TADF and X-ray Radioluminescence of New Cu(I) Halide Complexes: Different Halide Effects on These Processes
Abstract
1. Introduction
2. Results and Discussion
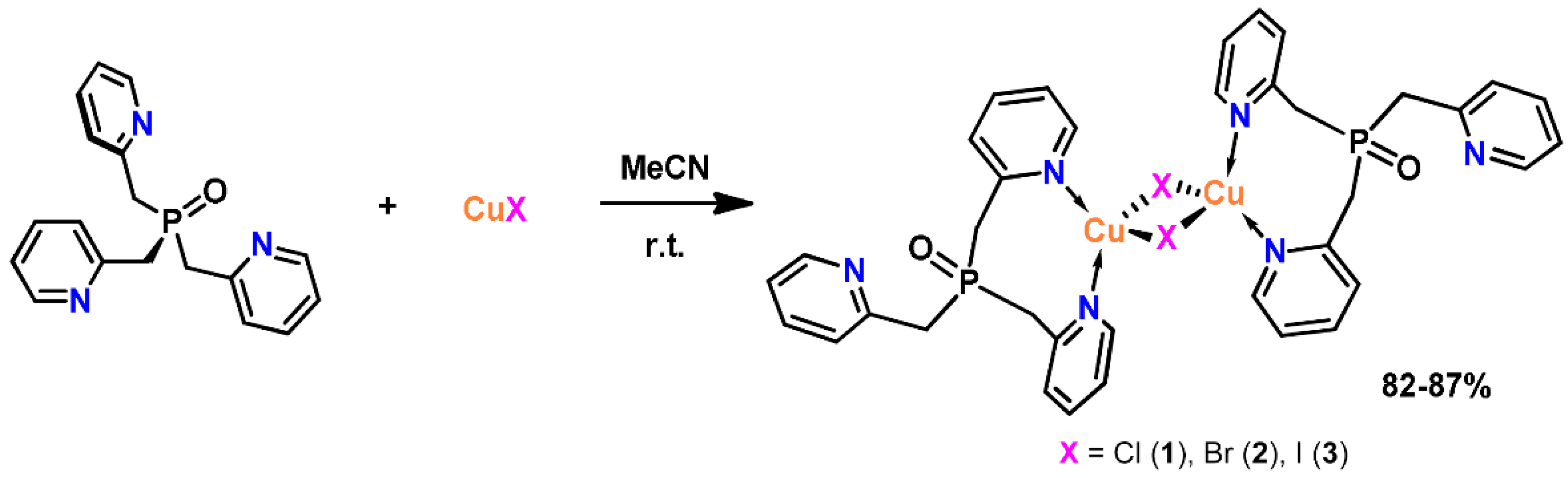
2.1. Synthesis and Characterization
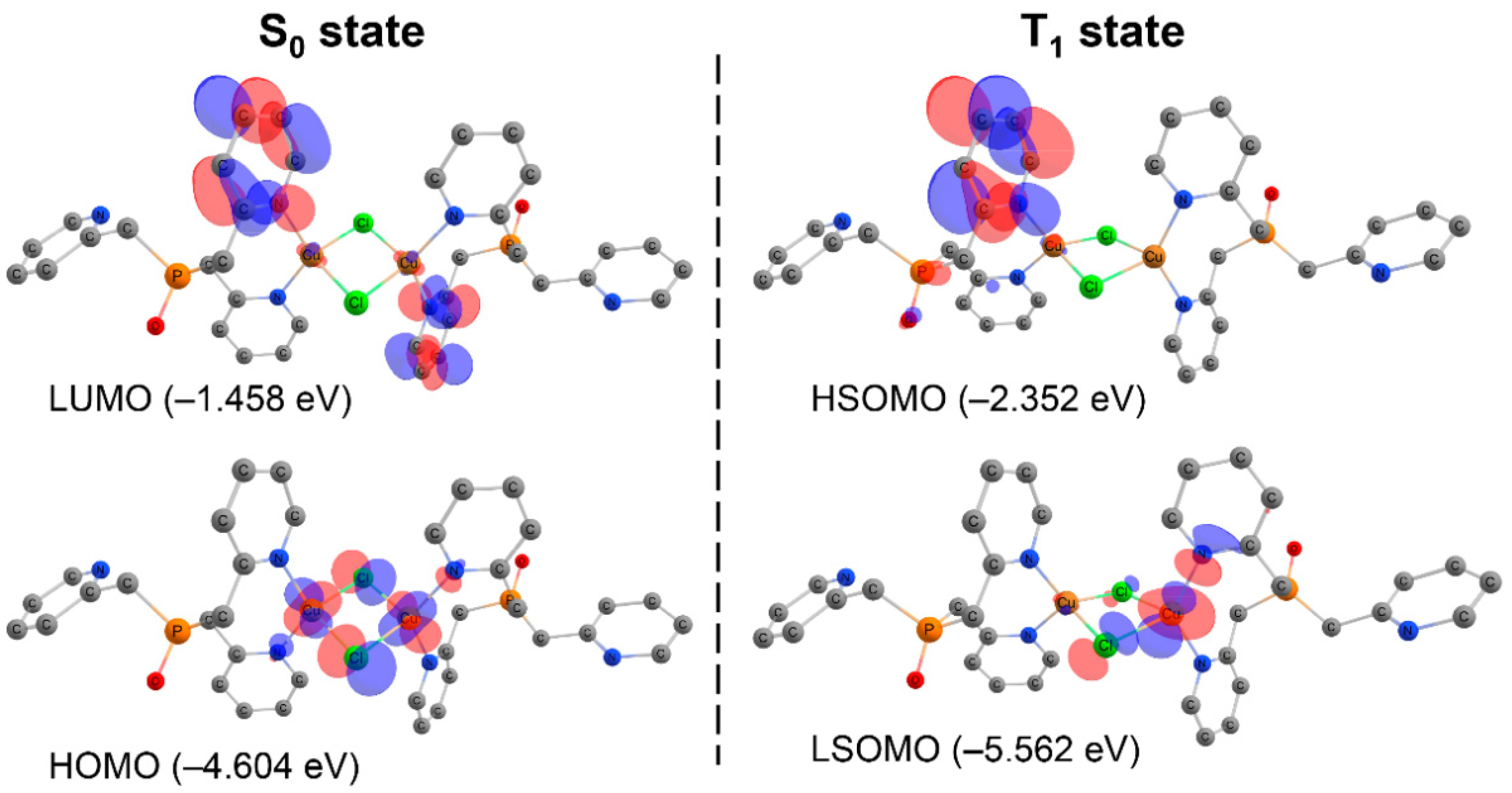
2.2. Theoretical Consideration
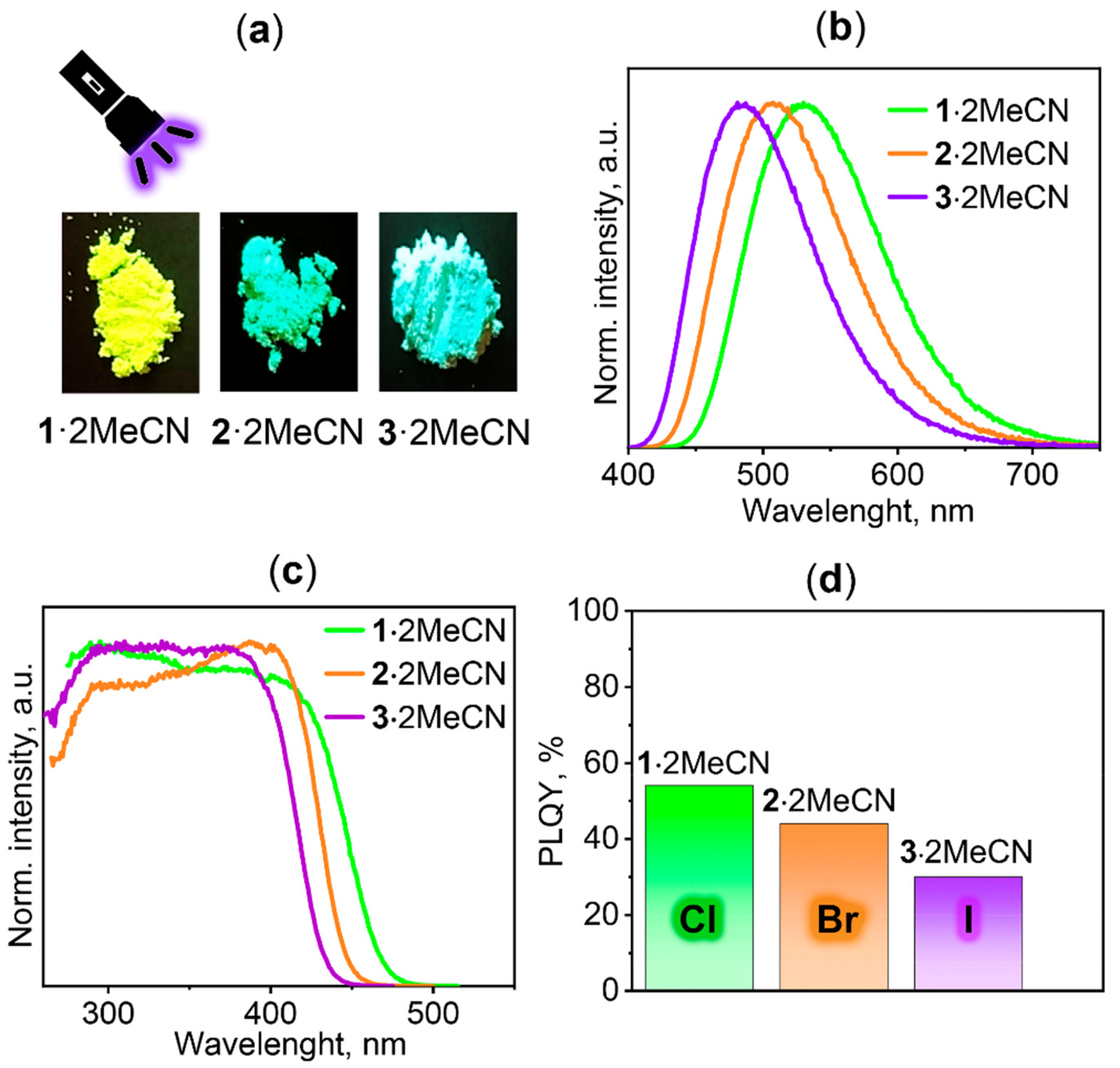
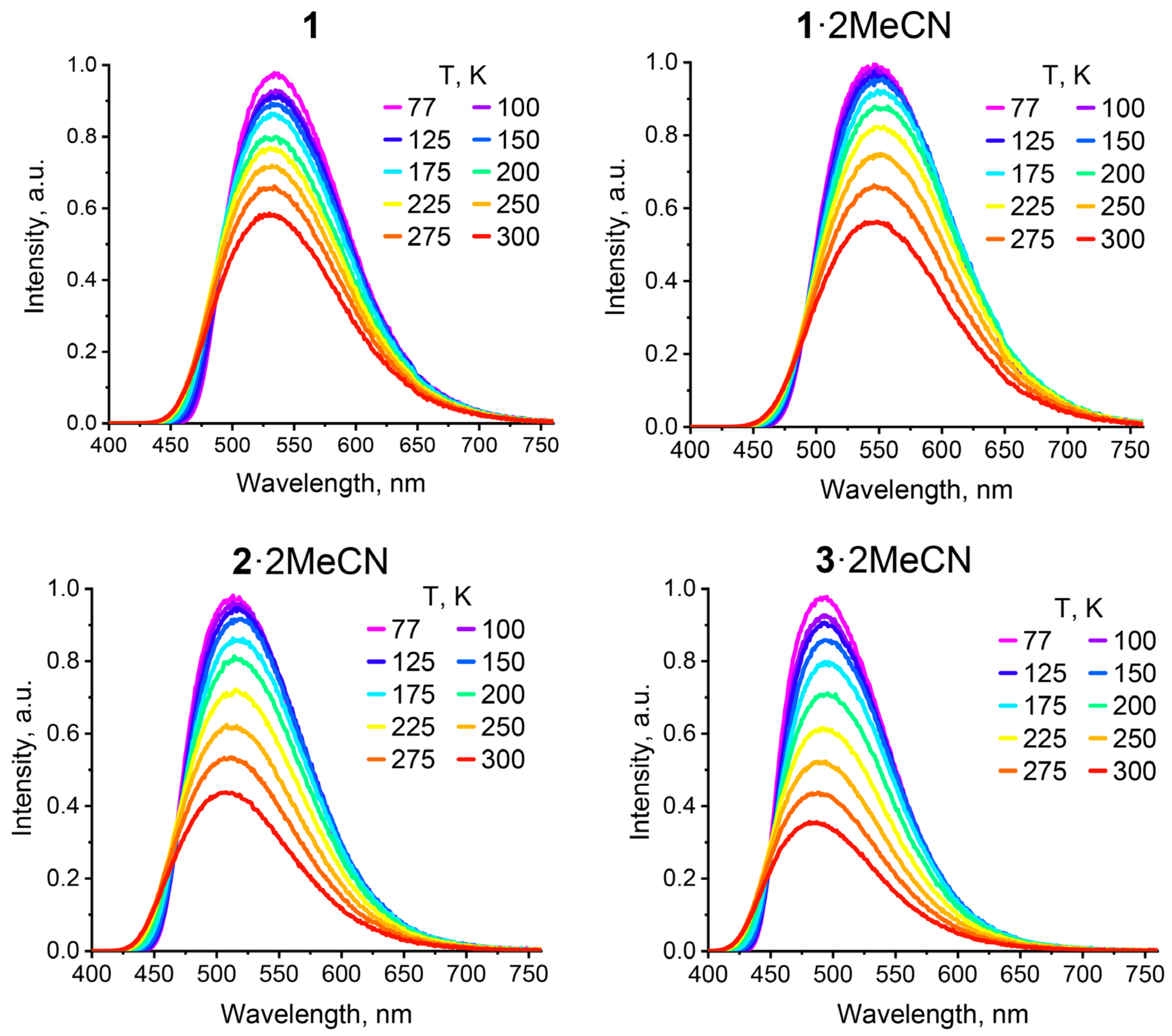
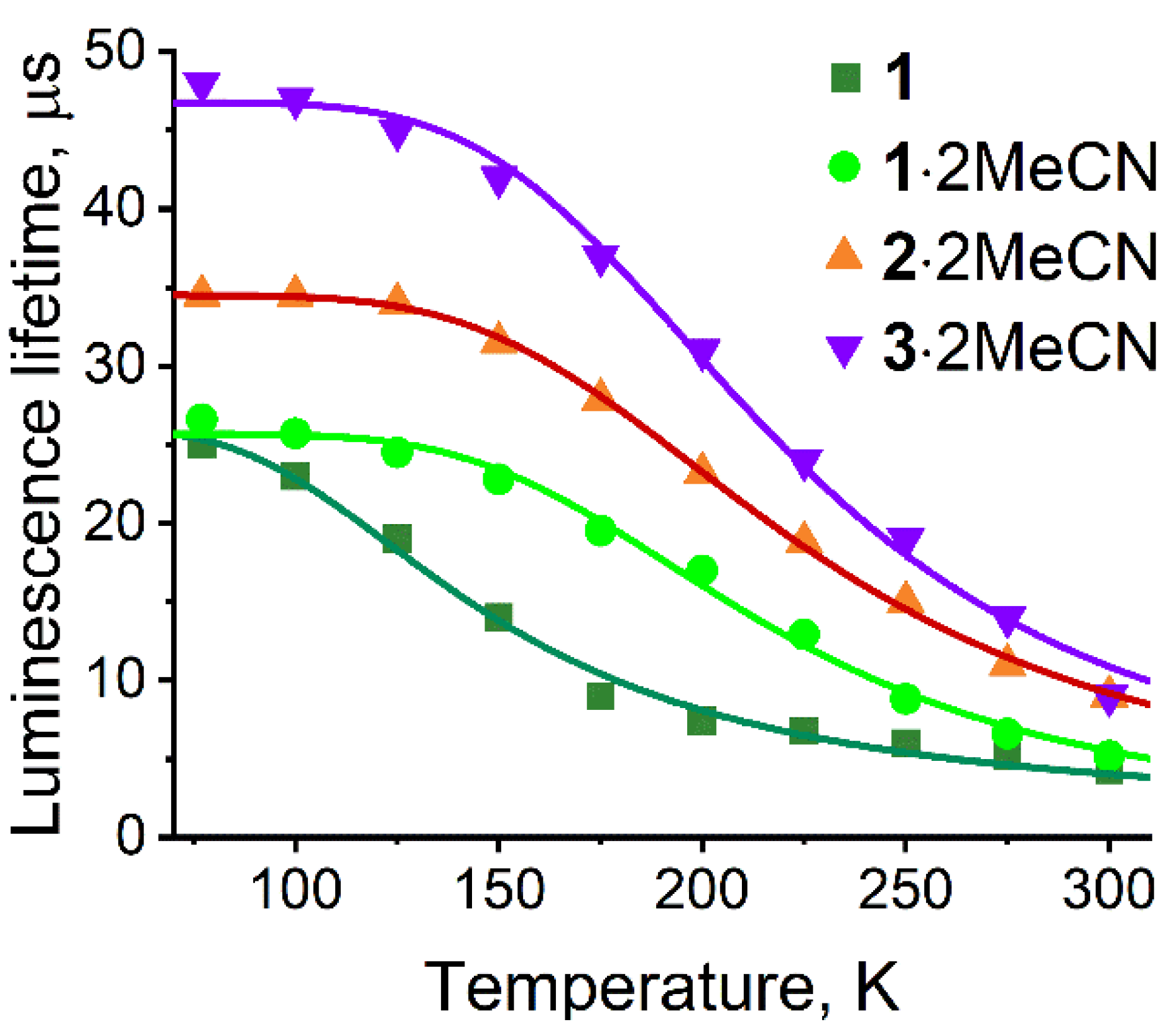
2.3. TADF Properties
2.4. X-ray Radioluminescence
3. Materials and Methods
3.1. General
3.2. Synthesis and Characterization Data
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shmelev, N.Y.; Okubazghi, T.H.; Abramov, P.A.; Komarov, V.Y.; Rakhmanova, M.I.; Novikov, A.S.; Gushchin, A.L. Intramolecular Aurophilic Interactions in Dinuclear Gold(I) Complexes with Twisted Bridging 2,2′-Bipyridine Ligands. Dalton Trans. 2021, 50, 12448–12456. [Google Scholar] [CrossRef] [PubMed]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Plyusnin, P.E.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Diiodine—Iodobismuthate(III) Hybrids Cat3{[Bi2I9](I2)3}: Syntheses, Stability, and Optical Properties. Inorg. Chem. 2020, 59, 17320–17325. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Gorokh, I.D.; Abramov, P.A.; Novikov, A.S.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. Chlorobismuthates Trapping Dibromine: Formation of Two-Dimensional Supramolecular Polyhalide Networks with Br2 Linkers. Eur. J. Inorg. Chem. 2017, 2017, 4925–4929. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Smolentsev, A.I.; Kozlova, S.G.; Fedin, V.P. [PtBi2I12]2−: The First Polyiodobismuthate Containing an Octahedral Heterometallic Unit. Dalton Trans. 2013, 42, 9818–9821. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fang, Y.; Li, J. Copper Iodide Based Hybrid Phosphors for Energy-Efficient General Lighting Technologies. Adv. Funct. Mater. 2018, 28, 1705593. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) Hybrid Inorganic-Organic Materials with Intriguing Stimuli Responsive and Optoelectronic Properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Wallesch, M.; Volz, D.; Zink, D.M.; Schepers, U.; Nieger, M.; Baumann, T.; Bräse, S. Bright Coppertunities: Multinuclear CuI Complexes with N-P Ligands and Their Applications. Chem. Eur. J. 2014, 20, 6578–6590. [Google Scholar] [CrossRef]
- Troyano, J.; Zamora, F.; Delgado, S. Copper(I)-Iodide Cluster Structures as Functional and Processable Platform Materials. Chem. Soc. Rev. 2021, 50, 4606–4628. [Google Scholar] [CrossRef]
- Hei, X.; Liu, W.; Zhu, K.; Teat, S.J.; Jensen, S.; Li, M.; O’Carroll, D.M.; Wei, K.; Tan, K.; Cotlet, M.; et al. Blending Ionic and Coordinate Bonds in Hybrid Semiconductor Materials: A General Approach toward Robust and Solution-Processable Covalent/Coordinate Network Structures. J. Am. Chem. Soc. 2020, 142, 4242–4253. [Google Scholar] [CrossRef]
- Enikeeva, K.R.; Shamsieva, A.V.; Strelnik, A.G.; Fayzullin, R.R.; Zakharychev, D.V.; Kolesnikov, I.E.; Dayanova, I.R.; Gerasimova, T.P.; Strelnik, I.D.; Musina, E.I.; et al. Green Emissive Copper(I) Coordination Polymer Supported by the Diethylpyridylphosphine Ligand as a Luminescent Sensor for Overheating Processes. Molecules 2023, 28, 706. [Google Scholar] [CrossRef]
- Dayanova, I.R.; Shamsieva, A.V.; Strelnik, I.D.; Gerasimova, T.P.; Kolesnikov, I.E.; Fayzullin, R.R.; Islamov, D.R.; Saifina, A.F.; Musina, E.I.; Hey-Hawkins, E.; et al. Assembly of Heterometallic AuICu2I2 Cores on the Scaffold of NPPN-Bridging Cyclic Bisphosphine. Inorg. Chem. 2021, 60, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Lescop, C. Coordination-Driven Supramolecular Synthesis Based on Bimetallic Cu(I) Precursors: Adaptive Behavior and Luminescence. Chem. Rec. 2021, 21, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Moutier, F.; Schiller, J.; Calvez, G.; Lescop, C. Self-Assembled Luminescent Cu(I) Tetranuclear Metallacycles Based on 3,3′-Bipyridine Ligands. Org. Chem. Front. 2021, 8, 2893–2902. [Google Scholar] [CrossRef]
- El Sayed Moussa, M.; Khalil, A.M.; Evariste, S.; Wong, H.L.; Delmas, V.; Le Guennic, B.; Calvez, G.; Costuas, K.; Yam, V.W.W.; Lescop, C. Intramolecular Rearrangements Guided by Adaptive Coordination-Driven Reactions toward Highly Luminescent Polynuclear Cu(I) Assemblies. Inorg. Chem. Front. 2020, 7, 1334–1344. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and Photophysics of Coordination Compounds: Copper. In Topics in Current Chemistry; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 280, pp. 69–115. ISBN 3540733469. [Google Scholar]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) Complexes—Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Yersin, H. (Ed.) Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Wiley-VCH: Weinheim, Germany, 2019; ISBN 978-3-527-33900-6. [Google Scholar]
- Ohara, H.; Kobayashi, A.; Kato, M. Simple and Extremely Efficient Blue Emitters Based on Mononuclear Cu(I)-Halide Complexes with Delayed Fluorescence. Dalton Trans. 2014, 43, 17317–17323. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhou, D.; Monkowius, U.; Yersin, H. Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence. Micromachines 2021, 12, 1500. [Google Scholar] [CrossRef]
- Hofbeck, T.; Monkowius, U.; Yersin, H. Highly Efficient Luminescence of Cu(I) Compounds: Thermally Activated Delayed Fluorescence Combined with Short-Lived Phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Demyanov, Y.V.; Rakhmanova, M.I.; Bagryanskaya, I.Y. Pyridylarsine-Based Cu(I) Complexes Showing TADF Mixed with Fast Phosphorescence: A Speeding-up Emission Rate Using Arsine Ligands. Dalton Trans. 2022, 51, 1048–1055. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Berezin, A.S.; Samsonenko, D.G.; Mazur, A.S.; Tolstoy, P.M.; Plyusnin, V.F.; Kolesnikov, I.E.; Artem’ev, A.V. New Cu(I) Halide Complexes Showing TADF Combined with Room Temperature Phosphorescence: The Balance Tuned by Halogens. Dalton Trans. 2020, 49, 3155–3163. [Google Scholar] [CrossRef]
- Schinabeck, A.; Chen, J.; Kang, L.; Teng, T.; Homeier, H.H.H.; Suleymanova, A.F.; Shafikov, M.Z.; Yu, R.; Lu, C.-Z.; Yersin, H. Symmetry-Based Design Strategy for Unprecedentedly Fast Decaying Thermally Activated Delayed Fluorescence (TADF). Application to Dinuclear Cu(I) Compounds. Chem. Mater. 2019, 31, 4392–4404. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. TADF: Enabling Luminescent Copper(I) Coordination Compounds for Light-Emitting Electrochemical Cells. J. Mater. Chem. C 2022, 10, 4456–4482. [Google Scholar] [CrossRef] [PubMed]
- Ravaro, L.P.; Zanoni, K.P.S.; de Camargo, A.S.S. Luminescent Copper(I) Complexes as Promising Materials for the next Generation of Energy-Saving OLED Devices. Energy Rep. 2020, 6, 37–45. [Google Scholar] [CrossRef]
- Hashimoto, M.; Igawa, S.; Yashima, M.; Kawata, I.; Hoshino, M.; Osawa, M. Highly Efficient Green Organic Light-Emitting Diodes Containing Luminescent Three-Coordinate Copper(I) Complexes. J. Am. Chem. Soc. 2011, 133, 10348–10351. [Google Scholar] [CrossRef]
- Perruchas, S. Molecular Copper Iodide Clusters: A Distinguishing Family of Mechanochromic Luminescent Compounds. Dalton Trans. 2021, 50, 12031–12044. [Google Scholar] [CrossRef]
- Kirakci, K.; Fejfarová, K.; Martinčík, J.; Nikl, M.; Lang, K. Tetranuclear Copper(I) Iodide Complexes: A New Class of X-Ray Phosphors. Inorg. Chem. 2017, 56, 4609–4614. [Google Scholar] [CrossRef]
- Lian, L.; Wang, X.; Zhang, P.; Zhu, J.; Zhang, X.; Gao, J.; Wang, S.; Liang, G.; Zhang, D.; Gao, L.; et al. Highly Luminescent Zero-Dimensional Organic Copper Halides for X-Ray Scintillation. J. Phys. Chem. Lett. 2021, 12, 6919–6926. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Wang, Z.; Liu, Z.-M.; Yu, H.-J.; Zhang, J.-H.; Wang, Y.; Mao, R.; Pan, M.; Su, C.-Y. Multiresponsive UV-One-Photon Absorption, Near-Infrared-Two-Photon Absorption, and X/γ-Photoelectric Absorption Luminescence in One [Cu4I4] Compound. Inorg. Chem. 2019, 58, 10736–10742. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Nikl, M.; Hamel, M.; Wu, H.; Qian, S.; Kucerkova, R.; Babin, V.; Ren, G.; Wu, Y. Preparation and Performance of Plastic Scintillators with Copper Iodide Complex-Loaded for Radiation Detection. Polymers 2022, 249, 124832. [Google Scholar] [CrossRef]
- Vinogradova, K.A.; Plyusnin, V.F.; Kupryakov, A.S.; Rakhmanova, M.I.; Pervukhina, N.V.; Naumov, D.Y.; Sheludyakova, L.A.; Nikolaenkova, E.B.; Krivopalov, V.P.; Bushuev, M.B. Halide Impact on Emission of Mononuclear Copper(I) Complexes with Pyrazolylpyrimidine and Triphenylphosphine. Dalton Trans. 2014, 43, 2953–2960. [Google Scholar] [CrossRef]
- Leitl, M.J.; Küchle, F.R.; Mayer, H.A.; Wesemann, L.; Yersin, H. Brightly Blue and Green Emitting Cu(I) Dimers for Singlet Harvesting in OLEDs. J. Phys. Chem. A 2013, 117, 11823–11836. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, J.; Jess, I.; Näther, C.; Necas, M.; Taborsky, P. Luminescence Properties of “Double-Stranded Staircase” Copper(I) Halide Coordination Polymers with N-Containing Ligands. New J. Chem. 2011, 35, 861–864. [Google Scholar] [CrossRef]
- Hofbeck, T.; Niehaus, T.A.; Fleck, M.; Monkowius, U.; Yersin, H. P∩N Bridged Cu(I) Dimers Featuring Both TADF and Phosphorescence. From Overview towards Detailed Case Study of the Excited Singlet and Triplet States. Molecules 2021, 26, 3415. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.K.; Vitale, M.; Ford, P.C. Photoluminescence Properties of the Structurally Analogous Tetranuclear Copper(I) Clusters Cu4X4(Dpmp)4 (X = I, Br, Cl; Dpmp = 2-(Diphenylmethyl)Pyridine). Inorg. Chem. 1993, 32, 869–874. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, R.; Liu, L.; Zhong, X.-X.; Wang, L.; Li, G.-H.; Li, F.-B.; Alamry, K.A.; Zhao, Y. From Deep Blue to Green Emitting and Ultralong Fluorescent Copper(I) Halide Complexes Containing Dimethylthiophene Diphosphine and PPh3 Ligands. Dalton Trans. 2019, 48, 11448–11459. [Google Scholar] [CrossRef]
- Araki, H.; Tsuge, K.; Sasaki, Y.; Ishizaka, S.; Kitamura, N. Luminescence Ranging from Red to Blue: A Series of Copper(I)−Halide Complexes Having Rhombic {Cu2(μ-X)2} (X = Br and I) Units with N-Heteroaromatic Ligands. Inorg. Chem. 2005, 44, 9667–9675. [Google Scholar] [CrossRef]
- Mondal, R.; Lozada, I.B.; Davis, R.L.; Williams, J.A.G.; Herbert, D.E. Site-Selective Benzannulation of N-Heterocycles in Bidentate Ligands Leads to Blue-Shifted Emission from [(P^N)Cu]2(μ-X)2 Dimers. Inorg. Chem. 2018, 57, 4966–4978. [Google Scholar] [CrossRef]
- Chen, K.; Shearer, J.; Catalano, V.J. Subtle Modulation of Cu4X4L2 Phosphine Cluster Cores Leads to Changes in Luminescence. Inorg. Chem. 2015, 54, 6245–6256. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent Copper(I) Complexes with Halogenido-Bridged Dimeric Core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, C.; Han, C.; Yang, H.; Wei, Y.; Xu, H. Balanced Dual Emissions from Tridentate Phosphine-Coordinate Copper(I) Complexes toward Highly Efficient Yellow OLEDs. Adv. Mater. 2016, 28, 5975–5979. [Google Scholar] [CrossRef]
- Gneuß, T.; Leitl, M.J.; Finger, L.H.; Rau, N.; Yersin, H.; Sundermeyer, J. A New Class of Luminescent Cu(I) Complexes with Tripodal Ligands—TADF Emitters for the Yellow to Red Color Range. Dalton Trans. 2015, 44, 8506–8520. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.K.; Hughes, R.P.; Glueck, D.S.; Royappa, A.T.; Rheingold, A.L.; Arthur, R.B.; Nicholas, A.D.; Patterson, H.H. Synthesis, Structure, and Luminescence of Copper(I) Halide Complexes of Chiral Bis(Phosphines). Inorg. Chem. 2017, 56, 12809–12820. [Google Scholar] [CrossRef] [PubMed]
- Zhanga, L.; Lia, B. HigRoom-Temperature Pure Blue-Emitting Phosphorescent Multinuclear Cu(I)-Based Emitters. J. Electrochem. Soc. 2009, 156, J174–J178. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Gusarova, N.K.; Shagun, V.A.; Malysheva, S.F.; Smirnov, V.I.; Borodina, T.N.; Trofimov, B.A. Complexation of Tris(2-Pyridyl)Phosphine Chalcogenides with Copper(I) Halides: The Selective Formation of Scorpionate Complexes, [Cu(N,N′,N″-2-Py3PX)Hal] (X = O, S and Se). Polyhedron 2015, 90, 1–6. [Google Scholar] [CrossRef]
- Malysheva, S.F.; Belogorlova, N.A.; Kuimov, V.A.; Litvintsev, Y.I.; Sterkhova, I.V.; Albanov, A.I.; Gusarova, N.K.; Trofimov, B.A. PCl3- and Organometallic-Free Synthesis of Tris(2-Picolyl)Phosphine Oxide from Elemental Phosphorus and 2-(Chloromethyl)Pyridine Hydrochloride. Tetrahedron Lett. 2018, 59, 723–726. [Google Scholar] [CrossRef]
- Hettstedt, C.; Unglert, M.; Mayer, R.J.; Frank, A.; Karaghiosoff, K. Methoxyphenyl Substituted Bis(Picolyl)Phosphines and Phosphine Oxides. Eur. J. Inorg. Chem. 2016, 2016, 1405–1414. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Shafikov, M.Z.; Schinabeck, A.; Antonova, O.V.; Berezin, A.S.; Bagryanskaya, I.Y.; Plusnin, P.E.; Yersin, H. Sky-blue thermally activated delayed fluorescence (TADF) based on Ag(I) complexes: Strong solvation-induced emission enhancement. Inorg. Chem. Front. 2019, 6, 3168–3176. [Google Scholar] [CrossRef]
- Siebrand, W. Radiationless Transitions in Polyatomic Molecules. I. Calculation of Franck—Condon Factors. J. Chem. Phys. 1967, 46, 440–447. [Google Scholar] [CrossRef]
- Robinson, G.W.; Frosch, R.P. Electronic Excitation Transfer and Relaxation. J. Chem. Phys. 1963, 38, 1187–1203. [Google Scholar] [CrossRef]
- EKalneus, V.; Melnikov, A.; Korolev, V.; Ivannikov, V.; Stass, D. A Low-Field Magnetically Affected Reaction Yield (MARY) Spectrometer with Spectral Fluorescence Resolution. Appl. Magn. Reson. 2013, 44, 81–96. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bruker Apex3 Software Suite: Apex3, SADABS-2016/2 and SAINT, Version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09; revision D.01; Carnegie Mellon University: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular-Dichroism Spectra Using Density-Functional Force-Fields. J. Chem. Phys. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Pritchard, B.; Altarawy, D.; Didier, B.; Gibson, T.; Windus, T. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Van Caillie, C.; Amos, R. Geometric derivatives of excitation energies using SCF and DFT. Chem. Phys. Lett. 1999, 308, 249–255. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Evtushok, D.; Melnikov, A.; Vorotnikova, N.; Vorotnikov, Y.; Ryadun, A.; Kuratieva, N.; Kozyr, K.; Obedinskaya, N.; Kretov, E.; Novozhilov, I.N.; et al. A comparative study of optical properties and X-ray induced luminescence of octahedral molybdenum and tungsten cluster complexes. Dalton Trans. 2017, 46, 11738–11747. [Google Scholar] [CrossRef]
- Stass, D.; Vorotnikova, N.; Shestopalov, M. Efficient light-emitting diodes from organic radicals with doublet emission. J. Appl. Phys. 2021, 129, 183102. [Google Scholar] [CrossRef]
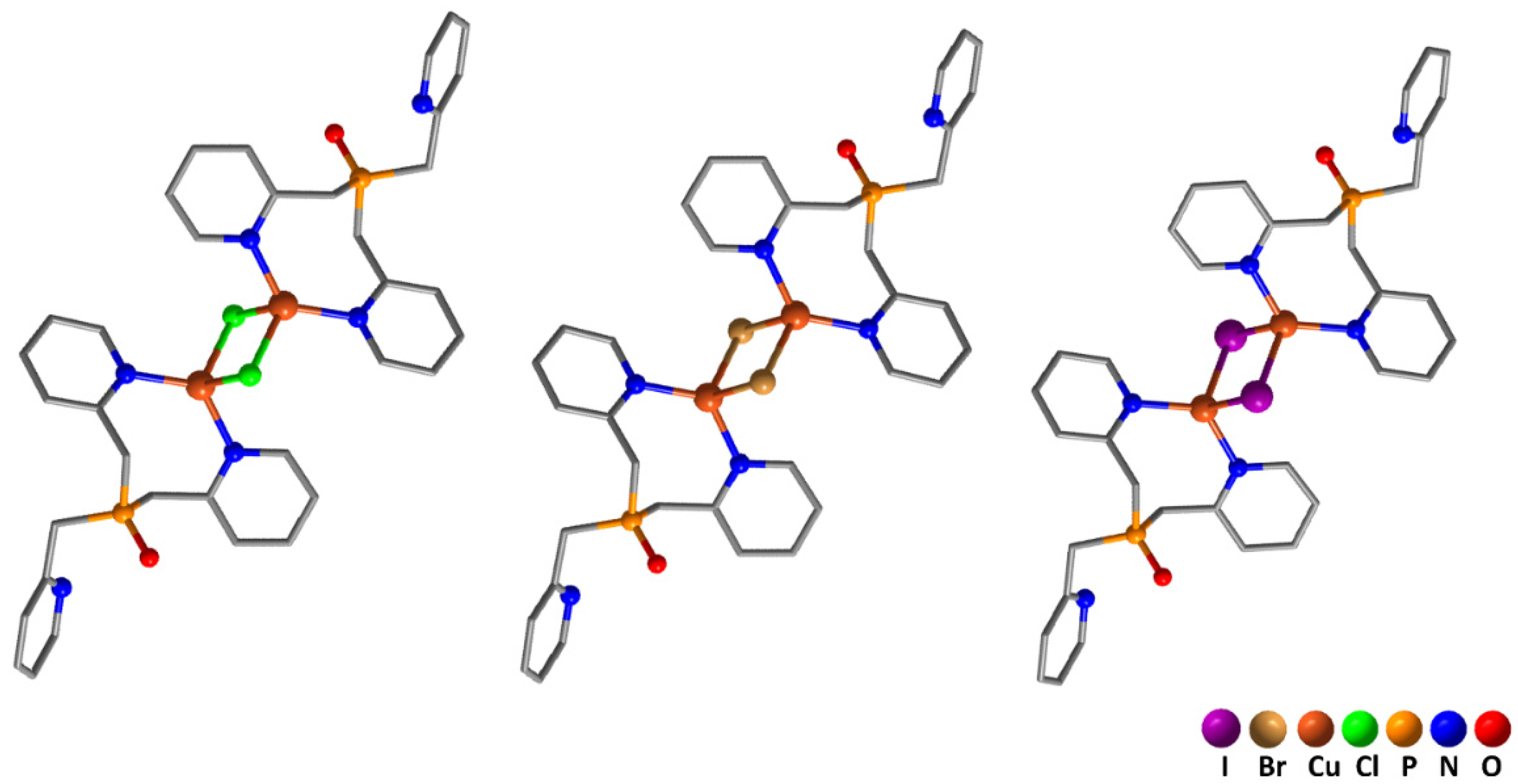

| 1·2MeCN | 2·2MeCN | 3·2MeCN | |
|---|---|---|---|
| Cu···Cu | 3.1220(4) | 3.2239(11) | 3.2739(8) |
| Cu–N | 2.0173(19), 2.030(2) | 2.019(5), 2.025(6) | 2.035(4), 2.048(4) |
| Cu–X (X = halide) | 2.3471(7), 2.5916(8) | 2.4894(11), 2.6973(14) | 2.6612(7), 2.7919(8) |
| 1·2MeCN | 1 (b) | 2·2MeCN | 3·2MeCN | |
|---|---|---|---|---|
| λmax (298 K), nm | 530 | 545 | 507 | 485 |
| ΦPL (298 K), % | 54 | 35 | 44 | 30 |
| τ (298 K), µs | 4.3 | 5.2 | 9 | 9 |
| λmax (77 K), nm | 535 | 547 | 514 | 492 |
| τ (77 K), µs | 25 | 26.6 | 34.5 | 48 |
| ∆E(S1–T1), cm−1 (c) | 762 | 391 | 734 | 765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artem'ev, A.V.; Baranov, A.Y.; Berezin, A.S.; Stass, D.V.; Hettstedt, C.; Kuzmina, U.A.; Karaghiosoff, K.; Bagryanskaya, I.Y. TADF and X-ray Radioluminescence of New Cu(I) Halide Complexes: Different Halide Effects on These Processes. Int. J. Mol. Sci. 2023, 24, 5145. https://doi.org/10.3390/ijms24065145
Artem'ev AV, Baranov AY, Berezin AS, Stass DV, Hettstedt C, Kuzmina UA, Karaghiosoff K, Bagryanskaya IY. TADF and X-ray Radioluminescence of New Cu(I) Halide Complexes: Different Halide Effects on These Processes. International Journal of Molecular Sciences. 2023; 24(6):5145. https://doi.org/10.3390/ijms24065145
Chicago/Turabian StyleArtem'ev, Alexander V., Andrey Yu. Baranov, Alexey S. Berezin, Dmitry V. Stass, Christina Hettstedt, Ul’yana A. Kuzmina, Konstantin Karaghiosoff, and Irina Yu. Bagryanskaya. 2023. "TADF and X-ray Radioluminescence of New Cu(I) Halide Complexes: Different Halide Effects on These Processes" International Journal of Molecular Sciences 24, no. 6: 5145. https://doi.org/10.3390/ijms24065145
APA StyleArtem'ev, A. V., Baranov, A. Y., Berezin, A. S., Stass, D. V., Hettstedt, C., Kuzmina, U. A., Karaghiosoff, K., & Bagryanskaya, I. Y. (2023). TADF and X-ray Radioluminescence of New Cu(I) Halide Complexes: Different Halide Effects on These Processes. International Journal of Molecular Sciences, 24(6), 5145. https://doi.org/10.3390/ijms24065145








