An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation
Abstract
1. Introduction
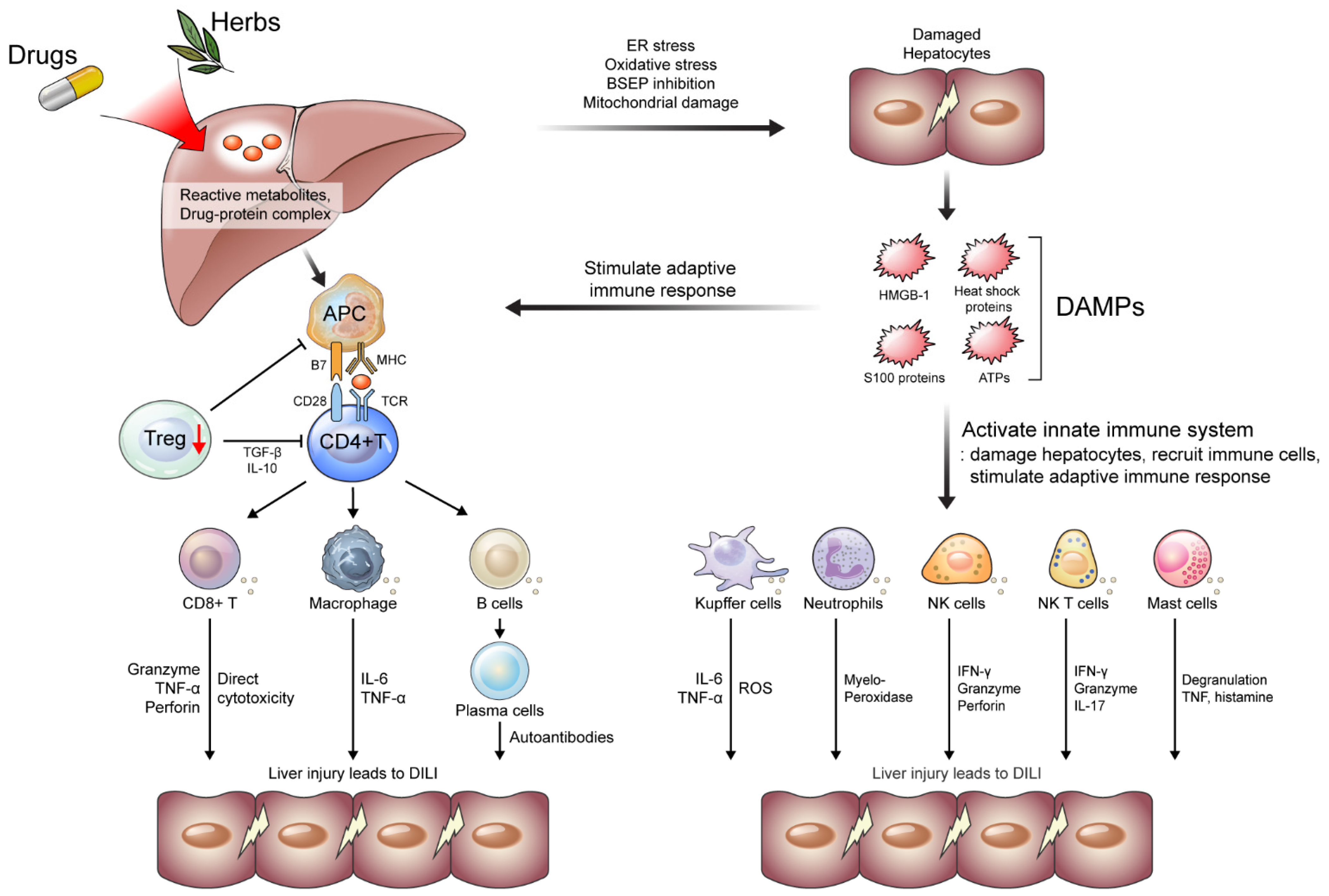
2. Immunological Perspective on DILI Mechanism
2.1. Danger Hypothesis
2.2. Innate Immune Systems in DILI
2.2.1. Kupffer Cells
2.2.2. Neutrophils
2.2.3. NK Cells
2.2.4. NK T Cells
2.2.5. Mast Cells
2.3. Adaptive Immune Systems in DILI
2.3.1. CD4+ and CD8+ T Cells
2.3.2. B Cells
2.3.3. Treg Cells
3. DILI Caused by Drugs Treating HCC and LT
3.1. DILI Caused by Drugs Treating HCC
3.1.1. Tyrosine Kinase Inhibitors
3.1.2. Immune Check Point Inhibitors
3.2. DILI Casued by Drugs for Treating LT
Immunosuppressants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vuppalanchi, R.; Liangpunsakul, S.; Chalasani, N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am. J. Gastroenterol. 2007, 102, 558–562. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 2014, 146, 914–928. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966; quiz 967. [Google Scholar] [CrossRef] [PubMed]
- Lammert, C.; Einarsson, S.; Saha, C.; Niklasson, A.; Bjornsson, E.; Chalasani, N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology 2008, 47, 2003–2009. [Google Scholar] [CrossRef]
- Sgro, C.; Clinard, F.; Ouazir, K.; Chanay, H.; Allard, C.; Guilleminet, C.; Lenoir, C.; Lemoine, A.; Hillon, P. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin. Liver Dis. 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Dugan, C.M.; Fullerton, A.M.; Roth, R.A.; Ganey, P.E. Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis. Toxicol. Sci. 2011, 120, 507–518. [Google Scholar] [CrossRef]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Aithal, G.; Watkins, P.; Andrade, R.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.; Wilke, R.; Avigan, M.; Kaplowitz, N.; et al. Case Definition and Phenotype Standardization in Drug-Induced Liver Injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int. J. Mol. Sci. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Pharmacogenomics of adverse drug reactions. Genome Med. 2013, 5, 5. [Google Scholar] [CrossRef]
- Osanlou, O.; Pirmohamed, M.; Daly, A.K. Chapter Seven-Pharmacogenetics of Adverse Drug Reactions. In Advances in Pharmacology; Brøsen, K., Damkier, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 155–190. [Google Scholar]
- Teschke, R. Treatment of Drug-Induced Liver Injury. Biomedicines 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e1347. [Google Scholar] [CrossRef]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-Margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying Hepatic Cell Death during Liver Damage: Ferroptosis—A Novel Form of Non-Apoptotic Cell Death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Kakisaka, K.; Yoshida, Y.; Suzuki, Y.; Sato, T.; Kuroda, H.; Miyasaka, A.; Takikawa, Y. Serum markers for mitochondrial dysfunction and cell death are possible predictive indicators for drug-induced liver injury by direct acting antivirals. Hepatol. Res. 2018, 48, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Ju, C.; Ramaiah, S.K.; Uetrecht, J.; Jaeschke, H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010, 115, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Kim, S.-H.; Naisbitt, D.J. Update on Advances in Research on Idiosyncratic Drug-Induced Liver Injury. Allergy Asthma Immunol. Res. 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, K.; Jacobs, J. Studies on the Sensitization of Animals with Simple Chemical Compounds. J. Exp. Med. 1935, 61, 643–656. [Google Scholar] [CrossRef]
- Kaplowitz, N.; DeLeve, L.D. Drug-Induced Liver Disease; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cho, T.; Uetrecht, J. How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem. Res. Toxicol. 2017, 30, 295–314. [Google Scholar] [CrossRef]
- Uetrecht, J. Mechanisms of idiosyncratic drug-induced liver injury. Adv. Pharmacol. 2019, 85, 133–163. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, X.; Liu, Y.; Liu, J.; Li, C.; Chen, L.; Chen, H.; Ouyang, D. The Immunological Mechanisms and Immune-Based Biomarkers of Drug-Induced Liver Injury. Front. Pharmacol. 2021, 12, 723940. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.A.; Holman, N.S.; Green, A.M.; Andersen, M.E.; LeCluyse, E.L. Co-culture of hepatocytes and Kupffer cells as an in vitro model of inflammation and drug-induced hepatotoxicity. J. Pharm. Sci. 2016, 105, 950–964. [Google Scholar] [CrossRef]
- Seo, H.-Y.; Kim, M.-K.; Lee, S.-H.; Hwang, J.S.; Park, K.-G.; Jang, B.K. Kahweol ameliorates the liver inflammation through the inhibition of NF-κB and STAT3 activation in primary Kupffer cells and primary hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Yang, L.; Xie, H.; Yang, Y.; Wang, H.; Wu, C.; Shen, T.; Zhu, Q. Bradykinin contributes to immune liver injury via B2R receptor-mediated pathways in trichloroethylene sensitized mice: A role in Kupffer cell activation. Toxicology 2019, 415, 37–48. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D.; et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Jaeschke, H.; Hasegawa, T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006, 26, 912–919. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- El-Benna, J.; Dang, P.M.; Gougerot-Pocidalo, M.A.; Elbim, C. Phagocyte NADPH oxidase: A multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. 2005, 53, 199–206. [Google Scholar]
- Klugewitz, K.; Adams, D.H.; Emoto, M.; Eulenburg, K.; Hamann, A. The composition of intrahepatic lymphocytes: Shaped by selective recruitment? Trends Immunol. 2004, 25, 590–594. [Google Scholar] [CrossRef]
- Frey, M.; Packianathan, N.B.; Fehniger, T.A.; Ross, M.E.; Wang, W.C.; Stewart, C.C.; Caligiuri, M.A.; Evans, S.S. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J. Immunol. 1998, 161, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Cella, M.; Porter, S.I.; Li, S.; Gurewitz, G.L.; Hong, H.S.; Johnson, R.P.; Oltz, E.M.; Colonna, M. Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell 2019, 176, 348–360.e312. [Google Scholar] [CrossRef]
- Radaeva, S.; Sun, R.; Jaruga, B.; Nguyen, V.T.; Tian, Z.; Gao, B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006, 130, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, F.; Obholzer, M.; Metzler, S.; Stöber, R.; Hengstler, J.G.; Watzl, C. Enhanced activation of human NK cells by drug-exposed hepatocytes. Arch. Toxicol. 2020, 94, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kondo, T.; Ohshima, T.; Fujiwara, H.; Iwakura, Y.; Mukaida, N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002, 16, 1227–1236. [Google Scholar] [CrossRef]
- Wallace, K.L.; Marshall, M.A.; Ramos, S.I.; Lannigan, J.A.; Field, J.J.; Strieter, R.M.; Linden, J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood 2009, 114, 667–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bendelac, A.; Lantz, O.; Quimby, M.E.; Yewdell, J.W.; Bennink, J.R.; Brutkiewicz, R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995, 268, 863–865. [Google Scholar] [CrossRef]
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.M.; Bezbradica, J.S.; Van Kaer, L.; Joyce, S. CD1d-Restricted Natural Killer T Cells. In eLS; Wiley: Hoboken, NJ, USA, 2016; pp. 1–27. [Google Scholar] [CrossRef]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-cell and CD8+ T-cell Signatures in Mice with Nonalcoholic Steatohepatitis. Hepatol. Commun. 2017, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef]
- Lin, Q.; Kuypers, M.; Liu, Z.; Copeland, J.K.; Chan, D.; Robertson, S.J.; Kontogiannis, J.; Guttman, D.S.; Banks, E.K.; Philpott, D.J.; et al. Invariant natural killer T cells minimally influence gut microbiota composition in mice. Gut Microbes 2022, 14, 2104087. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Mast cell ontogeny: An historical overview. Immunol. Lett. 2014, 159, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells--key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thorlacius, H. Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. Br. J. Pharmacol. 2005, 145, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Romani, L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol. Rev. 2018, 282, 188–197. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast Cells in Liver Fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Tolefree, J.A.; Garcia, A.J.; Farrell, J.; Meadows, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Francis, N.; Mirabel, J.; Francis, H. Alcoholic liver disease and mast cells: What’s your gut got to do with it? Liver Res. 2019, 3, 46–54. [Google Scholar] [CrossRef]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, J.; van Heemst, J.; van Heiningen, J.; Dorjée, A.L.; Schilham, M.W.; van der Beek, F.B.; Huizinga, T.W.J.; Schuerwegh, A.J.M.; Toes, R.E.M. Communication between human mast cells and CD4+ T cells through antigen-dependent interactions. Eur. J. Immunol. 2013, 43, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Z.; Leung, M.W.Y.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.C.; Eric Gershwin, M. Adaptive immunity in the liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Aithal, G.P. Immune-Mediated Drug-Induced Liver Injury. In Liver Immunology: Principles and Practice; Gershwin, M.E.M., Vierling, J., Tanaka, A.P., Manns, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 491–504. [Google Scholar]
- Dardalhon, V.; Korn, T.; Kuchroo, V.K.; Anderson, A.C. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008, 31, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Metkar, S.S.; Wang, B.; Ebbs, M.L.; Kim, J.H.; Lee, Y.J.; Raja, S.M.; Froelich, C.J. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J. Cell Biol. 2003, 160, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Amante, M.F.; Filippini, A.V.; Cejas, N.; Lendoire, J.; Imventarza, O.; Parisi, C. Dress syndrome and fulminant hepatic failure induced by lamotrigine. Ann. Hepatol. 2009, 8, 75–77. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Patel, A.M.; Liu, Y.S.; Davies, S.P.; Brown, R.M.; Kelly, D.A.; Scheel-Toellner, D.; Reynolds, G.M.; Stamataki, Z. The Role of B Cells in Adult and Paediatric Liver Injury. Front. Immunol. 2021, 12, 729143. [Google Scholar] [CrossRef]
- Metushi, I.G.; Sanders, C.; Lee, W.M.; Uetrecht, J. Detection of anti-isoniazid and anti-cytochrome P450 antibodies in patients with isoniazid-induced liver failure. Hepatology 2014, 59, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.A.; Shevach, E.M. Cutting edge: Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 2001, 167, 1137–1140. [Google Scholar] [CrossRef]
- Lee, S.K.; Park, M.J.; Choi, J.W.; Baek, J.A.; Kim, S.Y.; Choi, H.J.; You, Y.K.; Jang, J.W.; Sung, P.S.; Bae, S.H.; et al. Patient-Derived Avatar Mouse Model to Predict the Liver Immune Homeostasis of Long-Term Stable Liver Transplant Patients. Front. Immunol. 2022, 13, 817006. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.H.; Lee, S.K.; Kim, H.Y.; Jung, E.S.; Kim, D.G.; Choi, J.; Bae, S.H.; Yoon, S.K.; Chung, B.H.; et al. Serial Monitoring of Immune Markers Being Represented Regulatory T Cell/T Helper 17 Cell Ratio: Indicating Tolerance for Tapering Immunosuppression after Liver Transplantation. Front. Immunol. 2018, 9, 352. [Google Scholar] [CrossRef]
- Longhi, M.S.; Hussain, M.J.; Mitry, R.R.; Arora, S.K.; Mieli-Vergani, G.; Vergani, D.; Ma, Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J. Immunol. 2006, 176, 4484–4491. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R.; Holder, B.S.; Cardone, J.; Ma, Y.; Robson, S.C.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 2014, 59, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients With Myasthenia Gravis. Front. Immunol. 2021, 12, 803101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jhun, J.; Lee, S.Y.; Choi, S.; Choi, S.S.; Park, M.S.; Lee, S.Y.; Cho, K.H.; Lee, A.R.; Ahn, J.; et al. A decrease in functional microbiomes represented as Faecalibacterium affects immune homeostasis in long-term stable liver transplant patients. Gut Microbes 2022, 14, 2102885. [Google Scholar] [CrossRef]
- Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1826746. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Feng, M.; Gu, J.; Xia, Z.; Zhang, H.; Zheng, S.; Duan, Z.; Hu, R.; Wang, J.; Shi, W.; et al. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-β is critical for immune homeostasis following acute liver injury. J. Mol. Cell Biol. 2013, 5, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, L.; Zhang, L.; Jiang, Z. Effect of Adoptive Transfer or Depletion of Regulatory T Cells on Triptolide-induced Liver Injury. Front. Pharmacol. 2016, 7, 99. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Chen, Y.; Lian, Z.X.; Wei, H.; Tian, Z. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury. Int. Immunopharmacol. 2015, 25, 293–301. [Google Scholar] [CrossRef]
- Lee, S.K.; Park, M.J.; Jhun, J.Y.; Beak, J.A.; Choi, J.W.; Rye, J.Y.; Jang, J.W.; Bae, S.H.; Yoon, S.K.; Choi, H.J.; et al. Combination Treatment With Metformin and Tacrolimus Improves Systemic Immune Cellular Homeostasis by Modulating Treg and Th17 Imbalance. Front. Immunol. 2020, 11, 581728. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- Zhou, D.; Luan, J.; Huang, C.; Li, J. Tumor-Associated Macrophages in Hepatocellular Carcinoma: Friend or Foe? Gut Liver 2021, 15, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef]
- Okoye, I.S.; Houghton, M.; Tyrrell, L.; Barakat, K.; Elahi, S. Coinhibitory Receptor Expression and Immune Checkpoint Blockade: Maintaining a Balance in CD8(+) T Cell Responses to Chronic Viral Infections and Cancer. Front. Immunol. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e302–e310. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Sorafenib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Kuroda, D.; Hayashi, H.; Nitta, H.; Imai, K.; Abe, S.; Hashimoto, D.; Chikamoto, A.; Ishiko, T.; Beppu, T.; Baba, H. Successful treatment for sorafenib-induced liver dysfunction: A report of case with liver biopsy. Surg. Case Rep. 2016, 2, 4. [Google Scholar] [CrossRef]
- Van Hootegem, A.; Verslype, C.; Van Steenbergen, W. Sorafenib-induced liver failure: A case report and review of the literature. Case Rep. Hepatol. 2011, 2011, 941395. [Google Scholar] [CrossRef]
- Shah, R.R.; Morganroth, J.; Shah, D.R. Hepatotoxicity of tyrosine kinase inhibitors: Clinical and regulatory perspectives. Drug Saf. 2013, 36, 491–503. [Google Scholar] [CrossRef]
- Regorafenib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Lenvatinib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Cabozantinib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- AlAsmari, A.F.; Ali, N.; AlAsmari, F.; AlAnazi, W.A.; Alqahtani, F.; Alharbi, M.; Alotaibi, F.M.; Aldossari, A.A.; AlSwayyed, M.; Alanazi, M.M.; et al. Elucidation of the Molecular Mechanisms Underlying Sorafenib-Induced Hepatotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 7453406. [Google Scholar] [CrossRef]
- Han, D.; Shinohara, M.; Ybanez, M.D.; Saberi, B.; Kaplowitz, N. Signal transduction pathways involved in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 196, 267–310. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e416. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Maker, A.V.; Attia, P.; Rosenberg, S.A. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 2005, 175, 7746–7754. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Michot, J.M.; Rosmorduc, O.; Guettier, C.; Samuel, D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020, 2, 100170. [Google Scholar] [CrossRef] [PubMed]
- Remash, D.; Prince, D.S.; McKenzie, C.; Strasser, S.I.; Kao, S.; Liu, K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J. Gastroenterol. 2021, 27, 5376–5391. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Abdullah, A.; Neuman, M.G. Checkpoint Inhibitors and Hepatotoxicity. Biomedicines 2021, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Heinrich, B.; Steinberg, S.M.; Yu, S.J.; Greten, T.F. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J. Immunother. Cancer 2017, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A. Nivolumab (NIVO) + Ipilimumab (IPI) Combination Therapy in Patients (Pts) with Advanced Hepatocellular Carcinoma (aHCC): Results from CheckMate 040; American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Kelley, R.K.; Abou-Alfa, G.K.; Bendell, J.C.; Kim, T.-Y.; Borad, M.J.; Yong, W.-P.; Morse, M.; Kang, Y.-K.; Rebelatto, M.; Makowsky, M. Phase I/II Study of Durvalumab and Tremelimumab in Patients with Unresectable Hepatocellular Carcinoma (HCC): Phase I Safety and Efficacy Analyses; American Society of Clinical Oncology: Alexandria, VA, USA, 2017. [Google Scholar]
- Tsung, I.; Dolan, R.; Lao, C.D.; Fecher, L.; Riggenbach, K.; Yeboah-Korang, A.; Fontana, R.J. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment. Pharmacol. Ther. 2019, 50, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Yeh, M.M. Hepatotoxicity of immune checkpoint inhibitors: A histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 2018, 31, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yao, Z.; Zhou, X.; Zhang, W.; Zhang, X.; Zhang, F. Immune-related adverse events of checkpoint inhibitors: Insights into immunological dysregulation. Clin. Immunol. 2020, 213, 108377. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2020, 64, 29–35. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Mo, Z.S.; Yang, X.H.; Lin, B.L.; Peng, L.; Xu, Y.; Lei, C.Y.; Zhuang, X.D.; Lu, L.; et al. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure. Gut Microbes 2021, 13, 1921925. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Shamsaddini, A.; Fagan, A.; McGeorge, S.; Gavis, E.; Sikaroodi, M.; Brenner, L.A.; Wade, J.B.; Gillevet, P.M. Distinct gut microbial compositional and functional changes associated with impaired inhibitory control in patients with cirrhosis. Gut Microbes 2021, 13, 1953247. [Google Scholar] [CrossRef]
- Temraz, S.; Nassar, F.; Kreidieh, F.; Mukherji, D.; Shamseddine, A.; Nasr, R. Hepatocellular Carcinoma Immunotherapy and the Potential Influence of Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 7800. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Michot, J.-M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Barciela, M.; Barreira-Díaz, A.; Vidal-González, J.; Muñoz-Couselo, E.; Martínez-Valle, F.; Viladomiu, L.; Mínguez, B.; Ortiz-Velez, C.; Castells, L.; Esteban, R. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int. 2020, 40, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Setser, A. The NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new standard for oncology clinical trials. J. Clin. Oncol. 2004, 22, 6098. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef]
- Huffman, B.M.; Kottschade, L.A.; Kamath, P.S.; Markovic, S.N. Hepatotoxicity After Immune Checkpoint Inhibitor Therapy in Melanoma: Natural Progression and Management. Am. J. Clin. Oncol. 2018, 41, 760–765. [Google Scholar] [CrossRef]
- Iwamoto, K.; Ishitsuka, Y.; Tanaka, R.; Sekine, I.; Fujimoto, M. Azathioprine combination therapy for steroid-refractory hepatic immune system-related adverse events. Eur. J. Dermatol. 2017, 27, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Shang, Z.; Sun, X.; Kong, X.; Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf Causality Assessment Method: Emerging role of gut microbiota dysbiosis. Front. Med. (Lausanne) 2022, 9, 972518. [Google Scholar] [CrossRef]
- Adam, R.; McMaster, P.; O’Grady, J.G.; Castaing, D.; Klempnauer, J.L.; Jamieson, N.; Neuhaus, P.; Lerut, J.; Salizzoni, M.; Pollard, S.; et al. Evolution of liver transplantation in Europe: Report of the European Liver Transplant Registry. Liver Transplant. 2003, 9, 1231–1243. [Google Scholar] [CrossRef]
- Cvetkovski, F.; Hexham, J.M.; Berglund, E. Strategies for Liver Transplantation Tolerance. Int. J. Mol. Sci. 2021, 22, 2253. [Google Scholar] [CrossRef]
- Di Maira, T.; Little, E.C.; Berenguer, M. Immunosuppression in liver transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101681. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992, 13, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Everson, G.T. Everolimus and mTOR inhibitors in liver transplantation: Opening the “box”. Liver Transplant. 2006, 12, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Schmeding, M.; Kiessling, A.; Neuhaus, R.; Heidenhain, C.; Bahra, M.; Neuhaus, P.; Neumann, U.P. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation 2011, 92, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, S.W.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Immunological Markers, Prognostic Factors and Challenges Following Curative Treatments for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 10271. [Google Scholar] [CrossRef]
- Tacrolimus. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Sirolimus. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Cyclosporine. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Mycophenolate. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Warren, M.; Mitsinikos, T.; Yanni, G.; Sasaki, M.; Sasaki, A.T.; Thomas, D. Mycophenolate Mofetil Hepatotoxicity Associated With Mitochondrial Abnormality in Liver Transplant Recipients and Mice. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 463–470. [Google Scholar] [CrossRef]

| Drugs | Target for Drug Action | Hepatobiliary Manifestation * | Frequency |
|---|---|---|---|
| Hepatocellular carcinoma—Tyrosine Kinase inhibitors | |||
| Sorafenib | Inhibits VEGFR, PDGFR, and Raf | R-value ≥ 5 (hepatocellular injury) LiverTox category: B | Common |
| Lenvatinib | Inhibits VEGFR, FGF, PDGFR, cKit, and RET proto-oncogene | 2 < R < 5 (mixed liver injury) LiverTox category: D | Common |
| Regorafenib | Inhibits VEGFR, PDGF, RAF kinase, and c-Kit | R-value ≥ 5 (hepatocellular injury) LiverTox category: B | Common |
| Cabozantinib | Inhibits MET, VEGFR-2, and RET | 2 < R < 5 (mixed liver injury) LiverTox category: E | uncommon |
| Hepatocellular carcinoma—Immune checkpoint inhibitors and VEGF(R) inhibitors | |||
| Atezolizumab plus bevacizumab | Inhibits PD-L1, and VEGF | R-value ≥ 5 (hepatocellular injury) LiverTox category: B | 14% |
| Durvalumab plus tremelimumab | Inhibits PD-L1, and CTLA-4 | R-value ≥ 5 (hepatocellular injury) LiverTox category: B | 20% |
| Nivolumab | Inhibits PD-1 | R-value ≥ 5 (hepatocellular injury) LiverTox category: A | 15% |
| Ramucirumab | Inhibits VEGFR-2 | Infrequent liver injury LiverTox category: E | Rare |
| Nivolumab plus ipilimumab | Inhibits PD-1, and CTLA-4 | R-value ≥ 5 (hepatocellular injury) LiverTox category: A | 20% |
| Liver transplantation—Immunosuppressants | |||
| Cyclosporine | Calcineurin inhibition | R-value ≤ 2 (cholestatic liver injury) LiverTox category: C | 1–5% |
| Tacrolimus | Calcineurin inhibition | R-value ≥ 5 (hepatocellular injury) LiverTox category: C | 5–10% |
| Sirolimus/Everolimus | mTOR inhibition | 2 < R < 5 (mixed liver injury) LiverTox category: E | Rare |
| MMF | Antimetabolite (inhibit inosine monophosphate) | R-value ≥ 5 (hepatocellular injury) LiverTox category: D | Rare |
| Immune Cells | Mechanism | Refs. | |
| Treg cells | Reduction in Treg cells and anti-inflammatory cytokines | [107,108] | |
| Th1 cells | Increase in Th1 cells and pro-inflammatory cytokines causing activation of CTLs and macrophages | [107,108,109,110] | |
| CTLs | Stimulate proliferation of CD8+ T cells | [107,108,109,110] | |
| B cells | Ealy B cell changes including the elevation of CD21lo subtype may induce the autoreactive B cells | [111] | |
| Grade of DILI | Definition | Management | [116] |
| Grade 1 | Asymptomatic, T.bil > 1.5×ULN, AST or ALT > 1–3×ULN | Monitoring, continue ICI | [116,117,118,119] |
| Grade 2 | Asymptomatic, T.bil > 1.5–3×ULN, AST or ALT > 3–5×ULN | Discontinue ICI, start 0.5–1.0 mg/kg/day of prednisolone with a taper, consider restart after recovering from DILI | [116,117,118,119] |
| Grade 3 | Symptomatic, Fibrosis, Compensated cirrhosis, T.bil > 3–10×ULN, AST or ALT > 5–20×ULN | Discontinue ICI, 1–2 mg/kg/day of IV methylprednisolone with a taper, consider liver biopsy, consider restart after recovering from DILI | [116,117,118,119] |
| Grade 4 | Decompensated symptom (ascites, encephalopathy, coagulopathy), T.bil > 10×ULN, AST or ALT > 20×ULN | Permanently discontinue ICI, 1–2 mg/kg/of IV methylprednisolone with a taper, consider liver biopsy | [116,117,118,119] |
| Grade 5 | Death due to DILI | [116] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.K.; Choi, J.Y.; Jung, E.S.; Kwon, J.H.; Jang, J.W.; Bae, S.H.; Yoon, S.K. An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 5002. https://doi.org/10.3390/ijms24055002
Lee SK, Choi JY, Jung ES, Kwon JH, Jang JW, Bae SH, Yoon SK. An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation. International Journal of Molecular Sciences. 2023; 24(5):5002. https://doi.org/10.3390/ijms24055002
Chicago/Turabian StyleLee, Soon Kyu, Jong Young Choi, Eun Sun Jung, Jung Hyun Kwon, Jeong Won Jang, Si Hyun Bae, and Seung Kew Yoon. 2023. "An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation" International Journal of Molecular Sciences 24, no. 5: 5002. https://doi.org/10.3390/ijms24055002
APA StyleLee, S. K., Choi, J. Y., Jung, E. S., Kwon, J. H., Jang, J. W., Bae, S. H., & Yoon, S. K. (2023). An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation. International Journal of Molecular Sciences, 24(5), 5002. https://doi.org/10.3390/ijms24055002







