The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties
Abstract
:1. Calcium Phosphate Bioceramics—Biomaterial Widely Used for Bone Tissue Regeneration
2. Synthesis of Sintered Hydroxyapatite
3. Mechanical Properties of Sintered HA
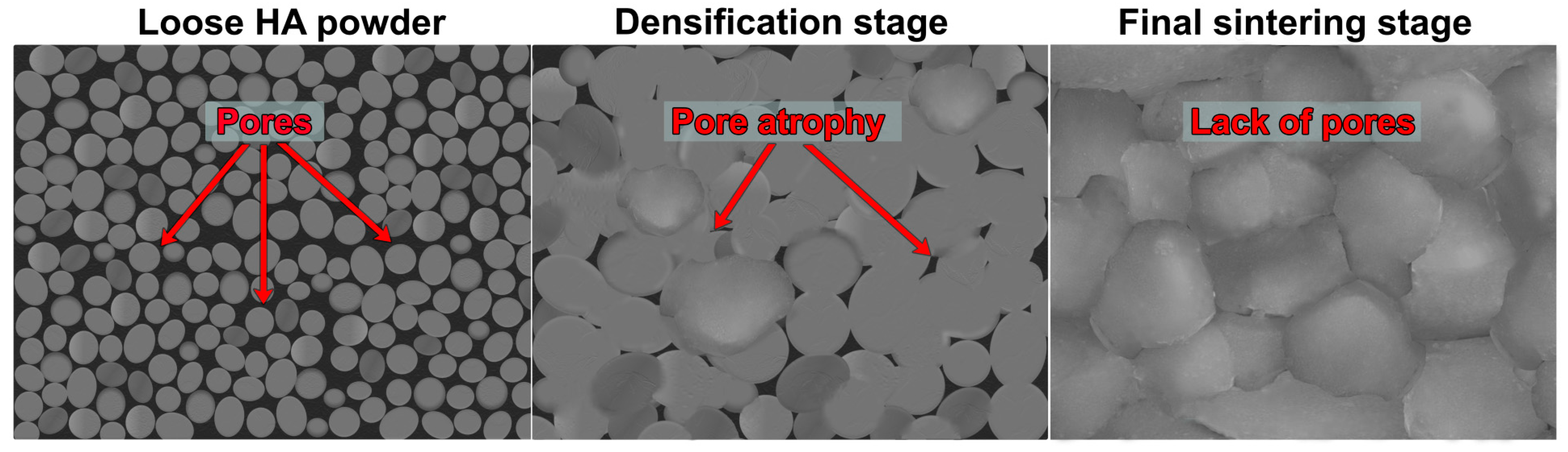
4. Microstructural Properties of Sintered HA
5. HA Biodegradability Dependent on the Sintering Temperature
6. HA Bioactivity Dependent on the Sintering Temperature
7. HA Biocompatibility Dependent on the Sintering Temperature


8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazimierczak, P.; Przekora, A. Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: A concise review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Przekora, A.; Palka, K.; Ginalska, G. Chitosan/β-1,3-glucan/calcium phosphate ceramics composites-Novel cell scaffolds for bone tissue engineering application. J. Biotechnol. 2014, 182–183, 46–53. [Google Scholar] [CrossRef]
- Sedel, L.; Viateau, V.; Petite, H.; Meunier, A.; Oudina, K.; de Pollak, C.; Bourguignon, M.; Guillemin, G.; Bensaïd, W. Tissue-engineered bone regeneration. Nat. Biotechnol. 2002, 18, 959–963. [Google Scholar] [CrossRef]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. 2002, 84, 454–464. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and bone healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Wenhao Wang, K.W.K.Y. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Sachot, N.; Engel, E.; Castano, O. Hybrid organic-inorganic scaffolding biomaterials for regenerative therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Escudero, A.; Espanol, M.; Ginebra, M.P. Synthetic bone graft substitutes: Calcium-based biomaterials. In Dental Implants and Bone Grafts; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–157. [Google Scholar] [CrossRef]
- Polo, L.; Díaz de Greñu, B.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial activity of commercial calcium phosphate based materials functionalized with vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol–gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple, and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malina, D.; Biernat, K.; Sobczak-Kupiec, A. Studies on sintering process of synthetic hydroxyapatite. Acta Biochim. Pol. 2013, 60, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- The, Y.C.; Tan, C.Y.; Ramesh, S.; Purbolaksono, J.; Tan, Y.M.; Chandran, H.; Teng, W.D.; Yap, B.K. Effect of calcination on the sintering behaviour of hydroxyapatite. Ceram.-Silik. 2014, 58, 320–325. [Google Scholar]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef] [PubMed]
- Obada, D.O.; Dauda, E.T.; Abifarin, J.K.; Dodoo-Arhin, D.; Bansod, N.D. Mechanical properties of natural hydroxyapatite using low cold compaction pressure: Effect of sintering temperature. Mater. Chem. Phys. 2020, 239, 122099. [Google Scholar] [CrossRef]
- Akash, A.; Mayo, M.J. Pore Growth during Initial-Stage Sintering. J. Am. Ceram. Soc. 2004, 82, 2948–2952. [Google Scholar] [CrossRef]
- Mazaheri, M.; Zahedi, A.M.; Haghighatzadeh, M.; Sadrnezhaad, S.K. Sintering of titania nanoceramic: Densification and grain growth. Ceram. Int. 2009, 35, 685–691. [Google Scholar] [CrossRef]
- Shi, J.L. Solid state sintering of ceramics: Pore microstructure models, densification equations and applications. J. Mater. Sci. 1999, 34, 3801–3812. [Google Scholar] [CrossRef]
- Thümmler, F.; Thomma, W. The sintering process. Metall. Rev. 1967, 12, 69–108. [Google Scholar] [CrossRef]
- German, R.M. Thermodynamics of sintering. In Sintering of Advanced Materials; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–32. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Kim, H.M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef]
- John, A.; Varma, H.K.; Kumari, T.V. Surface Reactivity of Calcium Phosphate Based Ceramics in a Cell Culture System. J. Biomater. Appl. 2003, 18, 63–78. [Google Scholar] [CrossRef]
- Rosa, A.L.; Beloti, M.M.; Oliveira, P.T.; Van Noort, R. Osseointegration and osseoconductivity of hydroxyapatite of different microporosities. J. Mater. Sci. Mater. Med. 2002, 13, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Klimek, K.; Wojcik, M.; Palka, K.; Ginalska, G. New method for HA/glucan bone scaffold preparation reduces cytotoxic effect of highly reactive bioceramics. Mater. Lett. 2017, 190, 213–216. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Reis, R.L. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: Influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef] [Green Version]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “false” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef]
- Gustavsson, J.; Ginebra, M.P.; Engel, E.; Planell, J. Ion reactivity of calcium-deficient hydroxyapatite in standard cell culture media. Acta Biomater. 2011, 7, 4242–4252. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Cent. Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]
- Gautam, G.; Kumar, S.; Kumar, K. Processing of biomaterials for bone tissue engineering: State of the art. Mater. Today Proc. 2021, 50, 2206–2217. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokopiev, O.; Sevostianov, I. Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. Mater. Sci. Eng. A 2006, 431, 218–227. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Ayatollahi, M.R.; Bushroa, A.R.; Herliansyah, M.K. Effect of sintering temperature on mechanical and tribological properties of hydroxyapatite measured by nanoindentation and nanoscratch experiments. Ceram. Int. 2014, 40, 9159–9164. [Google Scholar] [CrossRef]
- Oktar, F.N.; Genç, Y.; Göller, G.; Erkmen, E.Z.; Özyeǧin, L.S.; Toykan, D.; Demirkiran, H.; Haybat, H. Sintering of Synthetic Hydroxyapatite Compacts. Key Eng. Mater. 2004, 264–268, 2087–2090. [Google Scholar] [CrossRef]
- Benaqqa, C.; Chevalier, J.; Saâdaoui, M.; Fantozzi, G. Investigation of Crack Growth Process in Dense Hydroxyapatite Using the Double Torsion Method. In Fracture Mechanics of Ceramics; Springer: Boston, MA, USA, 2005; pp. 387–397. [Google Scholar] [CrossRef]
- Benaqqa, C.; Chevalier, J.; Saâdaoui, M.; Fantozzi, G. Slow crack growth in bioactive ceramics. Key Eng. Mater. 2004, 264–268, 1981–1984. [Google Scholar] [CrossRef]
- Benaqqa, C.; Chevalier, J.; Saädaoui, M.; Fantozzi, G. Slow crack growth behaviour of hydroxyapatite ceramics. Biomaterials 2005, 26, 6106–6112. [Google Scholar] [CrossRef]
- With, G.; Dijk, H.J.A.; Hattu, N.; Prijs, K. Preparation, microstructure and mechanical properties of dense polycrystalline hydroxy apatite. J. Mater. Sci. 1981, 16, 1592–1598. [Google Scholar] [CrossRef]
- Rootare, H.M.; Powers, J.M.; Craig, R.G. Sintered Hydroxyapatite Ceramic for Wear Studies. J. Dent. Res. 1978, 57, 777–783. [Google Scholar] [CrossRef]
- De Lange, G.L.; De Putter, C.; de Groot, K.; Burger, E.H. A Clinical, Radiographic, and Histological Evaluation of Permucosal Dental Implants of Dense Hydroxylapatite in Dogs. J. Dent. Res. 1989, 68, 509–518. [Google Scholar] [CrossRef]
- Chetty, A.; Wepener, I.; Marei, M.K.; El Kamary, Y.; Moussa, R.M. Hydroxyapatite: Synthesis, Properties, and Applications; Nova Biomedical: Waltham, MA, USA, 2012; pp. 91–132. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphate-Based Bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Bioceramics based on calcium orthophosphates (Review). Glas. Ceram. 2007, 64, 442–447. [Google Scholar] [CrossRef]
- Prokopiev, O.; Sevostianov, I.; Genin, J.; McGee, S.M.; Woodward, C. Microstructure and elastic properties of sintered hydroxyapatite. Int. J. Fract. 2004, 130, L183–L190. [Google Scholar] [CrossRef]
- Bailliez, S.; Nzihou, A. The kinetics of surface area reduction during isothermal sintering of hydroxyapatite adsorbent. Chem. Eng. J. 2004, 98, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.J.; Lu, Y.P.; Zhu, R.F.; Li, S.T.; Xiao, G.Y.; Zhao, G.F.; Xu, W.H. Effect of sintering on porosity, phase, and surface morphology of spray dried hydroxyapatite microspheres. J. Biomed. Mater. Res.—Part A 2008, 87, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.R.; Ducheyne, P. Effect of bioactive ceramic composition and structure on in vitro behavior. III. Porous versus dense ceramics. J. Biomed. Mater. Res. 1994, 28, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Ruys, A.J.; Sorrell, C.C.; Brandwood, A.; Milthorpe, B.K. Hydroxyapatite sintering characteristics: Correlation with powder morphology by high-resolution microscopy. J. Mater. Sci. Lett. 1995, 14, 744–747. [Google Scholar]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Corp, M.I. An Introduction to the Physical Characterization of Materials by Mercury Intrusion Porosimetry with Emphasis on Reduction and Presentation of Experimental Data. Mercury 2001, 22, 1–23. [Google Scholar]
- Bertoldi, S.; Farè, S.; Tanzi, M.C. Assessment of scaffold porosity: The new route of micro-CT. J. Appl. Biomater. Biomech. 2011, 9, 165–175. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Biodegradable bone implants in orthopedic applications: A review. Biocybern. Biomed. Eng. 2020, 40, 596–610. [Google Scholar] [CrossRef]
- Subash, A.; Basanth, A.; Kandasubramanian, B. Biodegradable polyphosphazene—Hydroxyapatite composites for bone tissue engineering. Int. J. Polym. Mater. 2022, 1–19. [Google Scholar] [CrossRef]
- Zara, S.; Susin, C.; Lee, J.; Fiorini, T.; Koo, K.-T.; Schüpbach, P.; Finger Stadler, A.; Wikesjö, U.M. Screening of Hydroxyapatite Biomaterials for Alveolar Augmentation Using a Rat Calvaria Critical-Size Defect Model: Bone Formation/Maturation and Biomaterials Resolution. Biomolecules 2022, 12, 1677. [Google Scholar] [CrossRef]
- Germaini, M.M.; Belhabib, S.; Guessasma, S.; Deterre, R.; Corre, P.; Weiss, P. Additive manufacturing of biomaterials for bone tissue engineering—A critical review of the state of the art and new concepts. Prog. Mater. Sci. 2022, 130, 100963. [Google Scholar] [CrossRef]
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef]
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Fulmer, M.T.; Ison, I.C.; Hankermayer, C.R.; Constantz, B.R.; Ross, J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002, 23, 751–755. [Google Scholar] [CrossRef]
- Stastny, P.; Sedlacek, R.; Suchy, T.; Lukasova, V.; Rampichova, M.; Trunec, M. Structure degradation and strength changes of sintered calcium phosphate bone scaffolds with different phase structures during simulated biodegradation in vitro. Mater. Sci. Eng. C 2019, 100, 544–553. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; Driessen, A.A.; de Groot, K.; van den Hooff, A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, H.; Sakurai, M.; Kashimoto, O.; Kikawa, T.; Suzuki, O. Comparative study on osteoconductivity by synthetic octacalcium phosphate and sintered hydroxyapatite in rabbit bone marrow. Calcif. Tissue Int. 2006, 78, 45–54. [Google Scholar] [CrossRef]
- Yamada, S.; Heymann, D.; Bouler, J.M.; Daculsi, G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Iwanaga, H.; Shibutani, T.; Moriwaki, Y.; Iwayama, Y. Osteoclastic responses to various calcium phosphates in cell cultures. J. Biomed. Mater. Res. 1999, 47, 424–433. [Google Scholar] [CrossRef]
- Hasegawa, M.; Doi, Y.; Uchida, A. Cell-mediated bioresorption of sintered carbonate apatite in rabbits. J. Bone Jt. Surg.—Ser. B 2003, 85, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, N.; Akagi, T.; Yamasaki, M.; Ozeki, T.; Nojima, T.; Hiramatsu, Y.; Nagai, N. Osteoclastic features of multinucleated giant cells responding to synthetic hydroxyapatite implanted in rat jaw bone. J. Electron Microsc. (Tokyo) 1992, 41, 141–146. [Google Scholar] [CrossRef]
- Goto, T.; Kojima, T.; Iijima, T.; Yokokura, S.; Kawano, H.; Yamamoto, A.; Matsuda, K. Resorption of synthetic porous hydroxyapatite and replacement by newly formed bone. J. Orthop. Sci. 2001, 6, 444–447. [Google Scholar] [CrossRef]
- Winter, M.; Griss, P.; de Groot, K.; Tagai, H.; Heimke, G.; Dijk, H.J.A.V.; Sawai, K. Comparative histocompatibility testing of seven calcium phosphate ceramics. Biomaterials 1981, 2, 159–160, 1N1. [Google Scholar] [CrossRef]
- Radin, S.R.; Ducheyne, P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J. Biomed. Mater. Res. 1993, 27, 35–45. [Google Scholar] [CrossRef]
- Nakamura, M.; Hentunen, T.; Salonen, J.; Nagai, A.; Yamashita, K. Characterization of bone mineral-resembling biomaterials for optimizing human osteoclast differentiation and resorption. J. Biomed. Mater. Res.—Part A 2013, 101, 3141–3151. [Google Scholar] [CrossRef]
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Banijamali, S.; Baino, F.; Kargozar, S.; Hill, R.G. Calcium carbonate: Adored and ignored in bioactivity assessment. Acta Biomater. 2019, 91, 35–47. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wang, Y.; Wang, H. Ultrafast bone-like apatite formation on bioactive tricalcium silicate cement using mussel-inspired polydopamine. Ceram. Int. 2019, 45, 3033–3043. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Dridi, A.; Riahi, K.Z.; Somrani, S. Mechanism of apatite formation on a poorly crystallized calcium phosphate in a simulated body fluid (SBF) at 37 °C. J. Phys. Chem. Solids 2021, 156, 110122. [Google Scholar] [CrossRef]
- Mezahi, F.Z.; Oudadesse, H.; Harabi, A.; Le Gal, Y.; Cathelineau, G. Sintering effects on physico chemical properties of bioactivity of natural and synthetic hydroxyapatite. J. Aust. Ceram. Soc. 2011, 47, 23–27. [Google Scholar]
- Cho, J.S.; Yoo, D.S.; Chung, Y.C.; Rhee, S.H. Enhanced bioactivity and osteoconductivity of hydroxyapatite through chloride substitution. J. Biomed. Mater. Res.—Part A 2014, 102, 455–469. [Google Scholar] [CrossRef]
- Dhal, J.; Fielding, G.; Bose, S.; Bandyopadhyay, A. Understanding bioactivity and polarizability of hydroxyapatite doped with tungsten. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2012, 100 B, 1836–1845. [Google Scholar] [CrossRef]
- Kitsugi, T.; Yamamuro, T.; Nakamura, T.; Kokubo, T.; Takagi, M.; Shibuya, T.; Takeuchi, H.; Ono, M. Bonding Behavior Between Two Bioactive Ceramics in Vivo. J. Biomed. Mater. Res. 1987, 21, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Jiang, G.; Bauer, J. The effect of structural characteristics on the in vitro bioactivity of hydroxyapatite. J. Biomed. Mater. Res. 2002, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Specifications for Innovative, Enabling Biomaterials Based on the Principles of Biocompatibility Mechanisms. Front. Bioeng. Biotechnol. 2019, 7, 255. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Laquerriere, P.; Kilian, L.; Bouchot, A.; Jallot, E.; Grandjean, A.; Guenounou, M.; Rard Balossier, G.; Frayssinet, P.; Bonhomme, P. Effect of Hydroxyapatite Sintering Temperature on Intracellular Ionic Concentrations of Monocytes: A TEM-Cryo-X-Ray Microanalysis Study. J. Biomed. Mater. Res. A 2001, 58, 238–246. [Google Scholar] [CrossRef]
- Wang, C.; Duan, Y.; Markovic, B.; Barbara, J.; Rolfe Howlett, C.; Zhang, X.; Zreiqat, H. Proliferation and bone-related gene expression of osteoblasts grown on hydroxyapatite ceramics sintered at different temperature. Biomaterials 2004, 25, 2949–2956. [Google Scholar] [CrossRef]
- Frayssinet, P.; Rouquet, N.; Fages, J.; Durand, M.; Vidalain, P.O.; Bonel, G. The influence of sintering temperature on the proliferation of fibroblastic cells in contact with HA-bioceramics. J. Biomed. Mater. Res. 1997, 35, 337–347. [Google Scholar] [CrossRef]
- Hyakuna, K.; Yamamuro, T.; Kotoura, Y.; Kakutani, Y.; Kitsugi, T.; Takagi, H.; Oka, M.; Kokubo, T. The influence of calcium phosphate ceramics and glass-ceramics on cultured cells and their surrounding media. J. Biomed. Mater. Res. 1989, 23, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.B.; Tan, M.H.; Kolli, T.N.; Zachariadou, C.; Farah, F.; Mohamed, F.F.; Chu, E.Y.; Foster, B.L. Bone Sialoprotein Is Critical for Alveolar Bone Healing in Mice. J. Dent. Res. 2022, 102, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Rustamov, V.; Keller, F.; Klicks, J.; Hafner, M.; Rudolf, R. Bone sialoprotein shows enhanced expression in early, high- proliferation stages of three-dimensional spheroid cell cultures of breast cancer cell line MDA-MB-231. Front. Oncol. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Rossi, M.; Battafarano, G.; Pepe, J.; Minisola, S.; Del Fattore, A. The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases. Int. J. Mol. Sci. 2019, 20, 4502. [Google Scholar] [CrossRef] [Green Version]
- Przekora, A.; Ginalska, G. Enhanced differentiation of osteoblastic cells on novel chitosan/β-1,3-glucan/bioceramic scaffolds for bone tissue regeneration. Biomed. Mater. 2015, 10, 015009. [Google Scholar] [CrossRef]
- Zalewska, J.; Przekora, A.; Pałka, K.; Belcarz, A. Gypsum-related compensation of ions uptake by highly porous hydroxyapatite ceramics—Consequences for osteoblasts growth and proliferation. Biomater. Adv. 2022, 133, 112665. [Google Scholar] [CrossRef]
- López-Pérez, P.M.; Marques, A.P.; Silva, R.M.P.D.; Pashkuleva, I.; Reis, R.L. Effect of chitosan membrane surface modification via plasma induced polymerization on the adhesion of osteoblast-like cells. J. Mater. Chem. 2007, 17, 4064–4071. [Google Scholar] [CrossRef] [Green Version]
- Tozer, E.C.; Hughes, P.E.; Loftus, J.C. Ligand binding and affinity modulation of integrins. Biochem. Cell Biol. 1996, 74, 785–798. [Google Scholar] [CrossRef]
- Hughes, P.E.; Pfaff, M. Integrin affinity modulation. Trends Cell Biol. 1998, 8, 359–364. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: Implication for bone tissue engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef] [PubMed]
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jojczuk, M.; Lukasiewicz, P.; Nogalski, A.; Ginalska, G. Highly porous fluorapatite/β-1,3-glucan composite for bone tissue regeneration: Characterization and in vitro assessment of biomedical potential. Int. J. Mol. Sci. 2021, 22, 10414. [Google Scholar] [CrossRef]
- Lobo, S.E.; Arinzeh, T.L. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef]



| Type of Biomaterial | Type of Mechanical Test | Temperature of Sintering | Mechanical Properties | Ref. | |
|---|---|---|---|---|---|
| Young’s Modulus (E, GPa) | Compression Strength (MPa) | ||||
| HA powder cylindrical specimens | Ultrasonic | 1140 °C | 16.30 | 5.26 | [44] |
| 1200 °C | 36.30 | 7.66 | |||
| 1300 °C | 59.80 | 13.25 | |||
| 1340 °C | 73.5 | 13.81 | |||
| HA powder pellets | Universal testing machine (UTM) | 900 °C | ≈1.6 | ≈0.39 | [24] |
| 1000 °C | ≈3.6 | ≈0.58 | |||
| 1100 °C | ≈4.25 | ≈0.85 | |||
| HA powder–sucrose specimens | Nanoindentation | 1000 °C | 48 | - | [45] |
| 1100 °C | 74 | - | |||
| 1200 °C | 153 | - | |||
| 1300 °C | 134 | - | |||
| 1400 °C | 89 | - | |||
| HA powder compacts | Universal test machine (DVT.e) | 1000 °C | - | 18.48 | [46] |
| 1100 °C | - | 30.47 | |||
| 1200 °C | - | 28.85 | |||
| 1300 °C | - | 29.13 | |||
| Type of Biomaterial | Temperature of Sintering | Porosity (%) | SSA (m2/g) | Density (g/cm3) | Ref. |
|---|---|---|---|---|---|
| HA powder cylindrical specimens | 1140 °C | ≈37 | - | ≈1.6 | [44] |
| 1200 °C | ≈23 | - | ≈2.4 | ||
| 1300 °C | ≈14 | - | ≈2.5 | ||
| 1340 °C | ≈13 | - | ≈2.6 | ||
| HA powder cylindrical samples | 1150 °C | - | - | ≈1.45 | [56] |
| 1200 °C | - | - | ≈2.1 | ||
| 1250 °C | - | - | ≈2.3 | ||
| 1300 °C | - | - | ≈2.35 | ||
| 1350 °C | - | - | ≈2.38 | ||
| HA powder cylindrical specimens | 800 °C | ≈41 | - | ≈1.15 | [18] |
| 900 °C | ≈31 | - | ≈1.3 | ||
| 1000 °C | ≈4 | - | ≈1.8 | ||
| 1100 °C | ≈4 | - | ≈2.4 | ||
| 1200 °C | ≈4 | - | ≈2.4 | ||
| 1300 °C | ≈2 | - | ≈2.5 | ||
| 1400 °C | ≈2 | - | ≈2.5 | ||
| HA powder pellets | 900 °C | 51.7 ± 0.06 | - | - | [24] |
| 1000 °C | 49.5 ± 0.18 | - | - | ||
| 1100 °C | 46.9 ± 0.10 | - | - | ||
| HA powder compacts | 1000 °C | - | - | 2.15 | [46] |
| 1100 °C | - | - | 2.53 | ||
| 1200 °C | - | - | 2.93 | ||
| 1300 °C | - | - | 3.03 | ||
| HA powder | 550 °C | - | 28 | - | [57] |
| 1000 °C | - | 3 | - | ||
| HA microspheres | 500 °C | - | 60.44 | - | [58] |
| 600 °C | - | 44.13 | - | ||
| 800 °C | - | 12.45 | - | ||
| 1000 °C | - | 3.7 | - | ||
| 1100 °C | - | 2.1 | - | ||
| Stoichiometric HA powder | 200 °C | - | 45.2 | - | [59] |
| 900 °C | - | 4.7 | - |
| Type of Biomaterial | Temperature of Sintering | Biodegradation/Bioabsorbability Environment | Duration of the Experiment | Observation | Ref. |
|---|---|---|---|---|---|
| Synthetic HA (Calcitite) | Not provided | Tris buffer solution (pH = 7.3) 37 °C | 120 h | Poor solubility. The least soluble among the tested apatite materials (Calcitite, BoneSource, Norian cranial repair system (CRS)) | [73] |
| HA scaffold | 1250 °C | McIlvaine buffer (pH = 5.5) 37 °C | 14 days | Low degradation rate, 3% of mass loss | [74] |
| HA cylindrical implants | 1270 °C | In vivo rabbit model | 24 weeks | No significant changes in shape and architecture of the implant | [71] |
| HA powder specimen | 1100 °C 1300 °C | In vivo rabbit model | 36 weeks | No signs of bioabsorbability | [75] |
| HA granules | 1150 °C | In vivo rabbit model | 12 weeks | No significant changes after implantation period | [76] |
| HA pellets | 1000 °C | In vitro neonatal rabbit osteoclasts | 48 h | No signs of material bioresorption | [77] |
| HA discs | 1200 °C | In vitro neonatal rabbit osteoclasts | 48 h | No signs of material bioresorption | [78] |
| Carbonate HA cylindrical implants | 1200 °C | In vivo rabbit model | 12 months | No signs of bioabsorbability | [79] |
| HA granules | 200 °C 1250 °C | In vivo rat model | 3 weeks | Greater number of multinucleated giant cells was observed on HA sintered at 200 °C than on HA sintered at 1250 °C, indicating better bioabsorbability | [80] |
| HA blocks | 900 °C | In vivo human | 79 months | HA was replaced by newly formed bone | [81] |
| HA granules | 900 °C | In vivo rat model | 6 months | Significant changes in material microstructure indicated its bioabsorbability | [82] |
| Type of Biomaterial | Temperature of Sintering | Apatite Precipitation Time | Experiment Environment | Observation | Ref. |
|---|---|---|---|---|---|
| HA polycrystals | 800 °C | 9 h | Simulated body fluid (SBF) | Process of apatite formation was slower for HA sintered at 1200 °C compared to HA sintered at 800 °C | [31] |
| 1200 °C | 12 h | ||||
| HA granules | 800 °C | 7 days | Simulated body fluid (SBF) | Higher sintering temperature resulted in prolonged apatite precipitation | [93] |
| 1200 °C | 30 days | ||||
| HA powder | 200 °C | Up to 24 h | Simulated physiologic solution (SPS) | Apatite layer was formed the fastest on the surface of HA sintered at low temperatures (200 °C and 600 °C) | [59] |
| 600 °C | |||||
| 900 °C | |||||
| 1100 °C | |||||
| HA powder | 1100 °C | 7 days | Simulated body fluid (SBF) | Chloride-substituted HA was characterized by higher bioactivity than pure HA powder | [94] |
| Chloride-substituted HA powder | |||||
| HA powder | 1250 °C | 7 days | Simulated body fluid (SBF) | Pure HA was characterized by smaller newly precipitated apatite layer after 7 days of experiment compared to HA with hexavalent tungsten | [95] |
| HA with hexavalent tungsten | |||||
| HA powder specimen | 800 °C | 1 month | In vivo rat model | Apatite layer was formed significantly faster on the surface of HA sintered at 800 °C and 1000 °C compared to HA sintered at 1200 °C | [96] |
| 1000 °C | 1 months | ||||
| 1200 °C | 3 months |
| Type of Biomaterial | Temperature of Sintering | Experimental Model | Cytotoxicity | Observations | Ref. |
|---|---|---|---|---|---|
| HA particles | 600 °C | Human monocytic cell line (U-937) | Toxic | High dissolution rate | [102] |
| 1180 °C | Non-toxic | Low dissolution rate | |||
| HA ceramic discs | 800 °C | Human osteoblast-like cells derived from osteosarcoma (SaOS-2) | Non-toxic | Reduced bone sialoprotein and osteocalcin expression, low cell proliferation rate | [103] |
| 1000 °C | High bone sialoprotein and osteocalcin expression, high cell proliferation rate | ||||
| 1200 °C | |||||
| HA discs | 850 °C | Mouse-established fibroblast cell line (L929) | Non-toxic | Negative cell growth rate | [104] |
| 1050 °C | Slightly reduced cell growth rate | ||||
| 1250 °C | Normal cell growth rate | ||||
| HA discs | 600 °C | Chinese hamster fibroblast cell line (V79) | Highly toxic | Lack of cell growth on the discs | [105] |
| 900 °C | Poor cell growth on the discs | ||||
| 1200 °C | Low toxicity | Normal cell growth up to 7 days | |||
| Hydroxyapatite-B-1,3-glucan composite | 800 °C | Mouse calvarial preosteoblast cell line (MC3T3-E1) | Toxic | High ionic reactivity of the biomaterial, significantly reduced preosteoblast growth on the composite | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. https://doi.org/10.3390/ijms24065083
Trzaskowska M, Vivcharenko V, Przekora A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. International Journal of Molecular Sciences. 2023; 24(6):5083. https://doi.org/10.3390/ijms24065083
Chicago/Turabian StyleTrzaskowska, Marta, Vladyslav Vivcharenko, and Agata Przekora. 2023. "The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties" International Journal of Molecular Sciences 24, no. 6: 5083. https://doi.org/10.3390/ijms24065083






