Synthesis of Ester-Containing Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)Arylaldehydes with Oxalates under Metal Free Conditions
Abstract
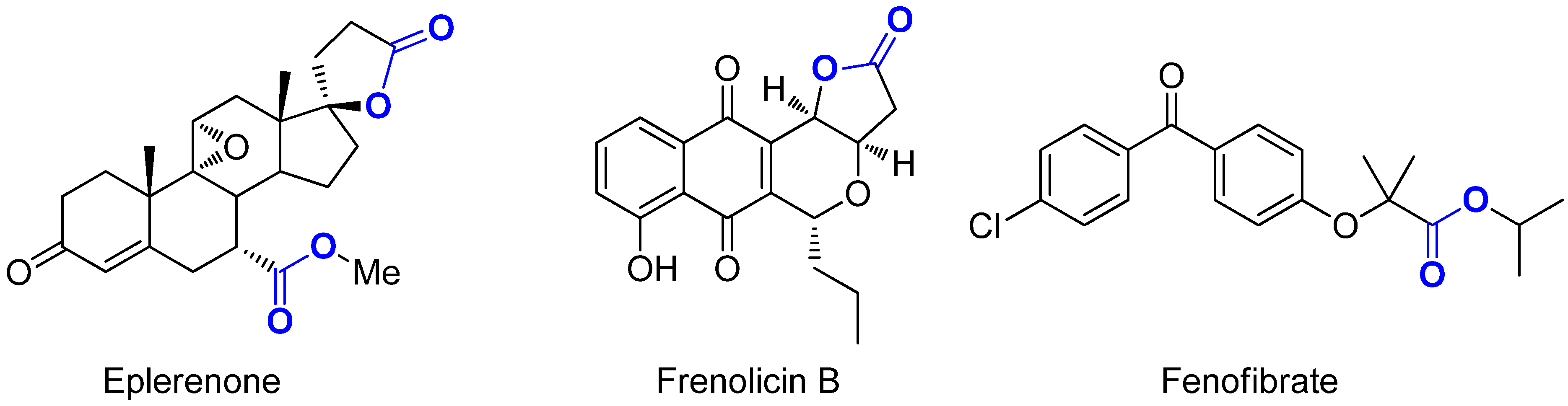
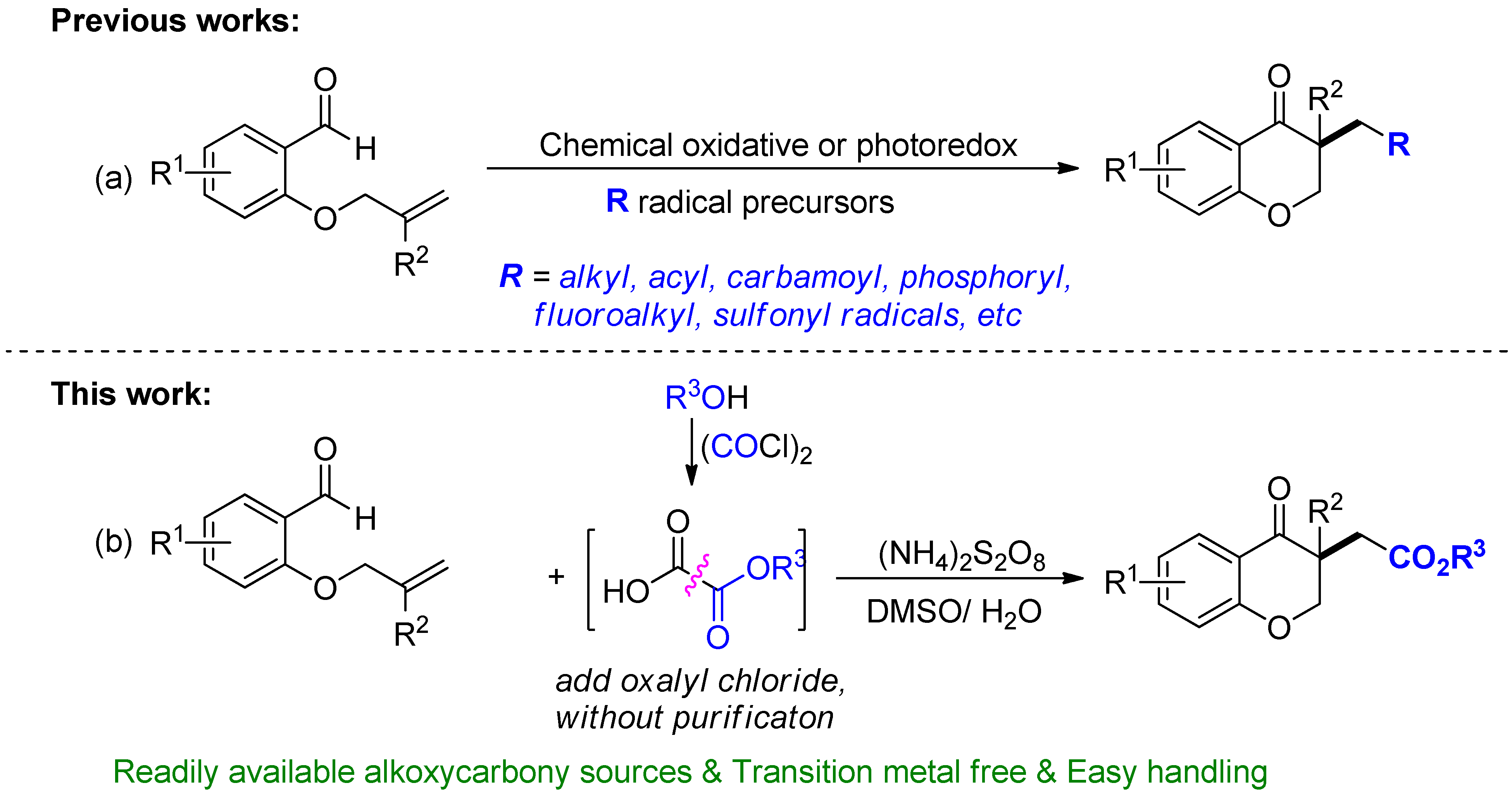
:1. Introduction
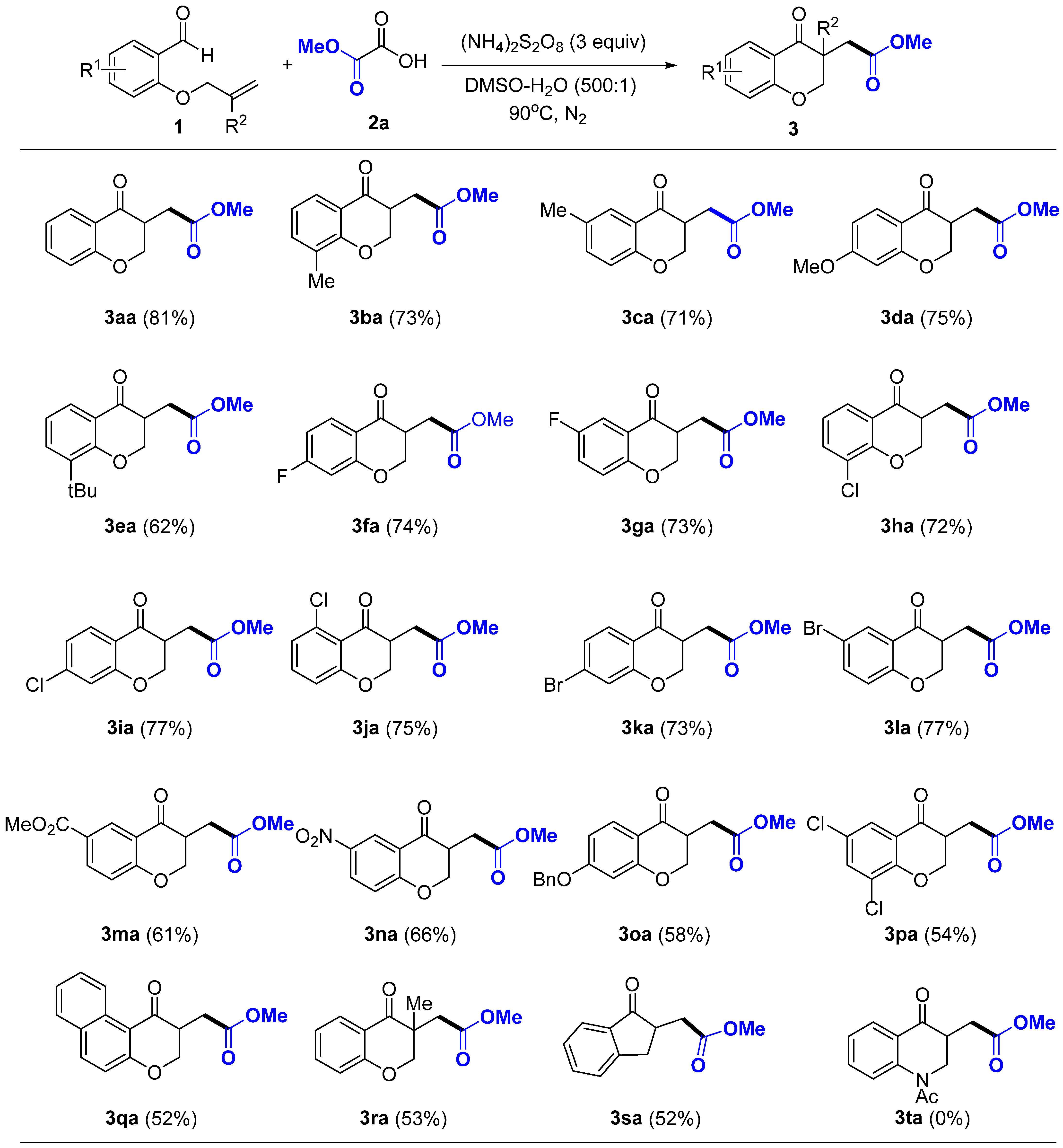
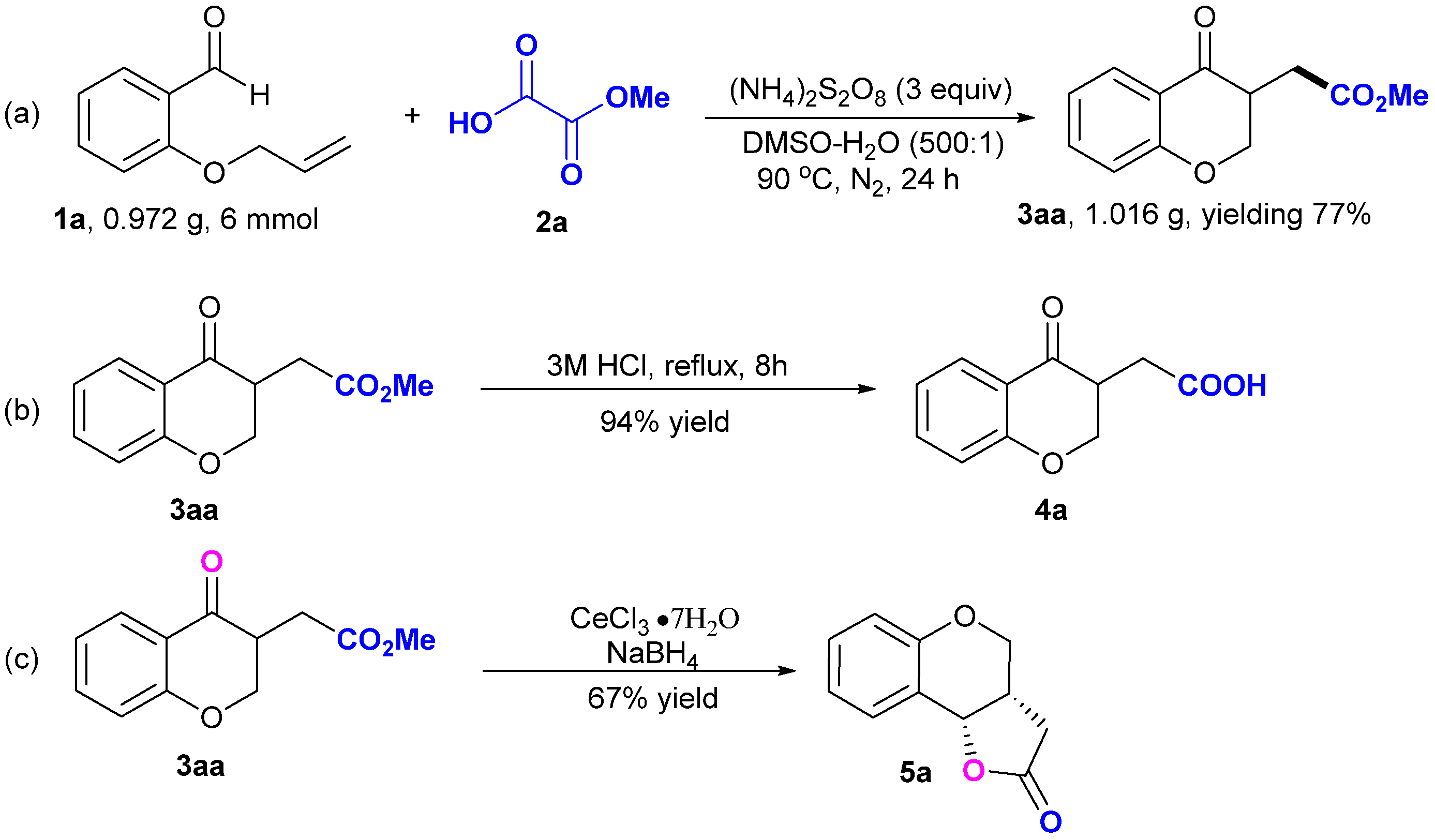
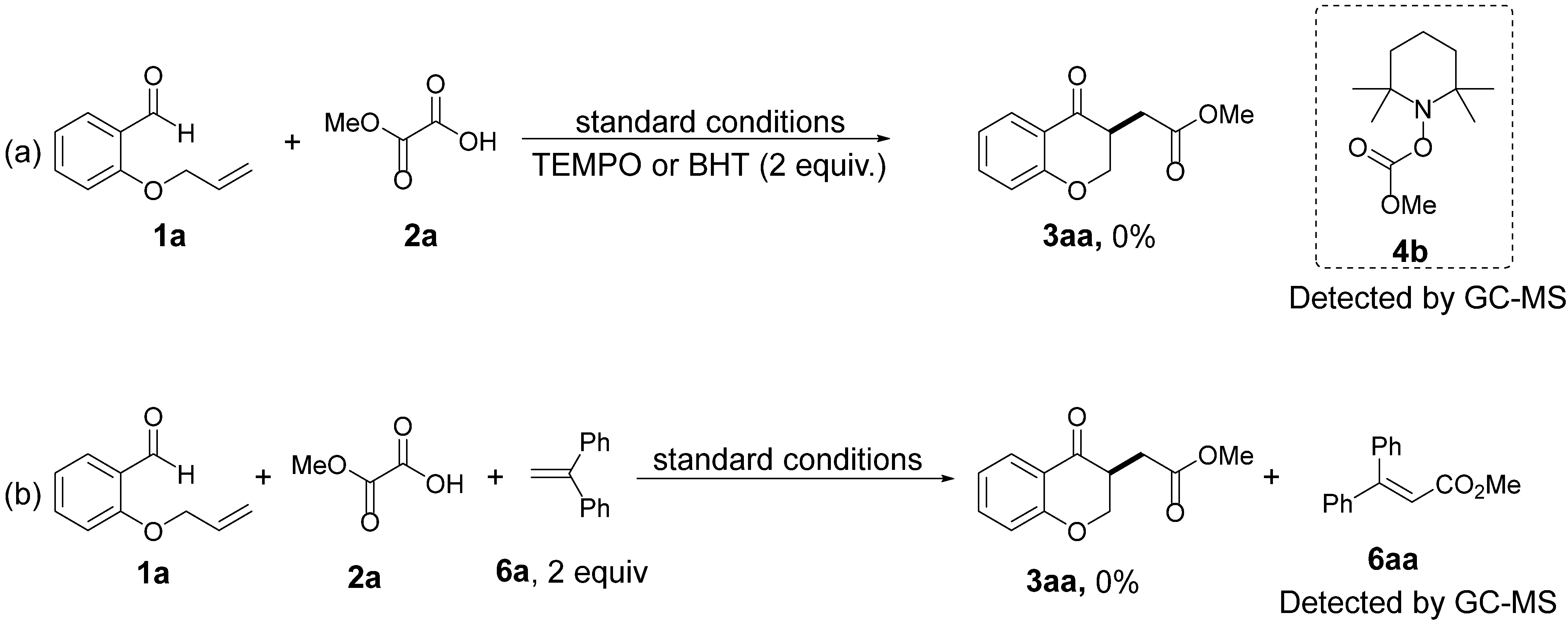
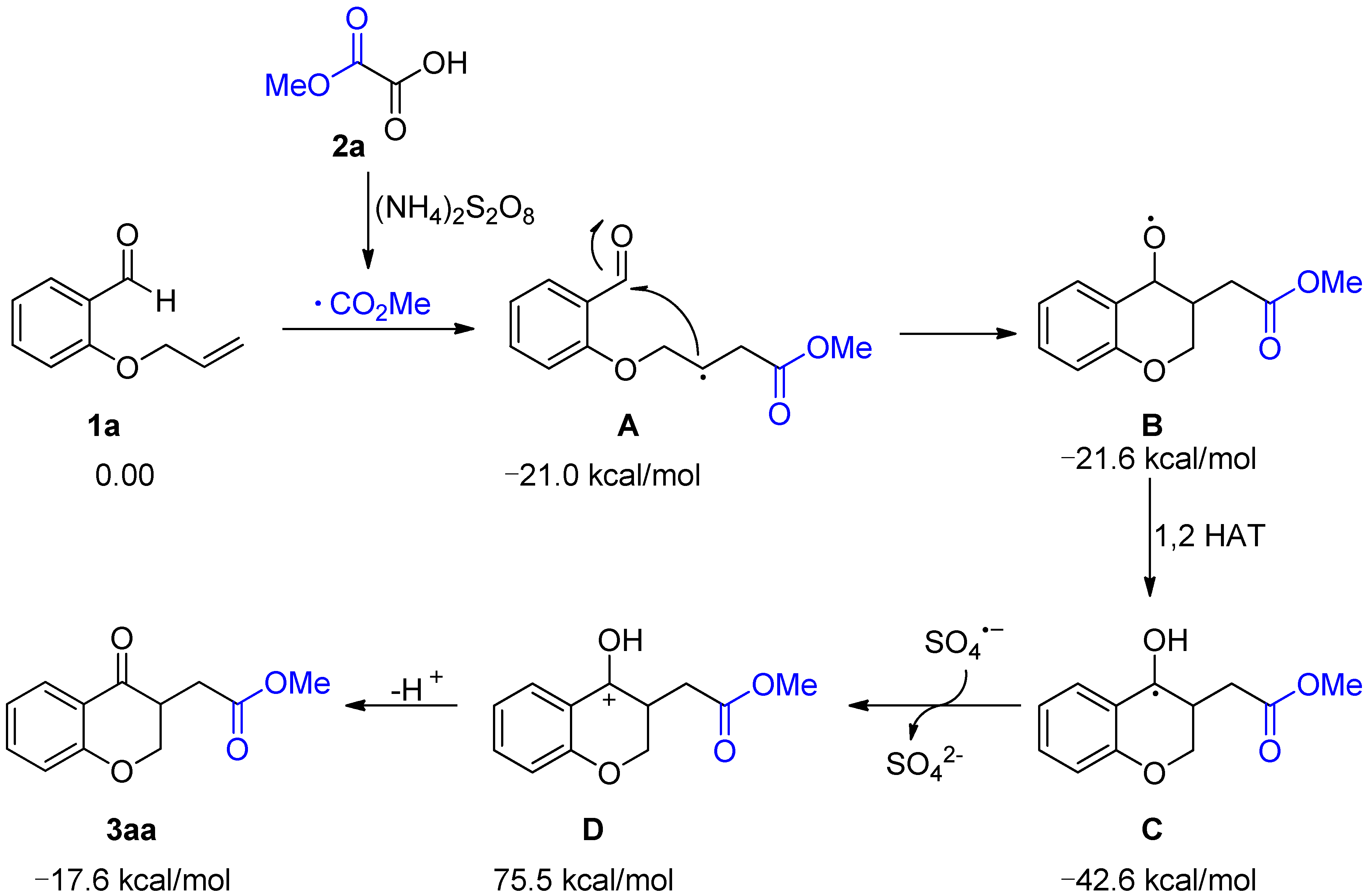
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Target Products 3
3.3. Gram-Scale Synthesis of 3aa
3.4. Characterization Data of Products 3aa–3sa and 3ab–3am
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wei, Z.; Jiao, H.; Jackstell, R.; Beller, M. Toward Green Acylation of (Hetero)arenes: Palladium-Catalyzed Carbonylation of Olefins to Ketones. ACS Cent. Sci. 2018, 4, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Xiao, J. From α-Arylation of Olefins to Acylation with Aldehydes: A Journey in Regiocontrol of the Heck Reaction. Acc. Chem. Res. 2011, 44, 614–626. [Google Scholar] [CrossRef]
- Ryu, I.; Sonoda, N. Free-Radical Carbonylations: Then and Now. Angew. Chem. Int. Ed. 1996, 35, 1050–1066. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mehta, P.H. Heterogeneous Catalysis in Esterification Reactions: Preparation of Phenethyl Acetate and Cyclohexyl Acetate by Using a Variety of Solid Acidic Catalysts. Ind. Eng. Chem. Res. 1994, 33, 2198–2208. [Google Scholar] [CrossRef]
- Zhang, J.; Leitus, G.; Ben-David, Y.; Milstein, D. Efficient Homogeneous Catalytic Hydrogenation of Esters to Alcohols. Angew. Chem. Int. Ed. 2006, 45, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Taffarel, E.; Chirayil, S.; Thummel, R.P. Synthesis and Properties of Ligands Based on Benzo[g]quinoline. J. Org. Chem. 1994, 59, 823–828. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Lei, A. Oxidative Carbonylation Reactions: Organometallic Compounds (R-M) or Hydrocarbons (R-H) as Nucleophiles. Angew. Chem. Int. Ed. 2011, 50, 10788–10799. [Google Scholar] [CrossRef]
- Ryu, I. Radical carboxylations of iodoalkanes and saturated alcohols using carbon monoxide. Chem. Soc. Rev. 2001, 30, 16–25. [Google Scholar] [CrossRef]
- Sang, R.; Hu, Y.; Razzaq, R.; Jackstell, R.; Franke, R.; Beller, M. State-of-the-art palladium-catalyzed alkoxycarbonylations. Org. Chem. Front. 2021, 8, 799–811. [Google Scholar] [CrossRef]
- Willis, M.C. Transition Metal Catalyzed Alkene and Alkyne Hydroacylation. Chem. Rev. 2010, 110, 725–748. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.-C.; Wu, X.-F. Carbonylative synthesis of heterocycles involving diverse CO surrogates. Chem. Commun. 2020, 56, 6016–6030. [Google Scholar] [CrossRef]
- Wu, X.-F.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account. Acc. Chem. Res. 2014, 47, 1041–1053. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef]
- Pan, C.; Fu, Y.; Ni, Q.; Yu, J.-T. Radical Decarboxylation/Annulation of Acrylamides with Aliphatic Acyl Peroxides. J. Org. Chem. 2017, 82, 5005–5010. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, Y.; Hao, W.; Yang, Y.; Sun, Y.; Xu, X. Catalyst-free aerobic radical cascade reactions of o-vinylphenylisocyanides with thiols to access 2-thio-substituted quinolines. Org. Chem. Front. 2022, 9, 6484–6489. [Google Scholar] [CrossRef]
- Yu, W.-Q.; Xiong, B.-Q.; Zhong, L.-J.; Liu, Y. Visible-light-promoted radical cascade alkylation/cyclization: Access to alkylated indolo/benzoimidazo[2,1-a]isoquinolin-6(5H)-ones. Org. Biomol. Chem. 2022, 20, 9659–9671. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Chen, X.-L.; Sun, K.; Zhu, H.-L.; Yuan, X.-Y.; Liu, Y.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Visible-light-induced metal-free cascade cyclization of N-arylpropiolamides to 3-phosphorylated, trifluoromethylated and thiocyanated azaspiro[4.5]trienones. Org. Chem. Front. 2021, 8, 760–766. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Xie, K.-C.; Liu, Y.-T.; Wang, H.; Yin, P.-C.; Qu, L.-B.; Chen, X.-L.; Yu, B. Visible-light-promoted catalyst-/additive-free synthesis of aroylated heterocycles in a sustainable solvent. Green Chem. 2022, 24, 1732–1737. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Zhu, H.-L.; Chen, X.-L.; Qu, L.-B.; Yu, B. Visible light-induced recyclable g-C3N4 catalyzed thiocyanation of C(sp2)–H bonds in sustainable solvents. Green Chem. 2021, 23, 3677–3682. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, W.; Liu, P.; Sun, P. Iron-Catalyzed Regioselective Alkoxycarbonylation of Imidazoheterocycles with Carbazates. J. Org. Chem. 2016, 81, 2482–2487. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Huang, H.; Wang, F.; Hu, X.; Zhang, X. Iron-Catalyzed Oxidative Radical Alkoxycarbonylation of Activated Alkenes with Carbazates toward Alkoxycarbonylated Benzimidazo[2,1-a]isoquinolin-6(5H)-ones. Synlett 2021, 32, 1219–1222. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Y.; Li, X.; Hong, G.; Fang, M.; Du, X. Iron-Catalyzed Arylalkoxycarbonylation of N-Aryl Acrylamides with Carbazates. J. Org. Chem. 2014, 79, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Han, J.; Zhang, H.; Zhu, C. Radical Arylalkoxycarbonylation of 2-Isocyanobiphenyl with Carbazates: Dual C–C Bond Formation toward Phenanthridine-6-carboxylates. J. Org. Chem. 2014, 79, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, X.; Zhuang, S.; Liu, P.; Sun, P. Photoredox Catalysis: Construction of Polyheterocycles via Alkoxycarbonylation/Addition/Cyclization Sequence. Org. Lett. 2017, 19, 3580–3583. [Google Scholar] [CrossRef]
- Ji, M.; Xu, L.; Luo, X.; Jiang, M.; Wang, S.; Chen, J.-Q.; Wu, J. Alkoxycarbonyl radicals from alkyloxalyl chlorides: Photoinduced synthesis of isoquinolinediones under visible light irradiation. Org. Chem. Front. 2021, 8, 6704–6709. [Google Scholar] [CrossRef]
- 2Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A Valid Scaffold in Medicinal Chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977–36999. [Google Scholar] [CrossRef] [Green Version]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef]
- Seifert, T.; Malo, M.; Kokkola, T.; Engen, K.; Fridén-Saxin, M.; Wallén, E.A.A.; Lahtela-Kakkonen, M.; Jarho, E.M.; Luthman, K. Chroman-4-one- and Chromone-Based Sirtuin 2 Inhibitors with Antiproliferative Properties in Cancer Cells. J. Med. Chem. 2014, 57, 9870–9888. [Google Scholar] [CrossRef]
- Fridén-Saxin, M.; Seifert, T.; Landergren, M.R.; Suuronen, T.; Lahtela-Kakkonen, M.; Jarho, E.M.; Luthman, K. Synthesis and Evaluation of Substituted Chroman-4-one and Chromone Derivatives as Sirtuin 2-Selective Inhibitors. J. Med. Chem. 2012, 55, 7104–7113. [Google Scholar] [CrossRef]
- Das, S.; Parida, S.K.; Mandal, T.; Sing, L.; De Sarkar, S.; Murarka, S. Organophotoredox-Catalyzed Cascade Radical Annulation of 2-(Allyloxy)arylaldehydes with N-(acyloxy)phthalimides: Towards Alkylated Chroman-4-one Derivatives. Chem. Asian J. 2020, 15, 568–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-K.; Cai, B.-G.; Yang, Q.-Q.; Wang, L.; Xuan, J. Visible-Light-Promoted Cascade Radical Cyclization: Synthesis of 1,4-Diketones Containing Chroman-4-One Skeletons. Chem. Asian J. 2019, 14, 3269–3273. [Google Scholar] [CrossRef]
- Hu, H.; Chen, X.; Sun, K.; Wang, J.; Liu, Y.; Liu, H.; Fan, L.; Yu, B.; Sun, Y.; Qu, L.; et al. Silver-Catalyzed Radical Cascade Cyclization toward 1,5-/1,3-Dicarbonyl Heterocycles: An Atom-/Step-Economical Strategy Leading to Chromenopyridines and Isoxazole-/Pyrazole-Containing Chroman-4-Ones. Org. Lett. 2018, 20, 6157–6160. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Du, J.-Y.; Li, Q.-L.; Gao, F.; Ma, C.-L. Visible-Light-Promoted Cascade Radical Cyclization: Synthesis of Chroman-4-ones and Dihydroquinolin-4-ones. J. Org. Chem. 2020, 85, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-C.; Liu, W.; Wu, H.-Y.; Zhou, Y.-B.; Ding, Q.; Peng, Y. Transition-metal-free synthesis of CMe2CF3-containing chroman-4-ones via decarboxylative trifluoroalkylation. Org. Chem. Front. 2020, 7, 487–491. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, W.; Xie, G.; Wang, X. Metal-free synthesis of phosphinoylchroman-4-ones via a radical phosphinoylation–cyclization cascade mediated by K2S2O8. Beilstein J. Org. Chem. 2020, 16, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, Y.; Zhang, J.; Yu, Y.; Tan, Z.; Zhu, G. Copper-catalyzed acyltrifluoromethylation of alkenes: Rapid access to trifluoroethyl indanones and related compounds. Chem. Commun. 2017, 53, 6440–6443. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, J.; Chen, L.; Zhang, L.; Zheng, L.; Wei, X. Silver-catalyzed cascade radical cyclization of 2-(allyloxy)arylaldehydes with cyclopropanols: Access to chroman-4-one derivatives. Org. Chem. Front. 2019, 6, 1471–1475. [Google Scholar] [CrossRef]
- 3Tang, L.; Yang, Z.; Chang, X.; Jiao, J.; Ma, X.; Rao, W.; Zhou, Q.; Zheng, L. K2S2O8-Mediated Selective Trifluoromethylacylation and Trifluoromethylarylation of Alkenes under Transition-Metal-Free Conditions: Synthetic Scope and Mechanistic Studies. Org. Lett. 2018, 20, 6520–6525. [Google Scholar]
- Xiao, Y.-M.; Liu, Y.; Mai, W.-P.; Mao, P.; Yuan, J.-W.; Yang, L.-R. A Novel and Facile Synthesis of Chroman-4-one Derivatives via Cascade Radical Cyclization Under Metal-free Condition. ChemistrySelect 2019, 4, 1939–1942. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Peng, S.; Yang, L.-H.; Liu, X.-W. Metal-Free Synthesis of Carbamoylated Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)arylaldehydes with Oxamic Acids. Molecules 2022, 27, 7049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wu, M.; Zhang, M.; Zhou, X. Visible-Light-Induced Difluoroacetylation of O-(Allyloxy)Aryl-Aldehydes: Access to Difluoroacetylated Chroman-4-ones. Asian J. Org. Chem. 2019, 8, 828–831. [Google Scholar] [CrossRef]
- Santoso, H.; Casana, M.I.; Donner, C.D. Exploring O-stannyl ketyl and acyl radical cyclizations for the synthesis of γ-lactone-fused benzopyrans and benzofurans. Org. Biomol. Chem. 2014, 12, 171–176. [Google Scholar] [CrossRef]
- Donner, C.D.; Casana, M.I. Synthesis of novel pyranoquinones using an acyl radical cyclization strategy. Tetrahedron Lett. 2012, 53, 1105–1107. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Liu, J.; Ruan, S.; Li, P. Facile synthesis of carbamoylated benzimidazo[2,1-a]isoquinolin-6(5H)-ones via radical cascade cyclization under metal-free conditions. Org. Biomol. Chem. 2021, 19, 3489–3496. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Tu, X.; Qin, B.; Huang, S.; Zhang, J.; Wu, J. Synthesis of Ester-Substituted Indolo[2,1-a]isoquinolines via Photocatalyzed Alkoxycarbonylation/Cyclization Reactions. Org. Lett. 2022, 24, 642–647. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03. 2016; Gaussian Inc.: Wallingford, CT, USA, 2016; Available online: https://scholar.google.com/scholar_lookup?title=Gaussian+16,+Revision+B.01&author=M.J.+Frisch&author=G.W.+Trucks&author=H.B.+Schlegel&author=G.E.+Scuseria&author=M.A.+Robb&publication_year=2016& (accessed on 1 December 2022).
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A At. Mol. Opt. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]

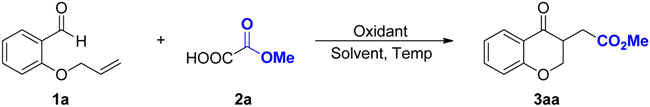 | ||||
|---|---|---|---|---|
| Entry | Oxidant | Solvent | Temp (°C) | Yield of 3aa b |
| 1 | (NH4)2S2O8 | DMSO | 80 | 71% |
| 2 | (NH4)2S2O8 | DMF | 80 | 0% |
| 3 | (NH4)2S2O8 | CH3CN | 80 | 0% |
| 4 | (NH4)2S2O8 | DCE | 80 | 0% |
| 5 | (NH4)2S2O8 | THF | 80 | 0% |
| 6 | (NH4)2S2O8 | H2O | 80 | 0% |
| 7 | (NH4)2S2O8 | DMSO/H2O (500:1) | 80 | 76% |
| 8 | (NH4)2S2O8 | DMSO/H2O (100:1) | 80 | 71% |
| 9 | (NH4)2S2O8 | DMSO/H2O (10:1) | 80 | 44% |
| 10 | (NH4)2S2O8 | DMSO/H2O (5:1) | 80 | 17% |
| 11 | K2S2O8 | DMSO/H2O (500:1) | 80 | 0% |
| 12 | Na2S2O8 | DMSO/H2O (500:1) | 80 | 0% |
| 13 | TBHP | DMSO/H2O (500:1) | 80 | 0% |
| 14 | Selectfluor | DMSO/H2O (500:1) | 80 | 0% |
| 15 | PhI(OAc)2 | DMSO/H2O (500:1) | 80 | 0% |
| 16 | (NH4)2S2O8 | DMSO/H2O (500:1) | 90 | 81% |
| 17 | (NH4)2S2O8 | DMSO/H2O (500:1) | 100 | 79% |
| 18 | (NH4)2S2O8 | DMSO/H2O (500:1) | 70 | 34% |
| 19 c | (NH4)2S2O8 | DMSO/H2O (500:1) | 90 | 57% |
| 20 d | (NH4)2S2O8 | DMSO/H2O (500:1) | 90 | 64% |
| 21 | DMSO/H2O (500:1) | 90 | 0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Liu, J.; Jiang, Z.-Q.; Yuan, L.; Liu, X.-W.; Xie, L.-Y. Synthesis of Ester-Containing Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)Arylaldehydes with Oxalates under Metal Free Conditions. Int. J. Mol. Sci. 2023, 24, 5028. https://doi.org/10.3390/ijms24055028
Peng S, Liu J, Jiang Z-Q, Yuan L, Liu X-W, Xie L-Y. Synthesis of Ester-Containing Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)Arylaldehydes with Oxalates under Metal Free Conditions. International Journal of Molecular Sciences. 2023; 24(5):5028. https://doi.org/10.3390/ijms24055028
Chicago/Turabian StylePeng, Sha, Jiao Liu, Ze-Qun Jiang, Lin Yuan, Xiao-Wen Liu, and Long-Yong Xie. 2023. "Synthesis of Ester-Containing Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)Arylaldehydes with Oxalates under Metal Free Conditions" International Journal of Molecular Sciences 24, no. 5: 5028. https://doi.org/10.3390/ijms24055028
APA StylePeng, S., Liu, J., Jiang, Z.-Q., Yuan, L., Liu, X.-W., & Xie, L.-Y. (2023). Synthesis of Ester-Containing Chroman-4-Ones via Cascade Radical Annulation of 2-(Allyloxy)Arylaldehydes with Oxalates under Metal Free Conditions. International Journal of Molecular Sciences, 24(5), 5028. https://doi.org/10.3390/ijms24055028







