnosis
6.3. Other Diagnostic Methods
7. Disease Management
7.1. Oral Health Education
7.2. Removal of Pathogenic Factors
7.3. Drug Therapy
- (1)
- (2)
- Antifibrotic drugs and proteolytic enzymes: exogenous antifibrotic factors and proteolytic enzymes can reverse the process of OSF fibrosis [93]. In clinical practice, hyaluronidase is often used in combination with hormones, and clinical studies have shown that dexamethasone combined with hyaluronidase is the clinical efficacy of local injection into lesions [93];
- (3)
- Peripheral vascular dilators: improve the microcirculation and hemorheology in the lesion area to improve clinical efficacy and relieve the symptoms of patients with OSF [94,95]. Clinical studies have shown that treatment with oral isoxsuprine and combined injections of dexamethasone and hyaluronidase are more effective in relieving OSF symptoms than treatment alone [95];
- (4)
7.4. Auxiliary Mouth Opening Training
7.5. Surgical Treatment
7.6. Hyperbaric Oxygen Therapy, Laser Therapy, Natural Compounds
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSF | oral submucosal fibrosis |
| OSCC | oral squamous cell carcinoma |
| OPMD | oral potentially malignant disorders |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| GRO-α | growth regulated oncogene α |
| IL-1β | interleukin-1β |
| IL-8 | interleukin-8 |
| TNF-α | tumor necrosis factor-α |
| TGF-β | transforming growth factor β |
| αvβ1 | alphavbeta1 |
| αvβ3 | alphavbeta3 |
| αvβ5 | alphavbeta5 |
| CTGF | connective tissue growth factors |
| BMFs | buccal mucosal fibroblasts |
| SOD | superoxide dismutase |
| GPx | glutathione peroxidase |
| LOX | lysyl oxidase |
| ECM | extracellular matrix |
| MMPs | matrix metalloproteinases |
| TIMPs | tissue inhibitor of matrix metalloproteinases |
| IL-1α | interleukin-1α |
| TNF-γ | tumor necrosis factor-γ |
| ANA | antinuclear antibody |
| SMA | smooth muscle antibody |
| GPCA | gastric parietal cell autoantibody |
| FN1 | fibronectin 1 |
| SNPs | single nucleotide polymorphisms |
| BQ | betel quid |
| PAI-1 | plasminogen activator inhibitor type-1 |
| ALK5 | anaplastic lymphoma kinase 5 |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| HIF-1α | hypoxia-induced factor-1α |
| ncRNAs | non-coding RNAs |
| miRNAs | microRNAs |
| lncRNAs | long non-coding RNAs |
| circRNAs | circular RNAs |
| fBMFs | fibrotic buccal mucosa fibroblasts |
| TPM1 | tropomyosin-1 |
| PDCD4 | programmed cell death factor 4 |
| ADSC-EVs | adipose-derived stem cell-derived extracellular vesicles |
| FOXF1 | forkhead box F1 |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| NF-κB | nuclear factor-kappa-B |
| STAT3 | signal transducer and activator of transcription 3 |
| IGF-1 | insulin-like growth factors 1 |
| FSP-1 | ferroptosis-suppressor-protein 1 |
| CSCs | cancer stem cells |
| PTEN | phosphatase and tensin homolog |
| AKT | protein kinase B |
| S6K | S6 kinase |
| EGF | epidermal growth factor |
| MDA | malondialdehyde |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| LTBP2 | latent transforming growth factor beta binding protein 2 |
| PI3K | phosphatidylinositide 3-kinases |
| mTOR | mammalian target of rapamycin |
| ATR-FTIR | attenuated total reflection-Fourier transform infrared spectroscopy |
| TGM2 | transglutaminase 2 |
| Rb | retinoblastoma |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2 associated X protein |
| c-Met | cellular-mesenchymal epithelial transition factor |
References
- Chen, P.Y.; Chao, S.C.; Hsieh, P.L.; Liao, Y.W.; Chu, P.M.; Harn, H.J.; Yu, C.C. Butylidenephthalide Abrogates the Snail-Induced Cancer Stemness in Oral Carcinomas. Int. J. Mol. Sci. 2022, 23, 6157. [Google Scholar] [CrossRef]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Chatterjee, R.; Chaudhuri, K. Oral submucous fibrosis: A global challenge. Rising incidence, risk factors, management, and research priorities. Periodontol. 2000 2019, 80, 200–212. [Google Scholar] [CrossRef]
- Singh, A.G.; Roy, S.; Oza, S.; Singhavi, H.; Chatterjee, K.; Chaturvedi, P. A contemporary narrative review to guide molecular epidemiology of oral submucous fibrosis. Int. J. Mol. Epidemiol. Genet. 2021, 12, 61–70. [Google Scholar] [PubMed]
- Cirillo, N.; Duong, P.H.; Er, W.T.; Do, C.T.N.; De Silva, M.E.H.; Dong, Y.; Cheong, S.C.; Sari, E.F.; McCullough, M.J.; Zhang, P.; et al. Are There Betel Quid Mixtures Less Harmful than Others? A Scoping Review of the Association between Different Betel Quid Ingredients and the Risk of Oral Submucous Fibrosis. Biomolecules 2022, 12, 664. [Google Scholar] [CrossRef]
- Chuang, S.L.; Wang, C.P.; Chen, M.K.; Su, W.W.; Su, C.W.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; Yen, A.M. Malignant transformation to oral cancer by subtype of oral potentially malignant disorder: A prospective cohort study of Taiwanese nationwide oral cancer screening program. Oral Oncol. 2018, 87, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Shao, Y.; Cornwell, W.; Wang, H.; Rogers, T.; Yang, X. Cigarette smoke modulates inflammation and immunity via ROS-regulated trained immunity and trained tolerance mechanisms. Antioxid. Redox Signal. 2022. [Google Scholar] [CrossRef]
- Saso, L.; Reza, A.; Ng, E.; Nguyen, K.; Lin, S.; Zhang, P.; Fantozzi, P.J.; Armagan, G.; Romeo, U.; Cirillo, N. A Comprehensive Analysis of the Role of Oxidative Stress in the Pathogenesis and Chemoprevention of Oral Submucous Fibrosis. Antioxidants 2022, 11, 868. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Hunter, K.D.; Fonseca, F.P.; Radhakrishnan, R. Emerging role of cellular senescence in the pathogenesis of oral submucous fibrosis and its malignant transformation. Head Neck 2021, 43, 3153–3164. [Google Scholar] [CrossRef]
- Shen, Y.W.; Shih, Y.H.; Fuh, L.J.; Shieh, T.M. Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments. Int. J. Mol. Sci. 2020, 21, 7231. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hsieh, P.L.; Liao, Y.W.; Peng, C.Y.; Yu, C.C.; Lu, M.Y. Arctigenin Reduces Myofibroblast Activities in Oral Submucous Fibrosis by LINC00974 Inhibition. Int. J. Mol. Sci. 2019, 20, 1328. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, B.; Zhu, X.; Goodman, M.; Gatewood, R.; Mendiola, P.; Quinata, K.; Paulino, Y. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS ONE 2017, 12, e0172196. [Google Scholar] [CrossRef] [Green Version]
- Divyambika, C.V.; Sathasivasubramanian, S.; Vani, G.; Vanishree, A.J.; Malathi, N. Correlation of Clinical and Histopathological Grades in Oral Submucous Fibrosis Patients with Oxidative Stress Markers in Saliva. Indian J. Clin. Biochem. 2018, 33, 348–355. [Google Scholar] [CrossRef]
- Bagewadi, S.B.; Hirpara, D.R.; Paliwal, A.; Raiyani, B.D.; Hafiz, A.; Vasra, H.D.; Pampaniya, H.M. Estimation of Salivary Copper, Zinc, Iron, and Copper-to-zinc Ratio in Oral Submucous Fibrosis Patients: A Case-control Study. J. Contemp. Dent. Pract. 2022, 23, 303–306. [Google Scholar]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Shetty, S.S.; Sharma, M.; Kabekkodu, S.P.; Kumar, N.A.; Satyamoorthy, K.; Radhakrishnan, R. Understanding the molecular mechanism associated with reversal of oral submucous fibrosis targeting hydroxylysine aldehyde-derived collagen cross-links. J. Carcinog. 2021, 20, 9. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef]
- Saxena, R.; Prasoodanan, P.K.V.; Gupta, S.V.; Gupta, S.; Waiker, P.; Samaiya, A.; Sharma, A.K.; Sharma, V.K. Assessing the Effect of Smokeless Tobacco Consumption on Oral Microbiome in Healthy and Oral Cancer Patients. Front. Cell. Infect. Microbiol. 2022, 12, 841465. [Google Scholar] [CrossRef]
- Arakeri, G.; Rai, K.K.; Hunasgi, S.; Merkx, M.A.W.; Gao, S.; Brennan, P.A. Oral submucous fibrosis: An update on current theories of pathogenesis. J. Oral Pathol. Med. 2017, 46, 406–412. [Google Scholar] [CrossRef]
- Kamath, V.V.; Krishnamurthy, S.; Satelur, K.P.; Rajkumar, K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis: An immunohistochemical observation. Indian J. Med. Paediatr. Oncol. 2015, 36, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.P.; Wu, K.J.; Chen, H.M.; Deng, Y.T. Arecoline activates latent transforming growth factor beta1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallate. J. Formos. Med. Assoc. 2018, 117, 527–534. [Google Scholar] [CrossRef]
- Cheng, R.H.; Wang, Y.P.; Chang, J.Y.; Pan, Y.H.; Chang, M.C.; Jeng, J.H. Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. Int. J. Mol. Sci. 2020, 21, 8104. [Google Scholar] [CrossRef]
- Evans, R.A.; Tian, Y.C.; Steadman, R.; Phillips, A.O. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp. Cell Res. 2003, 282, 90–100. [Google Scholar] [CrossRef]
- Angadi, P.V.; Kale, A.D.; Hallikerimath, S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J. Oral Pathol. Med. 2011, 40, 208–213. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Yang, P.Y.; Ho, D.C.; Chen, S.H.; Hsieh, P.L.; Liao, Y.W.; Tsai, L.L.; Yu, C.C.; Fang, C.Y. Down-regulation of miR-29c promotes the progression of oral submucous fibrosis through targeting tropomyosin-1. J. Formos. Med. Assoc. 2022, 121, 1117–1122. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- Anila Namboodiripad, P.C. Cystatin C: Its role in pathogenesis of OSMF. J. Oral Biol. Craniofac. Res. 2014, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Chua, N.Q.E.; Dang, S.; Davis, A.; Chong, K.W.; Prime, S.S.; Cirillo, N. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int. J. Mol. Sci. 2022, 23, 1637. [Google Scholar] [CrossRef]
- Oliveira, N.G.; Ramos, D.L.; Dinis-Oliveira, R.J. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: An updated review. Arch. Toxicol. 2021, 95, 375–393. [Google Scholar] [CrossRef]
- Islam, S.; Muthumala, M.; Matsuoka, H.; Uehara, O.; Kuramitsu, Y.; Chiba, I.; Abiko, Y. How Each Component of Betel Quid Is Involved in Oral Carcinogenesis: Mutual Interactions and Synergistic Effects with Other Carcinogens-a Review Article. Curr. Oncol. Rep. 2019, 21, 53. [Google Scholar] [CrossRef]
- Li, J.; Yao, M.; Zhu, X.; Li, Q.; He, J.; Chen, L.; Wang, W.; Zhu, C.; Shen, T.; Cao, R.; et al. YAP-Induced Endothelial-Mesenchymal Transition in Oral Submucous Fibrosis. J. Dent. Res. 2019, 98, 920–929. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, H.; Hu, Z.; Zhao, R.; Hu, Y.; Chen, W.; Han, Y.; Yang, L.; Hu, X.; Wang, C.; et al. Experimental study on TGF-β1-mediated CD147 expression in oral submucous fibrosis. Oral Dis. 2018, 24, 993–1000. [Google Scholar] [CrossRef]
- He, X.F.; Wang, H.; Tian, Y.; Zhang, T.; Qiu, Z.P.; Cui, X.J.; Zhou, J.S.; Yan, X.L.; Wu, Y.W.; Pan, Y.S.; et al. Evaluation of Copper Levels in Dental Calculus of OSF Patients with Chewing Dried Areca-Nut Quids in Hunan Province of Mainland China. Biol. Trace Elem. Res. 2022, 201, 677–682. [Google Scholar] [CrossRef]
- Flevaris, P.; Vaughan, D. The Role of Plasminogen Activator Inhibitor Type-1 in Fibrosis. Semin. Thromb. Hemost. 2017, 43, 169–177. [Google Scholar] [CrossRef]
- Chakravorty, N. Non-coding RNAs: The silent regulators of health and diseases. Mol. Biol. Rep. 2022, 49, 6971–6973. [Google Scholar] [CrossRef]
- Liao, Y.W.; Tsai, L.L.; Lee, Y.H.; Hsieh, P.L.; Yu, C.C.; Lu, M.Y. miR-21 promotes the fibrotic properties in oral mucosa through targeting PDCD4. J. Dent. Sci. 2022, 17, 677–682. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.; Xiao, Y.; Shi, B.; Wu, H.; Liu, D. Adipose-Derived Stem Cell-Derived Extracellular Vesicles Inhibit the Fibrosis of Fibrotic Buccal Mucosal Fibroblasts via the MicroRNA-375/FOXF1 Axis. Stem Cells Int. 2021, 2021, 9964159. [Google Scholar] [CrossRef]
- Chattopadhyay, E.; Singh, R.; Ray, A.; Roy, R.; De Sarkar, N.; Paul, R.R.; Pal, M.; Aich, R.; Roy, B. Expression deregulation of mir31 and CXCL12 in two types of oral precancers and cancer: Importance in progression of precancer and cancer. Sci. Rep. 2016, 6, 32735. [Google Scholar] [CrossRef] [PubMed]
- Chickooree, D.; Zhu, K.; Ram, V.; Wu, H.J.; He, Z.J.; Zhang, S. A preliminary microarray assay of the miRNA expression signatures in buccal mucosa of oral submucous fibrosis patients. J. Oral Pathol. Med. 2016, 45, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, T.; Fukagawa, T.; Suzuki, Y.; Takahashi, Y.; Sawada, G.; Ishibashi, M.; Kurashige, J.; Sudo, T.; Tanaka, F.; Shibata, K.; et al. Contrasting expression patterns of histone mRNA and microRNA 760 in patients with gastric cancer. Clin. Cancer Res. 2013, 19, 6438–6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Agarwal, P.; Thangjam, G.S.; Radhesh, R.; Rao, S.G.; Kondaiah, P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors 2011, 29, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Yu, C.C.; Liao, Y.W.; Hsieh, P.L.; Ohiro, Y.; Chu, P.M.; Huang, Y.C.; Yu, C.H.; Tsai, L.L. miR-10b regulated by Twist maintains myofibroblasts activities in oral submucous fibrosis. J. Formos. Med. Assoc. 2020, 119, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Yu, C.C.; Chen, P.Y.; Hsieh, P.L.; Peng, C.Y.; Liao, Y.W.; Yu, C.H.; Lin, K.H. miR-200c inhibits the arecoline-associated myofibroblastic transdifferentiation in buccal mucosal fibroblasts. J. Formos. Med. Assoc. 2018, 117, 791–797. [Google Scholar] [CrossRef]
- Liu, C.M.; Liao, Y.W.; Hsieh, P.L.; Yu, C.H.; Chueh, P.J.; Lin, T.; Yang, P.Y.; Yu, C.C.; Chou, M.Y. miR-1246 as a therapeutic target in oral submucosa fibrosis pathogenesis. J. Formos. Med. Assoc. 2019, 118, 1093–1098. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.J.; Du, C.; Cao, Q.; Li, M.; Feng, H. Target regulation of micro-RNA-203 to the expression of collagen type IV; alpha 4 and its role in oral submucous fibrosis. Zhonghua Kou Qiang Yi Xue Za Zhi 2016, 51, 526–531. [Google Scholar] [CrossRef]
- Lee, Y.H.; Liao, Y.W.; Lu, M.Y.; Hsieh, P.L.; Yu, C.C. LINC00084/miR-204/ZEB1 Axis Mediates Myofibroblastic Differentiation Activity in Fibrotic Buccal Mucosa Fibroblasts: Therapeutic Target for Oral Submucous Fibrosis. J. Pers. Med. 2021, 11, 707. [Google Scholar] [CrossRef]
- Yu, C.C.; Liao, Y.W.; Hsieh, P.L.; Chang, Y.C. Targeting lncRNA H19/miR-29b/COL1A1 Axis Impedes Myofibroblast Activities of Precancerous Oral Submucous Fibrosis. Int. J. Mol. Sci. 2021, 22, 2216. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, Y.; Li, Z.; Zhu, Y.; He, Z.; Zhang, C. Exosome-derived long non-coding RNA ADAMTS9-AS2 suppresses progression of oral submucous fibrosis via AKT signalling pathway. J. Cell. Mol. Med. 2021, 25, 2262–2273. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; Gonzalez-Moles, M.A.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Murthy, V.; Mylonas, P.; Carey, B.; Yogarajah, S.; Farnell, D.; Addison, O.; Cook, R.; Escudier, M.; Diniz-Freitas, M.; Limeres, J.; et al. Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1793. [Google Scholar] [CrossRef]
- Jayasooriya, P.R.; Nadeeka Jayasinghe, K.A.; Mudiyanselage Tilakaratne, W. Relationship between thickness of fibrosis and epithelial dysplasia in oral submucous fibrosis. J. Investig. Clin. Dent. 2011, 2, 171–175. [Google Scholar] [CrossRef]
- Mehrtash, H.; Duncan, K.; Parascandola, M.; David, A.; Gritz, E.R.; Gupta, P.C.; Mehrotra, R.; Amer Nordin, A.S.; Pearlman, P.C.; Warnakulasuriya, S.; et al. Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 2017, 18, e767–e775. [Google Scholar] [CrossRef]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care (Engl.) 2020, 29, e13207. [Google Scholar] [CrossRef]
- Ramôa, C.P.; Eissenberg, T.; Sahingur, S.E. Increasing popularity of waterpipe tobacco smoking and electronic cigarette use: Implications for oral healthcare. J. Periodontal Res. 2017, 52, 813–823. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Arumugam, S.; Hsu, H.T.; Chung, C.M.; Lee, C.P.; Tsai, M.H.; Yeh, K.T.; Luo, S.Y.; Ko, Y.C. Arecoline N-oxide initiates oral carcinogenesis and arecoline N-oxide mercapturic acid attenuates the cancer risk. Life Sci. 2021, 271, 119156. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Myong, N.H. Loss of E-cadherin and Acquisition of Vimentin in Epithelial-Mesenchymal Transition are Noble Indicators of Uterine Cervix Cancer Progression. Korean J. Pathol. 2012, 46, 341–348. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [Green Version]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland. Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.N.; Lin, C.W.; Yang, S.F.; Chang, Y.C. Oral submucous fibrosis stimulates invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by activating MMP-2 and IGF-IR. J. Cell. Mol. Med. 2021, 25, 9814–9825. [Google Scholar] [CrossRef]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Ghuwalewala, S.; Ghatak, D.; Das, P.; Dey, S.; Sarkar, S.; Alam, N.; Panda, C.K.; Roychoudhury, S. CD44(high)CD24(low) molecular signature determines the Cancer Stem Cell and EMT phenotype in Oral Squamous Cell Carcinoma. Stem Cell Res. 2016, 16, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Sterz, C.M.; Kulle, C.; Dakic, B.; Makarova, G.; Böttcher, M.C.; Bette, M.; Werner, J.A.; Mandic, R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010, 46, 116–122. [Google Scholar] [CrossRef]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1(+) Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e626. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, R.; Hallikeri, K.; Sudhakaran, A. PTEN and alpha-SMA Expression and Diagnostic Role in Oral Submucous Fibrosis and Oral Squamous Cell Carcinoma with Concomitant Oral Submucous Fibrosis. J. Oral Maxillofac. Res. 2021, 12, e3. [Google Scholar] [CrossRef]
- Richter, P.; Umbreit, C.; Franz, M.; Berndt, A.; Grimm, S.; Uecker, A.; Böhmer, F.D.; Kosmehl, H.; Berndt, A. EGF/TGFβ1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J. Oral Pathol. Med. 2011, 40, 46–54. [Google Scholar] [CrossRef]
- Chen, S.C.; Liu, C.M.; Hsieh, P.L.; Liao, Y.W.; Lin, Y.J.; Yu, C.C.; Yu, C.H. E3 ligase carboxyl-terminus of Hsp70-interacting protein (CHIP) suppresses fibrotic properties in oral mucosa. J. Formos. Med. Assoc. 2020, 119, 595–600. [Google Scholar] [CrossRef]
- Kavitha, L.; Ranganathan, K.; Shyam, S.; Fathima, J.H.S.; Umesh, W.; Warnakulasuriya, S. Immunohistochemical biomarkers in oral submucous fibrosis: A scoping review. J. Oral Pathol. Med. 2022, 51, 594–602. [Google Scholar] [CrossRef]
- Nag, R.; Paul, R.R.; Pal, M.; Chatterjee, J.; Das, R.K. Epithelial Distribution of E-Cadherin, p63, and Mitotic Figures in ApoTome Images to Determine the Oncogenic Potentiality of Oral Submucous Fibrosis. Microsc. Microanal. 2020, 26, 1198–1210. [Google Scholar] [CrossRef]
- Kamala, K.A.; Kanetkar, S.R.; Datkhile, K.D.; Sankethguddad, S. Expression of Ki67 Biomarker in Oral Submucous Fibrosis with Clinico-Pathological Correlations: A Prospective Study. Asian Pac J Cancer Prev 2022, 23, 253–259. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lee, S.S.; Chang, Y.C. Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J. Oral Pathol. Med. 2015, 44, 669–673. [Google Scholar] [CrossRef]
- Ishida, T.; Hijioka, H.; Kume, K.; Miyawaki, A.; Nakamura, N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncol. Lett. 2013, 6, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Chu, P.M.; Hsieh, P.L.; Yang, H.W.; Chueh, P.J.; Huang, Y.F.; Liao, Y.W.; Yu, C.C. Glabridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGF-beta/smad signaling. Environ. Toxicol. 2018, 33, 248–255. [Google Scholar] [CrossRef]
- Bale, R.; Kattappagari, K.K.; Vidya, D.; Vuddandi, S.; Gummalla, C.; Baddam, V.R.R. Oral submucous fibrosis: A quantitative assessment of serum malondialdehyde, superoxide dismutase and correlation with clinical staging. J. Oral Maxillofac. Pathol. 2017, 21, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Reis, P.P.; Tomenson, M.; Cervigne, N.K.; Machado, J.; Jurisica, I.; Pintilie, M.; Sukhai, M.A.; Perez-Ordonez, B.; Grénman, R.; Gilbert, R.W.; et al. Programmed cell death 4 loss increases tumor cell invasion and is regulated by miR-21 in oral squamous cell carcinoma. Mol. Cancer 2010, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, C.; Li, N.; Wang, F.; Xu, Y.; Shen, Z.; Yang, L.; Li, Z.; He, C. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Hsieh, P.L.; Peng, C.Y.; Liao, Y.W.; Yu, C.H.; Yu, C.C. LncRNA MEG3 inhibits self-renewal and invasion abilities of oral cancer stem cells by sponging miR-421. J. Formos. Med. Assoc. 2021, 120, 1137–1142. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Yu, C.C. Oral Fibrosis and Oral Cancer: From Molecular Targets to Therapeutics. Int. J. Mol. Sci. 2022, 23, 6110. [Google Scholar] [CrossRef]
- Shaikh, S.; Yadav, D.K.; Rawal, R. Saliva based non invasive screening of Oral Submucous Fibrosis using ATR-FTIR spectroscopy. J. Pharm. Biomed. Anal. 2021, 203, 114202. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Purohit, B.M. ABO Blood Groups and Its Association with Oral Cancer, Oral Potentially Malignant Disorders and Oral Submucous Fibrosis- A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2021, 22, 1703–1712. [Google Scholar] [CrossRef]
- Pant, I.; Kumar, N.; Khan, I.; Rao, S.G.; Kondaiah, P. Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis. PLoS ONE 2015, 10, e0129252. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Liao, Y.W.; Lu, M.Y.; Yang, C.M.; Hsieh, P.L.; Yu, C.C. Positive Feedback Loop of SNAIL-IL-6 Mediates Myofibroblastic Differentiation Activity in Precancerous Oral Submucous Fibrosis. Cancers 2020, 12, 1611. [Google Scholar] [CrossRef]
- Humayun, S.; Prasad, V.R. Expression of p53 protein and ki-67 antigen in oral premalignant lesions and oral squamous cell carcinomas: An immunohistochemical study. Natl. J. Maxillofac. Surg. 2011, 2, 38–46. [Google Scholar] [CrossRef]
- Bazarsad, S.; Zhang, X.; Kim, K.Y.; Illeperuma, R.; Jayasinghe, R.D.; Tilakaratne, W.M.; Kim, J. Identification of a combined biomarker for malignant transformation in oral submucous fibrosis. J. Oral Pathol. Med. 2017, 46, 431–438. [Google Scholar] [CrossRef]
- Pal, U.S. Early diagnosis of oral submucous fibrosis is a boon in the prevention of oral cancer. Natl. J. Maxillofac. Surg. 2021, 12, 295–296. [Google Scholar] [CrossRef]
- James, L.; Shetty, A.; Rishi, D.; Abraham, M. Management of Oral Submucous Fibrosis with Injection of Hyaluronidase and Dexamethasone in Grade III Oral Submucous Fibrosis: A Retrospective Study. J. Int. Oral Health 2015, 7, 82–85. [Google Scholar]
- Liu, J.; Chen, F.; Wei, Z.; Qiu, M.; Li, Z.; Dan, H.; Chen, Q.; Jiang, L. Evaluating the efficacy of pentoxifylline in the treatment of oral submucous fibrosis: A meta-analysis. Oral Dis. 2018, 24, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Bhadage, C.J.; Umarji, H.R.; Shah, K.; Välimaa, H. Vasodilator isoxsuprine alleviates symptoms of oral submucous fibrosis. Clin. Oral Investig. 2013, 17, 1375–1382. [Google Scholar] [CrossRef]
- Guo, J.; Xie, H.; Wu, H.; Liang, M. Efficacy of Lycopene in the Treatment of Oral Submucous Fibrosis: A Meta-analysis of Randomized Controlled Trials. J. Evid. Based Dent. Pract. 2020, 20, 101471. [Google Scholar] [CrossRef]
- More, C.B.; Jatti Patil, D.; Rao, N.R. Medicinal management of oral submucous fibrosis in the past decade- A systematic review. J. Oral Biol. Craniofac. Res. 2020, 10, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Nerkar Rajbhoj, A.; Kulkarni, T.M.; Shete, A.; Shete, M.; Gore, R.; Sapkal, R. A Comparative Study to Evaluate Efficacy of Curcumin and Aloe Vera Gel along with Oral Physiotherapy in the Management of Oral Submucous Fibrosis: A Randomized Clinical Trial. Asian Pac. J. Cancer Prev. 2021, 22, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Peng, J.; Ma, L. Oral opening training increases oral opening in patients with oral submucous fibrosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021, 46, 731–735. [Google Scholar] [CrossRef]
- Pal, U.S.; Maurya, H.; Ganguly, R.; Singh, R.K.; Kumar, V. Complications of platysma myocutaneous flap in patients with oral submucosal fibrosis: A systematic review. J. Oral Biol. Craniofac. Res. 2022, 12, 421–426. [Google Scholar] [CrossRef]
- Dasukil, S.; Jena, A.K.; Boyina, K.K.; Grover, S.; Arora, G.; Ahmed, Z.U. Functional outcome of two different grafting techniques in the surgical management of oral submucous fibrosis: A comparative evaluation. Oral Maxillofac. Surg. 2022, 26, 477–483. [Google Scholar] [CrossRef]
- Anehosur, V.; Singh, P.K.; Dikhit, P.S.; Vadera, H. Clinical Evaluation of Buccal Fat Pad and Nasolabial Flap for Oral Submucous Fibrosis Intraoral Defects. Craniomaxillofac. Trauma Reconstr. 2021, 14, 196–200. [Google Scholar] [CrossRef]
- Sharma, M.; Radhakrishnan, R. Limited mouth opening in oral submucous fibrosis: Reasons, ramifications, and remedies. J Oral Pathol. Med. 2017, 46, 424–430. [Google Scholar] [CrossRef]
- Gondivkar, D.S.M.; Gadbail, D.A.R.; Sarode, D.S.C.; Gondivkar, D.R.S.; Patil, S.; Gaikwad, D.R.N.; Dinh-Toi, C.; Yuwanati, D.M. Treatment outcomes of laser therapy in oral submucous fibrosis-a systematic review. J. Oral Biol. Craniofac. Res. 2020, 10, 253–258. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, H.; Li, T. Multi-target pharmacological mechanisms of Salvia miltiorrhiza against oral submucous fibrosis: A network pharmacology approach. Arch. Oral Biol. 2021, 126, 105131. [Google Scholar] [CrossRef]
- Bohra, A.; Maheswari, T.N.U.; Harsh, A.; Garg, A. Black Turmeric and Aloe Vera in the Management of Oral Submucous Fibrosis: A Prospective Clinical Study. Asian Pac. J. Cancer Prev. 2021, 22, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.P.; Chen, H.M.; Lin, H.Y.; Yang, H.; Chang, J.Z. Epigallocatechin-3-gallate inhibits transforming-growth-factor-β1-induced collagen synthesis by suppressing early growth response-1 in human buccal mucosal fibroblasts. J. Formos. Med. Assoc. 2017, 116, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Al-Maweri, S.A. Efficacy of curcumin for management of oral submucous fibrosis: A systematic review of randomized clinical trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 300–308. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Ashraf, S.; Lingam, A.S.; Alqutaibi, A.; Abdulrab, S.; Alaizari, N.; Halboub, E. Aloe vera in treatment of oral submucous fibrosis: A systematic review and meta-analysis. J. Oral Pathol. Med. 2019, 48, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, A.; Patil, B.; Asha, V.R. Evaluation of efficacy of aloe vera in the treatment of oral submucous fibrosis-A clinical study. J. Oral Pathol. Med. 2017, 46, 50–55. [Google Scholar] [CrossRef]
- Chen, P.Y.; Ho, D.C.; Liao, Y.W.; Hsieh, P.L.; Lu, K.H.; Tsai, L.L.; Su, S.H.; Yu, C.C. Honokiol inhibits arecoline-induced oral fibrogenesis through transforming growth factor-beta/Smad2/3 signaling inhibition. J. Formos. Med. Assoc. 2021, 120, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Shih, Y.H.; Wang, T.H.; Lan, W.C.; Li, P.J.; Jhuang, H.S.; Hsia, S.M.; Shen, Y.W.; Chen, M.Y.-C.; Shieh, T.M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2021, 120, 827–837. [Google Scholar] [CrossRef]
- Peng, C.Y.; Yu, C.C.; Huang, C.C.; Liao, Y.W.; Hsieh, P.L.; Chu, P.M.; Yu, C.H.; Lin, S.S. Magnolol inhibits cancer stemness and IL-6/Stat3 signaling in oral carcinomas. J. Formos. Med. Assoc. 2022, 121, 51–57. [Google Scholar] [CrossRef] [PubMed]
- More, C.B.; Gavli, N.; Chen, Y.; Rao, N.R. A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: An evidence based approach. J. Oral Maxillofac. Pathol. 2018, 22, 382–391. [Google Scholar] [CrossRef] [PubMed]


| Methods | Principles | Name | Ref |
|---|---|---|---|
| Oral health education | Prevention is better than cure | ||
| Removal of pathogenic factors | Intervention | ||
| Chemical drug therapy | Glucocorticoids: inhibit the production of inflammatory factors, promote the apoptosis of inflammatory cells, are anti-inflammatory and inhibit fibrosis | Triamcinolone (mixture of triamcinolone 30 mg + lidocaine 20 mg, local injection once a week, eight times as a course of treatment) Dexamethasone (mixture of dexamethasone 4 mg + hyaluronidase 1500 IU + chymotrypsin 5000 IU, two injections per week for six to eight weeks) | [92,93,99,114] |
| Antifibrotic drugs and proteolytic enzymes: proteolytic, hydrolyzes hyaluronic acid and reduces collagen formation | Hyaluronidase (hydrocortisone acetate 25 mg/mL + hyaluronidase 1500 IU, local injection once a week for six weeks) | [93,98] | |
| Peripheral vasodilator: improves microcirculation and hemorheology | Pentoxifylline (400 mg, three times daily for three to four months) Isoxuprine (10 mg, three times daily for four to six weeks) | [94,95,114] | |
| Oxidants and nutrients: antioxidant and deactivation of free radicals | Lycopene (8 mg, once a day for half a year) Vitamin A (50,000 IU, once a day for half a year) Vitamin B, C, D Vitamin E (400 mg, once a day for half a year) | [96,97,114] | |
| Oral physiotherapy exercises | Physical therapy | Mouth opening training | [98,99] |
| Surgical treatment | The fibrous bands were excised and different flaps were implanted | Fiber strip excision | [92] |
| Hyperbaric oxygen therapy | Increase blood oxygen content, improve local ischemia and hypoxia, promote neovascularization and collateral circulation in the damaged area | Hyperbaric oxygen | [103] |
| Laser therapy | Ease mouth opening difficulty, burning sensation and increase cheek flexibility | Laser loosens the fiber band | [104] |
| Natural compounds | Anti-ibrosis, anti-inflammation and anti-tumor | Arctigenin | [12] |
| Invigorate the circulation of blood | Salvia miltiorrhiza (mixture of salvia miltiorrhiza 300 mg + lidocaine 20 mg, local injection once a week, eight times as a course of treatment) | [99,105] | |
| Anti-inflammatory, antioxidant, anti-ulcer and antifibrotic | Black turmeric (black turmeric 0.5 mg + Aloe vera gel 0.5 mg, three times a day for three months) | [106] | |
| Anti-inflammatory, antioxidant | Glabridin | [80] | |
| Antioxidant, inhibit collagen synthesis | Epigallocatechin gallate | [23,107] | |
| Modulates the inflammatory response, decreases collagen generation and induces apoptosis | Curcumin (300 mg, once daily, six to eight months) | [98,108,114] | |
| Antioxidant, anti-inflammatory, immunomodulatory and anti-tumor | Aloe vera (5 mg of Aloe vera gel, smearing, six weeks) | [98,106,109,110] | |
| Anti-inflammatory, antifibrotic and anti-cancer | Honokiol | [111,112] |
| miRNA | Change | Targets | Roles | Ref |
|---|---|---|---|---|
| miR-29c | Downregulation | TPM1 binds directly to miR-29c. | Downregulation of miR-29c affects TPM1 and myofibroblast activity. | [28] |
| miR-204 | Downregulation | Predicted target: MMP9, PLAUR, SERPINE1, SNAI2, COL5A3. | MiR-204 is considered to be a tumor suppressor. Restoring its expression can significantly inhibit cell proliferation, migration, invasion and tumor formation, and significantly increase the rate of cell apoptosis. | [41] |
| miR-509-5p | Downregulation | Predicted target: BMPR2, CDH6, HAS3, PARD6B, TIMP3, THBS1, THBS2. | Inhibited cell proliferation and migration, promote apoptosis in OSF. | [42] |
| miR-610 | Downregulation | Predicted target: CDH1, DSC2, KRAS, MMP19, MAPK1, TIMP3, MMP24. | It could cause collagen defect of OSF. | [42] |
| miR-760 | Downregulation | Histone mRNA binds directly to miR-760. Predicted target: CDH4, COX10, IL6, IL6R, IGF1R, TIMP2, TGM2. | MiR-760 can be used as a predictor of precancerous lesions. KEGG pathway analysis has demonstrated the role of the hedgehog signaling pathway and ubiquitin-mediated proteolysis. | [42,43] |
| miR-200C | Downregulation | ZEB1 binds directly to miR-200c. | Overexpression of miR-200c inhibits the expression of ZEB1 and α-SMA, and it is a key factor in tumorigenesis and cancer metastasis. | [46] |
| miR-203 | Downregulation | COL4A4 and miR-203 were negatively correlated. | It could inhibit fibrosis and epithelial–mesenchymal transition. | [48] |
| miR-21 | Upregulation | PDCD4 binds directly to miR-21. | MiR-21 inhibition of PDCD4 can regulate myofibroblast activation of BMFs and increase the invasiveness of oral cancer. | [39,82] |
| miR-31 | Upregulation | Predicted target: DMD, CXCL12, WASF3. | High expression of mir31 can affect the immortalization or transformation of oral keratinocytes, damage DNA repair genes, and cause genomic instability and EMT transition. | [41] |
| miR-623 | Upregulation | Predicted target: MAPK1, MAPK11, MAPK4, MMP1, MMP8, TIMP2, IL10. | It may be a profibrotic miRNA. The KEGG pathway included gap junction, TGF-β signaling pathway, insulin signaling pathway, chemokine signaling pathway, and cytokine–cytokine receptor interaction. | [42] |
| miR-455 | Upregulation | BMP7 was a negative modulator of fibrosis. Predicted target: BMPR, DSC1, MAPK14, MAPK11, IGF1, TIMP2, TGM3, BMP7. | It may have a promoting role in the OSF. | [42,44] |
| miR-10b | Upregulation | Regulated by Twist. | MiR-10b regulates the activation of myofibroblasts and the expression of α-SMA. | [45] |
| miR-1246 | Upregulation | Type I collagen and miR-1246 were positively correlated. | The activity of collagen gel contraction and the migration ability of fibroblasts was inhibited by inhibiting miR-1246. | [47] |
| RNA | Change | Pathways | Roles | Ref |
|---|---|---|---|---|
| LINC00974 | Upregulation | TGF-β/Smad pathway | It promotes the development of oral fibrosis. | [12] |
| LINC00084 | Upregulation | LINC00084/miR-204/ZEB1 axis | LINC00084 regulates myofibroblast activation through miR-204-ZEB1. | [49] |
| lncRNA H19 | Upregulation | IncRNAH19/miR-29b/COL1A1 axis | Chewing betel nut can upregulate the expression of H19 in BMFs by activating TGF-β1. lncRNA H19 acts as a molecular sponge for miR29b and causes oral fibrosis by interfering with the binding of miR-29b to collagen type I. | [50] |
| circEPSTI1 | Upregulation | circEPSTI1/miR-942-5p/LTBP2 axis and PI3K/Akt/mTOR pathway | CircEPSTI1/miR-942-5p/LTBP2 axis activates EMT and PI3K/Akt/mTOR signaling pathways to promote OSCC proliferation, migration and invasion. | [83] |
| lncRNA MEG3 | Downregulation | IncRNA MEG3/miR-421 | IncRNA MEG3 affects the characteristics of cancer stem cells through the molecular sponge miR-421. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Ning, Y.; Zhou, L.; Zhu, Y. Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. Int. J. Mol. Sci. 2023, 24, 4992. https://doi.org/10.3390/ijms24054992
Qin X, Ning Y, Zhou L, Zhu Y. Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. International Journal of Molecular Sciences. 2023; 24(5):4992. https://doi.org/10.3390/ijms24054992
Chicago/Turabian StyleQin, Xiaofeng, Yujie Ning, Liming Zhou, and Youming Zhu. 2023. "Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets" International Journal of Molecular Sciences 24, no. 5: 4992. https://doi.org/10.3390/ijms24054992
APA StyleQin, X., Ning, Y., Zhou, L., & Zhu, Y. (2023). Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. International Journal of Molecular Sciences, 24(5), 4992. https://doi.org/10.3390/ijms24054992





