Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway
Abstract
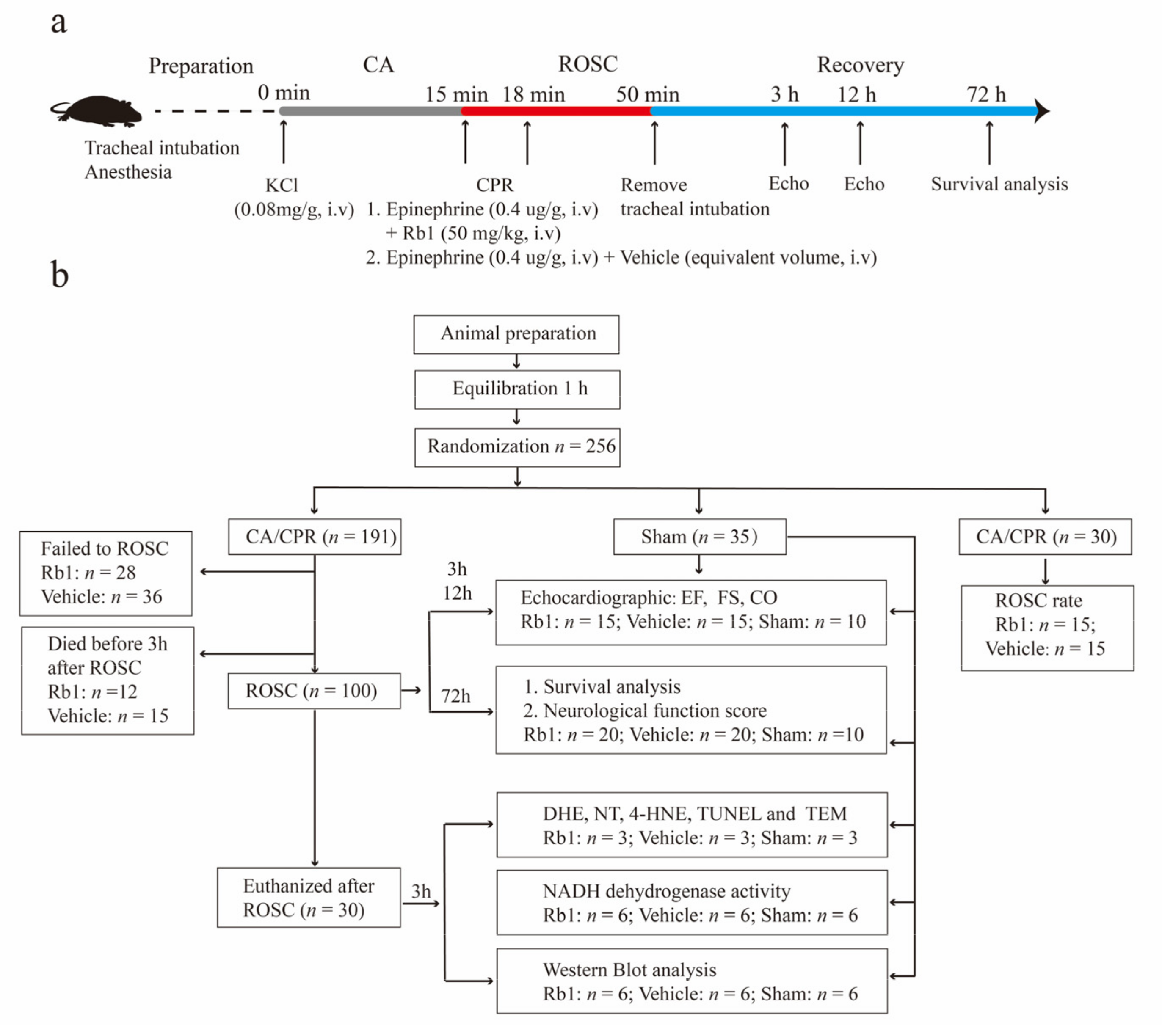
:1. Introduction
2. Results
2.1. Baseline and Procedural Characteristics of the Animals
2.2. Gn-Rb1 Treatment Improved the Prognosis of CA/CPR Mice
2.3. Gn-Rb1 Attenuated Myocardial Oxidative Stress Following CA/CPR
2.4. Gn-Rb1 Improves Mitochondrial Homeostasis and Energy Metabolism following CA/CPR
2.5. Gn-Rb1 Activates the Keap1/Nrf2 Signaling Pathway
2.6. Gene Knockdown of Nrf2 Attenuates the Ameliorative Effect of Gn-Rb1 on Oxidative Stress after Hypoxia/Reoxygenation(H/R)
2.7. Gene Knockdown of Nrf2 Attenuates the Ameliorative Effect of Gn-Rb1 on Mitochondrial Injury and Metabolic Destabilization after Hypoxia/Reoxygenation
2.8. Gn-Rb1 Treatment Improved Neurological Outcomes
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Experimental Animals
4.3. Echocardiography
4.4. Immunohistochemistry
4.5. Dihydroethidium (DHE) Staining
4.6. Transmission Electron Microscopy (TEM)
4.7. Western Blot Analysis
4.8. Real-Time Quantitative PCR
4.9. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labelling (TUNEL) Assay
4.10. Mitochondrial Isolation
4.11. Cell Culture and Transfection
4.12. Detection of Cellular Reactive Oxygen Species
4.13. Detection of Mitochondrial ROS (mROS)
4.14. Assessment of Neurological Function
4.15. Cellular ATP Assay
4.16. Mitochondrial Membrane Potential Examination
4.17. NADH Dehydrogenase Activity Assays
4.18. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdalla, M.; Mohamed, A.; Mohamed, W.; Khtab, K.; Cattoni, H.; Salih, M. Targeted temperature management after cardiac arrest: Updated meta-analysis of all-cause mortality and neurological outcomes. Int. J. Cardiol. Heart Vasc. 2019, 24, 100400. [Google Scholar] [CrossRef] [PubMed]
- Fazel Bakhsheshi, M.; Wang, Y.; Keenliside, L.; Lee, T.Y. A new approach to selective brain cooling by a Ranque-Hilsch vortex tube. Intensive Care Med. Exp. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- National Center for Cardiovascular Diseases. Annual report on cardiovascular health and diseases in China. J. Cardiovasc. Pulm. Dis. 2021, 40, 1005–1009. [Google Scholar]
- Sharp, W.W. Dynamin-related protein 1 as a therapeutic target in cardiac arrest. J. Mol. Med. 2015, 93, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.L.; Zhang, L.; Jin, Z.; Kasumov, T.; Chen, Y.R. Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am. J. Physiol. Cell Physiol. 2022, 322, C12–C23. [Google Scholar] [CrossRef]
- Okuma, Y.; Becker, L.B.; Hayashida, K.; Aoki, T.; Saeki, K.; Nishikimi, M.; Shoaib, M.; Miyara, S.J.; Yin, T.; Shinozaki, K. Effects of Post-Resuscitation Normoxic Therapy on Oxygen-Sensitive Oxidative Stress in a Rat Model of Cardiac Arrest. J. Am. Heart Assoc. 2021, 10, e018773. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. The Preparation of Ginsenoside Rg5, Its Antitumor Activity against Breast Cancer Cells and Its Targeting of PI3K. Nutrients 2020, 12, 246. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Lu, Q.; Wu, J.; Li, Y.; Sun, J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: A randomized, placebo-controlled study. Inflammopharmacology 2017, 25, 33–40. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, X.; Chen, Y.H.; Chen, Y.; Jiang, W.; Zheng, H.; Huang, F.Q.; Liu, B.; Zhou, W.; Qi, L.W.; et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics 2021, 11, 1703–1720. [Google Scholar] [CrossRef]
- Fan, H.J.; Tan, Z.B.; Wu, Y.T.; Feng, X.R.; Bi, Y.M.; Xie, L.P.; Zhang, W.T.; Ming, Z.; Liu, B.; Zhou, Y.C. The role of ginsenoside Rb1, a potential natural glutathione reductase agonist, in preventing oxidative stress-induced apoptosis of H9C2 cells. J. Ginseng Res. 2020, 44, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Fu, F.; Lai, Q.; Zhang, L.; Liu, T.; Yu, B.; Kou, J.; Li, F. Cardioprotective effect of ginsenoside Rb1 via regulating metabolomics profiling and AMP-activated protein kinase-dependent mitophagy. J. Ginseng Res. 2022, 46, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.Q.; Shan, C.S.; Shi, Y.H.; Wang, Y.; Zheng, G.Q. Combined Use of Emodin and Ginsenoside Rb1 Exerts Synergistic Neuroprotection in Cerebral Ischemia/Reperfusion Rats. Front. Pharmacol. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, M.; Yuan, H.; Meng, C.; Zhang, B.; Wu, H. Ginsenoside Rb1 protects against spinal cord ischemia-reperfusion injury in rats by downregulating the Bax/Bcl-2 ratio and caspase-3 and p-Ask-1 levels. Exp. Mol. Pathol. 2018, 105, 229–235. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, L.; Li, S.; Yang, G. Protective effect of ginsenoside Rb1 against lung injury induced by intestinal ischemia-reperfusion in rats. Molecules 2013, 18, 1214–1226. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Liu, H.M.; Lei, S.Q.; Su, W.T.; Duan, W.N.; Wu, Y.; Xia, Z.Y.; Xia, Z.Y. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. PLoS ONE 2013, 8, e80859. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.C.; Pan, C.S.; Yan, L.; Li, L.; Hu, B.H.; Chang, X.; Liu, Y.Y.; Fan, J.Y.; Sun, K.; Li, Q.; et al. Ginsenoside Rb1 protects against ischemia/reperfusion- induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci. Rep. 2017, 7, 44579. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Zheng, G.Q.; Wang, Y. Ginsenoside Rb1 for Myocardial Ischemia/Reperfusion Injury: Preclinical Evidence and Possible Mechanisms. Oxid. Med. Cell Longev. 2017, 2017, 6313625. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.Y.; Liu, D.H.; Wu, L.; Yu, X.G.; Wang, M.; Shi, G.Y.; Wen, R.H.; Zhou, B.; Hao, B.S.; Liu, Y.; et al. Ginsenoside Rb1 Ameliorates Age-Related Myocardial Dysfunction by Regulating the NF-B Signaling Pathway. Am. J. Chin. Med. 2020, 48, 1369–1383. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, S.; Zou, X.; Jing, Y.; Yang, R.; Li, S.; Wang, F. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim. 2017, 66, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wang, J.; Zhao, R.; Zhang, X.; Mei, Y. Ginsenoside-Rb1 Improved Diabetic Cardiomyopathy through Regulating Calcium Signaling by Alleviating Protein O-GlcNAcylation. J. Agric. Food Chem. 2019, 67, 14074–14085. [Google Scholar] [CrossRef]
- Shoaib, M.; Kim, N.; Choudhary, R.C.; Yin, T.; Shinozaki, K.; Becker, L.B.; Kim, J. Increased plasma disequilibrium between pro- and anti-oxidants during the early phase resuscitation after cardiac arrest is associated with increased levels of oxidative stress end-products. Mol. Med. 2021, 27, 135. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Berry, B.J.; Wojtovich, A.P. Physiologic Implications of Reactive Oxygen Species Production by Mitochondrial Complex I Reverse Electron Transport. Antioxidants 2019, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Guaricci, A.I.; Bulzis, G.; Pontone, G.; Scicchitano, P.; Carbonara, R.; Rabbat, M.; De Santis, D.; Ciccone, M.M. Current interpretation of myocardial stunning. Trends Cardiovasc. Med. 2018, 28, 263–271. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 2, 4376. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Shen, J.; Yang, B.; Dai, W.; Yan, J.; Maimouni, S.; Daguplo, H.Q.; Coppola, S.; Gao, Y.; et al. The Ubiquitin E3 Ligase TRIM21 Promotes Hepatocarcinogenesis by Suppressing the p62-Keap1-Nrf2 Antioxidant Pathway. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1369–1385. [Google Scholar] [CrossRef]
- Tan, M.K.; Lim, H.J.; Bennett, E.J.; Shi, Y.; Harper, J.W. Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol. Cell 2013, 52, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Xia, Z.Y. Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced renal injury by activating Nrf2/ARE pathway. Molecules 2012, 17, 7195–7205. [Google Scholar] [CrossRef]
- Ye, J.; Yao, J.P.; Wang, X.; Zheng, M.; Li, P.; He, C.; Wan, J.B.; Yao, X.; Su, H. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury. Mol Med. Rep. 2016, 13, 3083–3091. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Bailén, M.; Aguayo de Hoyos, E.; Ruiz-Navarro, S.; Díaz-Castellanos, M.A.; Rucabado-Aguilar, L.; Gómez-Jiménez, F.J.; Martínez-Escobar, S.; Moreno, R.M.; Fierro-Rosón, J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 2005, 6, 175–181. [Google Scholar] [CrossRef]
- Xu, T.; Tang, W.; Ristagno, G.; Wang, H.; Sun, S.; Weil, M.H. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit. Care Med. 2008, 36, 188–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, M.; Ho, K.M.; Hong, Y. Subphenotypes of Cardiac Arrest Patients Admitted to Intensive Care Unit: A latent profile analysis of a large critical care database. Sci. Rep. 2019, 9, 13644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Miao, Y.; Zhang, T.; Mu, N.; Ruan, L.; Duan, J.; Zhu, Y.; Zhang, R. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J. Pharm. Pharmacol. 2018, 70, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Yang, P.; Jiang, Y.L.; Lin, Z.; Pu, Y.W.; Xie, L.Q.; Sun, L.; Lu, D. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomed. Pharmacother. 2020, 125, 109913. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.W.; Lu, P.; Peng, L.; Jiang, W. Ginsenoside Rb1 Inhibits Cardiomyocyte Autophagy via PI3K/Akt/mTOR Signaling Pathway and Reduces Myocardial Ischemia/Reperfusion Injury. Am. J. Chin. Med. 2021, 49, 1913–1927. [Google Scholar] [CrossRef]
- Li, G.; Qian, W.; Zhao, C. Analyzing the anti-ischemia-reperfusion injury effects of ginsenoside Rb1 mediated through the inhibition of p38α MAPK. Can. J. Physiol. Pharmacol. 2016, 94, 97–103. [Google Scholar] [CrossRef]
- Rathore, P.; Arora, I.; Rastogi, S.; Akhtar, M.; Singh, S.; Samim, M. Collagen Nanoparticle-Mediated Brain Silymarin Delivery: An Approach for Treating Cerebral Ischemia and Reperfusion-Induced Brain Injury. Front. Neurosci. 2020, 14, 538404. [Google Scholar] [CrossRef]
- Peng, S.; Lu, X.F.; Qi, Y.D.; Li, J.; Xu, J.; Yuan, T.Y.; Wu, X.Y.; Ding, Y.; Li, W.H.; Zhou, G.Q.; et al. LCZ696 Ameliorates Oxidative Stress and Pressure Overload-Induced Pathological Cardiac Remodeling by Regulating the Sirt3/MnSOD Pathway. Oxid. Med. Cell Longev. 2020, 2020, 9815039. [Google Scholar] [CrossRef]
- Ye, J.T.; Li, F.T.; Huang, S.L.; Xue, J.L.; Aihaiti, Y.; Wu, H.; Liu, R.X.; Cheng, B. Effects of ginsenoside Rb1 on spinal cord ischemia-reperfusion injury in rats. J. Orthop. Surg. Res. 2019, 14, 259. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, X.; Wang, Y.; Mu, P.; Chen, C.; Huang, P.; Liu, D. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 2019, 19, 3633–3641. [Google Scholar] [CrossRef]
- Tsai, M.S.; Huang, C.H.; Wang, C.H.; Cheng, H.J.; Wu, S.N.; Chang, W.T.; Chen, W.J. Post-Cardiac Arrest Hydrocortisone Use Ameliorates Cardiac Mitochondrial Injury in a Male Rat Model of Ventricular Fibrillation Cardiac Arrest. J. Am. Heart Assoc. 2021, 10, e019837. [Google Scholar] [CrossRef]
- Kohlhauer, M.; Panel, M.; Roches, M.V.D.; Faucher, E.; Abi Zeid Daou, Y.; Boissady, E.; Lidouren, F.; Ghaleh, B.; Morin, D.; Tissier, R. Brain and Myocardial Mitochondria Follow Different Patterns of Dysfunction After Cardiac Arrest. Shock 2021, 56, 857–864. [Google Scholar] [CrossRef]
- Donnino, M.W.; Liu, X.; Andersen, L.W.; Rittenberger, J.C.; Abella, B.S.; Gaieski, D.F.; Ornato, J.P.; Gazmuri, R.J.; Grossestreuer, A.V.; Cocchi, M.N.; et al. National Post Arrest Research Consortium (NPARC) Investigators. Characterization of mitochondrial injury after cardiac arrest (COMICA). Resuscitation 2017, 113, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Chen, H.; Qin, J.; Chen, N.; Lu, S.; Jin, J.; Li, Y. Baicalin Improves Cardiac Outcome and Survival by Suppressing Drp1-Mediated Mitochondrial Fission after Cardiac Arrest-Induced Myocardial Damage. Oxid. Med. Cell Longev. 2021, 2021, 8865762. [Google Scholar] [CrossRef]
- Sharp, W.W.; Beiser, D.G.; Fang, Y.H.; Han, M.; Piao, L.; Varughese, J.; Archer, S.L. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit. Care Med. 2015, 43, e38–e47. [Google Scholar] [CrossRef]
- Ni, X.C.; Wang, H.F.; Cai, Y.Y.; Yang, D.; Alolga, R.N.; Liu, B.; Li, J.; Huang, F.Q. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022, 54, 102363. [Google Scholar] [CrossRef]
- Su, L.J.; Ren, Y.C.; Chen, Z.; Ma, H.F.; Zheng, F.; Li, F.; Zhang, Y.Y.; Gong, S.S.; Kou, J.P. Ginsenoside Rb1 improves brain, lung, and intestinal barrier damage in middle cerebral artery occlusion/reperfusion (MCAO/R) micevia the PPARγ signaling pathway. Chin. J. Nat. Med. 2022, 20, 561–571. [Google Scholar] [CrossRef]
- Xie, W.; Wang, X.; Xiao, T.; Cao, Y.; Wu, Y.; Yang, D.; Zhang, S. Protective Effects and Network Analysis of Ginsenoside Rb1 Against Cerebral Ischemia Injury: A Pharmacological Review. Front. Pharmacol. 2021, 12, 604811. [Google Scholar] [CrossRef]
- Huang, X.P.; Qiu, Y.Y.; Wang, B.; Ding, H.; Tang, Y.H.; Zeng, R.; Deng, C.Q. Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn. Mag. 2014, 10, 402–409. [Google Scholar]
- Li, F.; Lv, Y.N.; Tan, Y.S.; Shen, K.; Zhai, K.F.; Chen, H.L.; Kou, J.P.; Yu, B.Y. An integrated pathway interaction network for the combination of four effective compounds from ShengMai preparations in the treatment of cardio-cerebral ischemic diseases. Acta Pharmacol. Sin. 2015, 36, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Czibik, G.; Bellahcene, M.; Aksentijević, D.; Smith, A.C.; Mitchell, S.J.; Dodd, M.S.; Kirwan, J.; Byrne, J.J.; Ludwig, C.; et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012, 15, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.Q.; Li, Y.J.; Wang, Y.H.; Wang, Z.; Fang, L.; Shen, L.; Lu, Y.Q.; Shen, L.H.; He, B. Salvianolic Acid B Improves Postresuscitation Myocardial and Cerebral Outcomes in a Murine Model of Cardiac Arrest: Involvement of Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 1605456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hou, H.; Lin, V.; Ho, D.; Tran, K.; Che, B.; May, A.; Zhang, J.; Lu, Z.; Lu, Z.; et al. Probucol Protects Rats from Cardiac Dysfunction Induced by Oxidative Stress following Cardiopulmonary Resuscitation. Oxid. Med. Cell Longev. 2017, 2017, 1284804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascia, G.; Arbelo, E.; Hernandez-Ojeda, J.; Solimene, F.; Brugada, R.; Brugada, J. Brugada Syndrome and Exercise Practice: Current Knowledge, Shortcomings and Open Questions. Int. J. Sport. Med. 2017, 38, 573–581. [Google Scholar]
- Klein, A.; Grand, J.; Meyer, M.A.S.; Wiberg, S.; Mogelvang, R.; Vejlstrup, N.; Schousboe, B.; Gjedsted, J.; Oestergaard, M.; Wanscher, M.; et al. Global myocardial oedema in resuscitated out-of-hospital cardiac arrest patients assessed by cardiac magnetic resonance: A pilot study. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 53–57. [Google Scholar] [CrossRef]
- Qin, C.X.; May, L.T.; Li, R.; Cao, N.; Rosli, S.; Deo, M.; Alexander, A.E.; Horlock, D.; Bourke, J.E.; Yang, Y.H.; et al. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat. Commun. 2017, 8, 14232. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, K.; Bagchi, A.; Miyazaki, Y.; Hirai, S.; Seth, D.; Silverman, M.G.; Rezoagli, E.; Marutani, E.; Mori, N.; Magliocca, A.; et al. Improvement in Outcomes After Cardiac Arrest and Resuscitation by Inhibition of S-Nitrosoglutathione Reductase. Circulation 2019, 139, 815–827. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Geng, N.; Chen, T.; Xiao, Q.; Zhang, H.; Huo, H.; Jiang, L.; Shao, Q.; He, B. Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway. Int. J. Mol. Sci. 2023, 24, 5059. https://doi.org/10.3390/ijms24055059
Chen L, Geng N, Chen T, Xiao Q, Zhang H, Huo H, Jiang L, Shao Q, He B. Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway. International Journal of Molecular Sciences. 2023; 24(5):5059. https://doi.org/10.3390/ijms24055059
Chicago/Turabian StyleChen, Long, Na Geng, Taiwei Chen, Qingqing Xiao, Hengyuan Zhang, Huanhuan Huo, Lisheng Jiang, Qin Shao, and Ben He. 2023. "Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway" International Journal of Molecular Sciences 24, no. 5: 5059. https://doi.org/10.3390/ijms24055059
APA StyleChen, L., Geng, N., Chen, T., Xiao, Q., Zhang, H., Huo, H., Jiang, L., Shao, Q., & He, B. (2023). Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway. International Journal of Molecular Sciences, 24(5), 5059. https://doi.org/10.3390/ijms24055059




