1. Introduction
Skin wounds have varying severity levels and originate from trauma or surgery or develop slowly over time, as with pressure ulcers. The subsequent healing of skin wounds can be suspended, delayed, or diminished for various reasons. For example, re-injury can occur, after which healed structures are lost, and the healing progress is reset. Alternatively, a chronic wound remains in the inflammatory phase, preventing the resolution of inflammation and (myo)fibroblasts from closing and restoring the skin. In the case of burn injuries, underlying structures, such as nerves and sweat glands, can be so profoundly damaged that their function is completely lost. At the same time, the patient simultaneously ends up with permanent scarring. The use of advanced wound dressings is recommended for the treatment of such complex wounds [
1,
2]. Besides acting as a cover for the wounded area, wound dressings can include valuable functionalities, such as protection against bacteria, absorption of wound exudate, or keeping the wound hydrated [
3,
4,
5,
6]. This may help properly heal the wound and prevent scarring or other complications. However, the production costs and the amount of money and time healthcare personnel require to apply and refresh wound dressings are tremendous. Recent studies on the economic burden on the United Kingdom’s National Health Service (NHS) showed that the cost of treating a wound in the clinic ranges from GBP 698 to GBP 3998. These costs increase by 35% on average when the wound remains unhealed [
7,
8]. Changing wound dressings are often painful, especially for burn victims. Thus, it should be evident that better wound dressing products are desired to improve wound treatment and help healthcare providers and patients by making it more convenient to apply and remove painlessly.
Recently, hydrogel wound dressings based on polyisocyanopeptide (PIC) polymers were reported as a potential product for treating skin injuries [
9,
10]. The benefit of this product comes from its reversible thermosensitive gelation behaviour and chemically modifiable polyethylene glycol (PEG) side chains [
11,
12,
13,
14,
15,
16]. The envisioned clinical application method involves a PIC solution that is cooled into the liquid state (<15 °C, although this is tuneable [
11]) and then poured or sprayed onto the wound. The product will flow into every corner of the wound and subsequently undergo in situ gelation triggered by body heat, allowing the PIC gel to form a protective barrier. While present, the gel supposedly prevents bacterial entry, hydrates the wound, and provides additional (bio-)functionalities, such as drug delivery [
9,
17]. Other material benefits of the PIC hydrogel include an extracellular matrix-mimicking strain stiffening response, adjustable stiffness, rheology and pore size, and the possibility of chemical functionalization [
9,
10,
15,
18].
In the clinic, burn injuries are typically washed with a shower head or similar irrigation techniques daily. At this moment, PIC gel may likely be conveniently and painlessly removed, as its largely water-based origin allows it to be flushed out easily. Furthermore, if the irrigation fluid’s temperature is below the gelation point, the gel may reverse back to liquid, further improving the efficiency of this step. Pain-free removal of a dressing is desirable, as the debridement and dressing changes performed in burn wound care are extremely painful to the patient but, unfortunately, necessary to prevent infection [
19]. Moreover, hydrogels such as PIC are lowly adherent and more likely to be atraumatic [
20]. Atraumatic dressings do not cause additional damage to the wound upon removal, and this lack of disturbance to the wound repair process is overall beneficial to the outcome [
21]. It was therefore postulated that the convenient application and removal of PIC dressings might facilitate wound repair by eliciting less disruption.
In this current study, the feasibility of the aforementioned application-and-removal approach is simulated in a splinted wound model in mice. Previously, it was determined that PIC gel may provide a microbial barrier function [
9] but may eventually become encased below the skin surface in the more deeply positioned tissues [
10]. Currently, it is unknown whether irrigation of the wound will effectively wash out PIC gel and if this leads to undesired adverse effects such as morphological damage or delayed healing. Furthermore, it remains to be answered how treatment with PIC gel impacts the healing of wounds for a period longer than seven days. Therefore, hairless immuno-competent mice were subjected to a splinted full-thickness skin defect, treated with PIC gel, and a secondary dressing. This model was chosen for its ease of use and controllable environment, typically resulting in undisturbed wound healing. Experimental groups included wounds receiving 1. a single dose of PIC gel after surgery and regularly subjected to a washing procedure and new doses of PIC gel, and 2. wounds treated only with a non-adhesive Tegaderm™ sheet and a bandage were used as a control group that simulated present clinical treatment routines [
22,
23]. The study consisted of 3 parts; first, an initial study in which the washing procedures were practised, and the wound healing status was assessed at 14, 21, and 28 days post-injury. Second, a SPECT/CT imaging study, in which the efficiency of removing
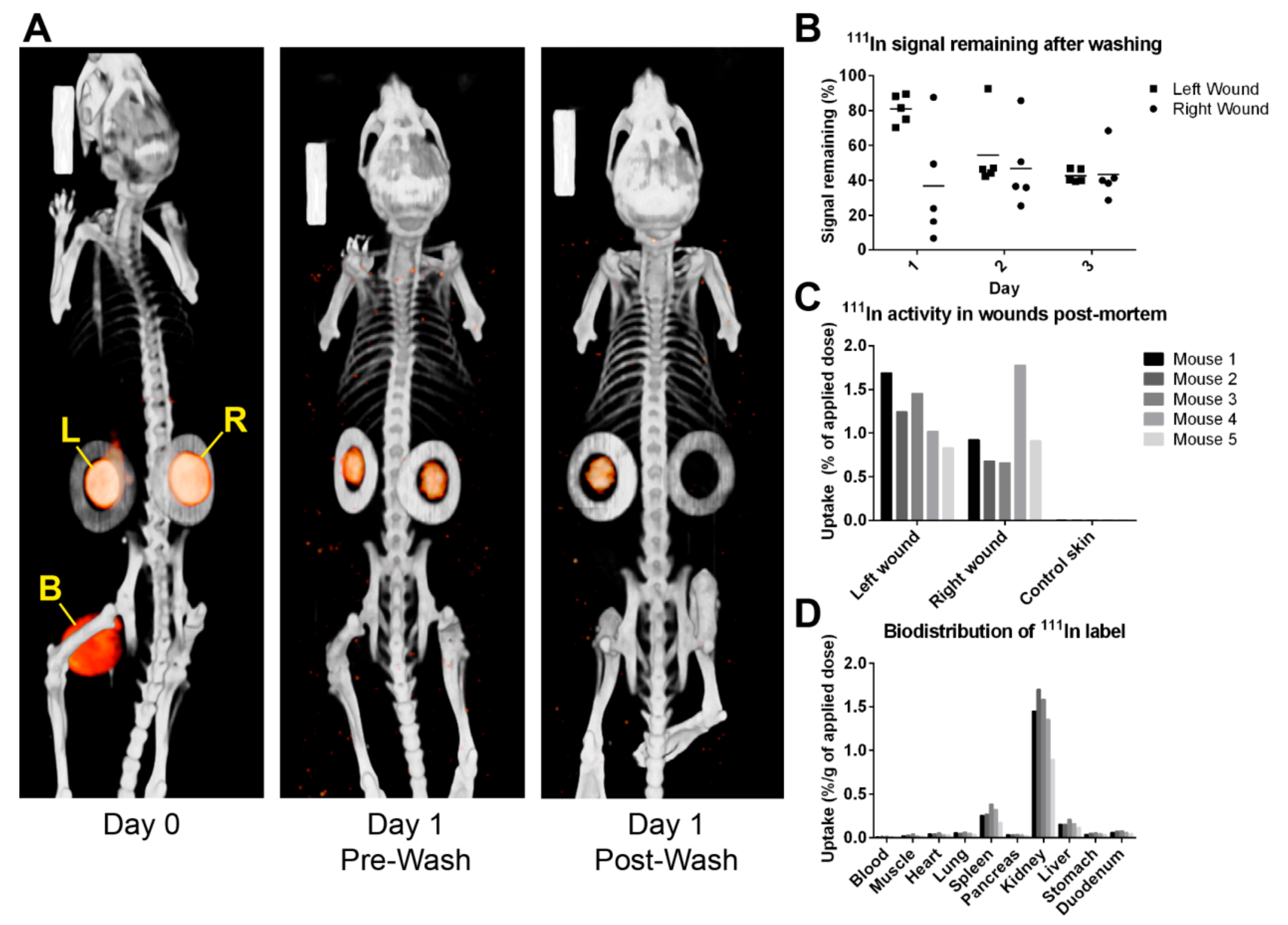
111In-labelled PIC-DTPA gel from wounds by cold saline rinsing was quantified. Third, a main wound healing study where the impact of a single or repeated dose of PIC hydrogel on wound size was analysed at 3, 7, and 14 days post-injury. The outcome of wound repair of these treatments was assessed by photography and (immuno-)histology. It was hypothesised that PIC gel does not interfere with wound healing up to 14 days and that a regular washing schedule does not interfere significantly with wound repair and prevents the encapsulation of PIC gel in the wound repair tissue.
3. Discussion
This study explored the feasibility of PIC hydrogel as a removable wound cover. The removal of the gel from wounds by washing with a cold solution was demonstrated. The impact of the material on wound repair was assessed at three critical moments during wound healing, namely during the inflammatory phase (day 3), proliferation phase (day 7), and remodelling phase (day 14). It was found that wounds treated with PIC gels performed on par with control wounds and the overall treatment benefits from regular dressing replacements.
Firstly, an initial study was performed to explore the wound model and washing procedures and identify the most appropriate time point representing the remodelling phase. Moreover, it was found that histological samples taken on days 21 or 28 after surgery showed such advanced healing that it was too difficult to identify where the original wound was made. Therefore, it was decided to follow up on wounds until day 14 in the main study to ensure that a reliable comparison between groups could be made. Moreover, it was discovered that PIC polymers that remained in the wound repair tissue calcified and triggered an immune response on days 14, 21, and 28. These reactions are undesirable, as heterotopic calcification can lead to additional complications [
24]. Moreover, giant cells were identified in small numbers around these calcifications. So far, it remains unclear whether these giant cells arrive first and trigger the calcification or vice versa. It is postulated that this process is linked to the dissipation of PIC’s liquid component (PBS), as this response has only been observed for 14 days or later in sections where the polymer is left behind.
Further research has to be performed to determine the long-term response to PIC gels/polymers and to discover if PIC polymers are degraded, expelled, or stay behind in the tissue. To that end, PIC’s material characteristics will play an important part because of its mimicry of natural extracellular matrix characteristics, such as stiffness and strain-stiffening, which may help prevent a negative host response [
25].
Tuning gels by adding biofunctional or chemical groups have also been shown to change the gel behaviour and properties in other model systems, which may influence cell–gel interactions, drug delivery, or wound repair [
18,
26,
27,
28,
29]. Here, an imaging study was performed using
111In-labelled PIC gel to determine the efficiency of gel removal from the wounds by cold saline washing. First, it was found that the
111In-DTPA PIC hydrogel could be easily detected using SPECT/CT. Based on results obtained here and in our previous study [
10], it is assumed that the signal present in the wounds 24 h after application of the labelled gel originates solely from the [
111In]In-DTPA-PIC. After this period, EDTA-bound
111In is expected to be cleared through the urine, and any remaining detectable signal should originate from PIC-bound
111In. Still, there is a possibility that some part of the signal originates from anything other than the PIC, such as radiolabelled polymer fragments or EDTA. However, this source is expected to be insignificant compared to the gel.
In addition, we explored different ways of washing to be used for the main study. The study showed that after adjusting the washing method, on average, 40 to 45% of PIC gel remained in the wounds after washing. The original intention was to consistently remove at least 90%, which was only achieved once. It was found that at these early time points the full-thickness wounds represent a very stringent model by having a deep pocket that retains the washing saline and gel like a reservoir. The washing saline was sometimes observed bouncing off the wound, making it difficult to remove all of the PIC gel, especially from the deeper corners. Since humans do not have loosened skin like mice, full-thickness wounds will likely have less gel remaining in the skin upon washing. Partial-thickness wounds would abolish this problem altogether. The remaining gel would also be pushed out because stem cells in the partially wounded skin would keep proliferating and differentiating.
In this study, it was chosen to use cold saline in the washing procedure. It was postulated that the temperature-induced switch of PIC from a gel into a liquid would facilitate easy removal. It remains unclear if the cold drop of saline added to the wounds before washing was sufficient to induce this transition. Moreover, the temperature employed (~4 °C) in this current study can be stressful, discomforting, and even delay wound repair in animals and patients [
30]. In a clinical setting, a good strategy may instead include first cooling the wound area with a “cold packing” before washing (thoroughly) with (luke)warm water. This could trigger the transition of PIC into a liquid, enabling an easier washout without being detrimental to the patient. Nevertheless, we decided to continue the cold washing in the main wound healing study, as we did not want to change the original study design. Still, a conscious effort was made to wash the wounds more efficiently by adjusting the saline stream angle and aiming into each side of the wound.
For the main wound healing study, the rate of wound closure and general tissue morphology were first assessed using photography and HE-stained sections. Photographic analyses showed that PIC-treated wounds were larger than their control counterparts on day 7. However, on day 14, the wounds in the refreshed PIC gel group were significantly smaller than the control and single PIC gel groups. This suggests that PIC gel or the washing procedure can accelerate wound closure. Re-epithelisation occurred at comparable rates in all groups, indicating that the PIC gel or washing procedures did not impede this process.
Furthermore, a previous study showed that PIC gel could become encased in the wound repair tissue [
10]. Part of the current study was to discover if regular wound-washing steps can help prevent this phenomenon. By washing away the old PIC gel and applying the new dose topically, it was postulated that PIC gel was less likely to get trapped in the more deeply positioned tissues. Histological evaluation of the imaging study showed minimal wound repair tissue development by day 3. Additionally, although PIC was still present, it was only observed to be positioned topically and not encapsulated in the wound tissue. The main study showed that wounds in the refreshed PIC group on day 14 displayed smaller and less encapsulated PIC gel than the single PIC group. By contrast, on day 7, the presence of PIC gel scores was, on average higher in the refreshed PIC group than in the single PIC group. In addition, on day 3, it was found that wounds in the imaging study typically had more PIC gel (average score 1.7) than wounds in the main healing study (average score 1.2). Both these findings can be attributed to new doses of PIC gel that were recently applied to these wounds. This effect disappears on day 14 because the wounds have closed considerably at this point, and the newly applied doses of PIC gel are positioned more topically where they are more easily washed away. Importantly, the formation of large, encapsulated compartments of PIC gel on day 14 occurred significantly less often in the refreshed PIC group. This suggests that, even though PIC gel is sometimes observed in larger volumes in earlier time points, the washing and replacement procedure may help prevent encapsulation of the gel and possibly reduce the odds of calcified deposits in the long term.
Furthermore, the washing procedures did not induce additional morphological damage and, on average, looked less structurally interrupted by PIC gel than wounds from the single PIC group. This suggests that PIC gels are atraumatic, i.e., do not cause damage to the wound bed upon removal [
20,
21]. This is a major benefit, as removing most dressing materials is painful. It has been shown to damage the newly formed epithelium and peri-wound area, which increases wound size and delays healing, especially when performed repeatedly in, e.g., chronic wounds [
31]. Lastly, the calcifications observed in the initial study were no longer observed in the main study. Additionally, the inflammatory responses, including giant cells, were less severe and frequent.
Next, immunohistologically and AZAN-stained sections were analysed for relevant wound repair cell types and collagen deposition, respectively. In general, granulocyte numbers were low. At day 3, granulocytes were also considerably lower in all groups of the main study compared to the imaging study. This possibly relates to introducing de novo contaminants during the DTPA-PIC-labelling procedure triggering an immune response. However, in the main study, an increased granulocyte influx was found for the refreshed PIC group on day 7 and the single PIC group on day 14. This can likely be attributed to immune responses against the foreign material rather than immune responses involved in wound repair. However, it must be stressed that no infections were noted in any mouse and that the overall presence of granulocytes was still considered low. Moreover, it was found that the wound tissue contained a considerable number of macrophages at all time points, but no significant differences were observed between the groups. Macrophage levels at day 3 were also highly comparable between the imaging and the main study.
Furthermore, using a non-parametric test, the single PIC group displayed significantly higher myofibroblast numbers on day 14 than the refreshed PIC group. Although this effect is only minimal, it suggests a slightly delayed healing in the single PIC group. Lastly, the treatments resulted in a comparable collagen deposition throughout the study. Only on day 14, a significant reduction in collagen expression between single PIC compared to refreshed PIC was found. This may be related to the deposition of connective tissue around PIC gels, which was present in larger volumes in this group at this time point. Overall, for the inflammatory and proliferation phase markers, wounds subjected to the dressing refreshment protocol performed similarly to the control group but better than those treated with a single dose of PIC. It was attempted to validate these findings with gene expression analysis using qPCR. However, while processing the results, it was concluded that the data were unreliable, as a portion of the harvested tissue included healthy cells. This was especially so for day 3, where healthy skin composed a larger percentage of the harvested tissue, clouding the wound cells’ expression patterns.
Finally, a limitation in this current study was the murine model. The mice’s small size and loose skin make it difficult to wash and remove the PIC gel effectively from the created full-thickness skin wounds. As was observed previously, PIC gel can leak easily into the subcutaneous layers of this mouse model after establishing a full-thickness skin wound and likely remain behind [
10]. Therefore, it is recommended that for follow-up studies, an animal model is used with a skin structure more similar to the human situation (e.g., the pig). Nevertheless, the murine model allowed the assessment of the initial impact of PIC hydrogel, applied either once or repeatedly, on wound healing up to two weeks. The obtained data provide reasonable evidence to confirm the merit of PIC hydrogel as a wound dressing. No apparent harmful interference with wound repair was found related to PIC gel or its removal, which opens options for future development into a clinically relevant product. In particular, PIC gels may be appropriate in hydrating (dry) burn wounds, and further molecular modification to introduce therapeutic options is encouraged.
4. Materials and Methods
4.1. Material Preparation
Triethylene glycol-grafted PIC polymers (Radboud University, Nijmegen, the Netherlands) were synthesised and prepared as described in detail previously [
9,
10,
15,
18,
32]. Synthesis of DTPA-functionalized PIC polymers (purchased from Noviocell BV, Oss, The Netherlands) and radioactive labelling with
111In was performed as described previously [
10]. For the imaging study, one batch of
111In-labelled PIC-DTPA gel was prepared in metal-free 2-(N-morpholino)ethanesulfonic acid (MES, Sigma Aldrich, Saint Louis, MI, USA) buffer at a final concentration of 4 mg/mL. For the main wound healing study, one large batch of PIC hydrogel was created at a concentration of 4 mg/mL using sterile PBS (Thermo Fisher Scientific, Waltham, MA, USA), which was aliquoted and stored at −20 °C for the duration of the experiment. The synthetic PIC hydrogel is a novel synthetic and thermoresponsive (liquid below 16 °C; gel above 16 °C) hydrogel. Detailed information about the morphology, pore size, and rheology can be found in the following references [
9,
10,
15,
18].
4.2. Animals
A total of 65 male mice were used, and the study was approved by the Committee for Animal Experiments of the Radboud University, Nijmegen, the Netherlands (DEC 2016-0103), and the guidelines set by the European Union in directive 2016/63/EU were followed. All mice were of strain SKH-1 (hairless) and supplied by Charles River Laboratories (Sulzfeld, Germany); all experiments were performed at the Central Animal Laboratory (CDL) of the Radboud University. Fifty-four animals were used in the main healing study, five for imaging with SPECT/CT and six for initial experiments. Mice were between 6 and 7 weeks of age at the start of the experiment and were housed individually in a temperature-regulated room (25 °C) on a 12 h light–dark cycle with fed food and water ad libitum.
4.3. Splinted Full-Thickness Wound Model
Mice were anaesthetised using an evaporated isoflurane/air mixture. Analgesia was provided (5 mg/kg Rimadyl, Zoetis Inc., Kalamazoo, MI, USA) half an hour before surgery and 24 h later. The dorsal skin was disinfected using Betadine (Meda Pharma B.V., Amstelveen, The Netherlands). Then, two full-thickness skin wounds were punched out with a Ø 4 mm biopsy punch (Kai medical, Seki City, Japan) by stretching a piece of folded dorsal skin and pressing the punch through all skin layers as described previously [
33]. Silicone ring splints (inner Ø 6 mm, outer Ø 12 mm, thickness 0.5 mm) were glued around the wound using cyanoacrylate gel (Bison, Goes, The Netherlands). While the glue was drying, 20 µL PIC gel was defrosted and applied directly to the wound in the cold liquid state using a pipette. After gelation of the gel was established, the wounds were covered in a cut-to-size sheet of Tegaderm™ (3M Medical, Diegem, Belgium) and a single wrap of Petflex
® no-chew bandage (Andover Healthcare Inc., Salisbury, MA, USA) covering only the mouse’s underbelly and back. For animals included for 14 days or longer in the experiment, these splints and secondary dressings were replaced (under anaesthesia) at 7 days after surgery. For the imaging study, [
111In] In-DTPA-PIC gel was kept defrosted on ice in a lead container.
4.4. Washing Protocol
To wash PIC gel out of the wounds, mice were placed under anaesthesia on a heating mat warmed to 37 °C. First, a droplet of fridge-cooled (~4 °C) sterile saline solution (Braun GmbH, Kronberg, Germany) was added to each wound to attempt reversing the gelation of the gel over a period of roughly one minute. Then, 25 mL of the cold saline was used for flushing and rinsing each wound. This method was performed using a 50 mL syringe (BD Plastipak™, Franklin Lakes, NJ, USA) with (18G Monoject™, Covidien, Tullamore, Ireland) to improve precision and pressure. The imaging study also attempted the method without a cannula but with a 10 mL syringe (BD Plastipak™). Attention was paid to changing the angle and pressure so that each corner of the wound was rinsed out properly. After washing, the cold saline-soaked padding was removed, and the body temperature was recovered with a heating lamp. During this time, new splints were glued in place. Subsequently, the lamp was turned off, and a new dose of PIC gel and the secondary dressing were re-applied.
4.5. Study Designs
The present study involved three sub-studies involving the aforementioned splinted full-thickness wound model (see
Table 1).
In the initial study, six mice received full-thickness skin wound injury and were treated with PIC gel and a secondary dressing. Three of the six mice underwent the washing protocol every third and fourth day in sequence, applying a new dose of PIC gel each time. Additionally, 2 mice (1 washed and 1 non-washed) were euthanised on days 14, 21, and 28 to determine the suitability of these time points as an indicator of wound healing conditions in the remodelling phase. Both wounds were harvested and processed for histological staining.
In the imaging study, five mice were subjected to the splinted full-thickness wound model and daily washing procedures up to three days post-injury. On day 0, the mice were operated, treated with the 111In-labelled PIC gel, and scanned with SPECT/CT. On the first and second day after applying PIC gel, the mice were imaged before and after washing the wounds, and a new dose of PIC was applied. Finally, on the third day after wounding, the protocol was repeated, but the animals were euthanised after the final scan and did not receive a new dose of PIC gel. The organs were harvested for biodistribution analysis, and subsequently, both wounds were processed for histological staining.
In the main wound healing study, 54 mice underwent the splinted full-thickness injury model. Mice were randomly assigned over three treatment groups and three time points using an online random number generator (
www.random.org, accessed on 21 November 2017). The ‘control’ group was a sham treatment in which the wounds were created, but mice only received a secondary dressing (Tegaderm™ and bandage) and no PIC gel. In the ‘single PIC’ group, mice received a single dose of PIC gel per wound and a secondary dressing following surgery. In the ‘refreshed PIC’ group, 20 µL of PIC gel and a secondary dressing was applied after wounding. Then, the mice underwent the washing protocol on days 3, 7, 10, and 14, ensuring that a fresh dose of PIC gel was always present. From each group, 6 mice were euthanised on days 3, 7, and 14 post-injury. An exception to the protocol was in the refreshed PIC group on day 3. The wounds of these mice were washed before harvesting to distinguish their treatment from the day 3 single PIC group. This contrasts with the refreshed PIC group on days 7 and 14, where wounds were not washed before harvest. One wound was processed for histological analysis, and the other was intended for gene expression analysis.
4.6. Wound Size Analysis
Photographs of the wounds were taken after surgery and sacrifice and at any intermediate point when bandages were uncovered (e.g., when washing or replacing the dressing). To this end, Tegaderm™ sheets had to be removed, typically causing splints to become undone as well. Pictures were thus taken of the skin in a relaxed state without splints. ImageJ [
34] was used to delineate the outer contours of the wound. These values were expressed as a percentage of wound area compared to day 0 (right after surgery).
4.7. SPECT/CT Imaging and Biodistribution
SPECT/CT imaging, [
111In]In-DTPA-PIC labelling, and analysis methods were carried out as described before [
10]. Briefly, indium was complexed to MES-dissolved DTPA-functionalised PIC in a fridge (4 °C) for over 3 h. Then, ethylenediamine-tetra-acetic acid (EDTA, Sigma Aldrich) was added to chelate any unlabelled
111In and the solution was diluted to 4 mg/mL using PBS. Applied doses were 7.8 ± 1.3 MBq on day 0, 6.0 ± 0.3 MBq on day 1, and 4.3 ± 0.3 MBq on day 2. When analysing the data, the SPECT signal originating from the wounds was quantified using Inveon Research Workplace (Siemens Healthcare, The Hague, The Netherlands) by positioning regions of interest on the wounds in the CT scan. The amount of signal was compared before-and-after washing, after correction for natural decay, and expressed as the amount of signal remaining. As each mouse bore two wounds, two wounds were analysed. Biodistribution analysis after dissection was also performed, as described previously [
10].
4.8. Tissue Harvesting
After sacrifice, a large skin flap surrounding and containing the wounds was excised using scissors and a scalpel. In the imaging study, wounds and control skin were harvested from this flap using a Ø 6 mm biopsy punch and then placed in 70% ethanol. Biodistribution analysis was first performed, and then the 111In was allowed to decay to safe values in 4% formaldehyde before processing the wounds for histology. In the main healing study, right wounds were harvested similarly and fixated immediately in 4% formaldehyde.
4.9. (Immuno-) Histology
Harvested tissue was fixated in 4% formaldehyde at room temperature for 24 h and then dehydrated and embedded in paraffin. Staining protocols for HE, Gr-1, F4/80, αSMA, and AZAN were identical to those published previously [
9,
10]. Von Kossa stainings were performed as follows: histological sections were deparaffinised in a xylene-alcohol-milliQ series, exposed to 5% silver nitrate under UV-illumination for 30 min, rinsed with milliQ, fixed with 2% sodium thiosulfate, and ultimately counter-stained with nuclear fast red. AZAN-stained images were scanned using a Pannoramic P250 digital slide scanner (3DHISTECH Ltd., Budapest, Hungary). Using Pannoramic Viewer (v1.15.4, 3DHISTECH Ltd.) software, one representative section was selected per sample, cropped, and exported at the highest quality settings for further analysis. All other histological sections were analysed under a microscope (Leica DM LB, Leica Microsystems B.V., Amsterdam, The Netherlands). Representative sections were photographed at 100×, 200×, or 400× zoom levels and (optionally) stitched together using a Zeiss Imager Z1 and an AxioCam MRc5 camera (Carl Zeiss Microimaging GmbH, Göttingen, Germany) using AxioVision V4.8.2. software’s MosaiQ plugin.
Histological results were scored blindly by authors RCV and FADTGW and presented as an average of the two. For all scorings, four representative sections of varying depth from each sample were investigated. Epithelial migration was scored on a scale of 0 to 4 in HE sections (no migration (0), partial migration (1), complete migration with no/partial keratinisation (2), complete migration with complete keratinisation (3), and hypertrophic (4)). The presence of PIC gel remnants in HE-stained sections was scored on a scale of 0 to 3 (absent (0), minor remnants (1), large remnants topically (2), and large remnants encapsulated within the wound repair tissue (3)). Cellular infiltration by granulocytes (GR-1 antibody), macrophages (F4/80 antibody), and myofibroblasts (αSMA antibody) were scored on a scale of 0 to 5 (cell type absent (0), mild presence (1), moderate presence (2), marked presence (3), very high presence (4), and wound tissue consists almost exclusively of this cell type (5)). Scores were treated as a scale, and a score of 0.5 could be awarded when the evaluator assessed the score to be slightly higher or lower than other sections of a similar score. The presence of giant cells in the HE sections was evaluated by eye. The percentage of blue-stained area in AZAN-stained sections, corresponding to collagenous structures, was calculated using ImageJ similarly as previously published [
9]. However, instead of using colour thresholds to isolate the blue signal, the colour deconvolution plugin was used. Five sections were randomly selected to determine the vectors that could separate the different coloured tissues in the whole data set. This resulted in three images depicting only the blue (collagen), red (nuclei, erythrocytes, muscle, glial tissue), or green/grey (artefacts) coloured pixels. The analysis was performed as follows: area deemed as wound tissue was isolated; healthy tissue parts and volumes of PIC gels were then cropped out; and, ultimately, the blue-positive area in the total wound tissue deemed as ‘Collagen content’ was calculated and expressed as a percentage.
4.10. Statistical Analysis
Statistical analysis was performed using IBM SPSS software (version 20, Armonk, NY, USA). In the imaging study, the results of histological investigations were compared between left and right wounds using the paired student’s t-test. In the main wound healing study, the control group was compared to the single PIC and to the refreshed PIC group. In addition, the single PIC and refreshed PIC groups were compared. Data were split by time point during analysis, as only the inter-group differences were of interest. Statistical tests were selected based on the nature and behaviour of the acquired data. The rate of wound closure and the histological scorings of granulocytes, macrophages, and collagen content were analysed by one-way ANOVA with Tukey post hoc test. The migration of epithelium and histological population of myofibroblasts were tested with the non-parametric Kruskal–Wallis test. The presence of PIC gel in HE-stained sections was analysed using Cross Tabulation and Fisher’s Exact Test. The statistical significance level α was set at 0.05.