Exploring the Potential of Vine Shoots as a Source of Valuable Extracts and Stable Lignin Nanoparticles for Multiple Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Extracts Resulting from the Pretreatment Process
2.1.1. Proanthocyanidins
2.1.2. Sugars
2.2. Cellulose and Hemicellulose Fraction
2.3. Characterization of Lignin from Vine Shoots Using Mild Acidolysis Extraction
2.3.1. Composition of the Isolated Lignin Samples
2.3.2. Proanthocyanidins Content in Lignin Samples
2.3.3. Molecular Weight and Polydispersity of Lignin Samples
2.3.4. Quantitative Carbon-13 Nuclear Magnetic Resonance (13C NMR)
2.3.5. Phosphorus-31 Nuclear Magnetic Resonance (31P NMR)
2.3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Lignin Nanoparticles—Size, Polydispersity Index, and Zeta-Potential
2.5. Antioxidant Activity of Soluble Lignin and Lignin Nanoparticles
| Sample | IC50 (mg/mL) | RSI (1/IC50) |
|---|---|---|
| LET (soluble lignin) | 0.017 | 59 |
| LETD (soluble lignin) | 0.016 | 63 |
| LWE (soluble lignin) | 0.030 | 33 |
| LWED (soluble lignin) | 0.026 | 38 |
| LET nanoparticles | 0.031 | 32 |
| LETD nanoparticles | 0.029 | 34 |
| LWE nanoparticles | 0.029 | 34 |
| LWED nanoparticles | 0.030 | 33 |
| Commercial antioxidants | ||
| Gallic acid | 0.002 (in this work) | 500 |
| Trolox | 0.003 (in this work) | 333 |
| Ascorbic acid | 0.005 (in this work) | 200 |
| Vitamin E | 0.0037 [47] | 263 |
| BHT | 0.038 [52] | 26 |
| BHA | 0.056 [52] | 18 |
3. Materials and Methods
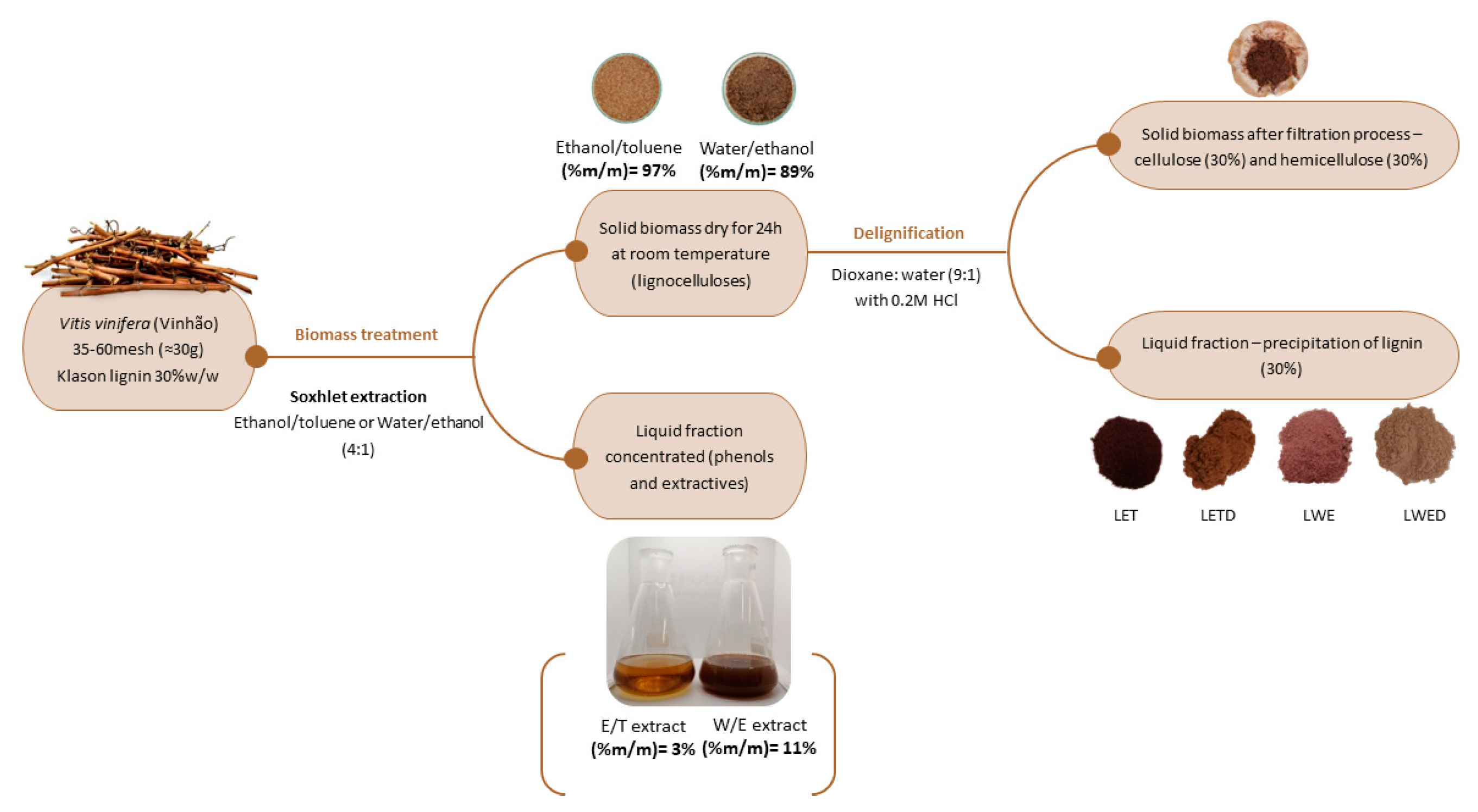
3.1. Preparation of Vine Shoots
3.2. Biomass Pre-Treatment and Lignin Isolation
3.3. Chemical Characterization of Extracts Obtained from Biomass Pretreatment
3.3.1. Total Polyphenol Content
3.3.2. Identification of Phenolic Compounds
3.3.3. Sugar Content
3.4. Chemical Characterization of Lignin Samples
3.4.1. Lignin Content
3.4.2. Inorganic Material, Carbohydrate, and Proanthocyanidin Content
3.4.3. 13C NMR and 31P NMR
3.5. Preparation of Lignin Nanoparticles
3.6. Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. State of the World Vitivinicultural Sector in 2019; International Organisation of Vine and Wine: Paris, France, 2020; pp. 1–15. [Google Scholar]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Florestal, I.M. Estudo do Impacto na Economia Local/Regional Autárquica pelo Aproveitamento e Valorização Energética e Económica das Podas; Ibero Massa Florestal, Lda: Aveiro, Portugal, 2014. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambiente, A.P.D. Portuguese National Inventory Report on Greenhouse Gases, 1990–2012; Portuguese Enviromental Agency: Amadora, Portugal, 2014. [Google Scholar]
- Briones, R.; Torres, L.; Saravia, Y.; Serrano, L.; Labidi, J. Liquefied agricultural residues for film elaboration. Ind. Crops Prod. 2015, 78, 19–28. [Google Scholar] [CrossRef]
- Rivas, S.; López, L.; Vila, C.; Parajó, J.C. Organosolv processing of vine shoots: Fractionation and conversion of hemicellulosic sugars into platform chemicals by microwave irradiation. Bioresour. Technol. 2021, 342, 125967. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Zwingelstein, M.; Draye, M.; Besombes, J.-L.; Piot, C.; Chatel, G. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct biorefinery from vine shoots: Bio-ethanol and lignin production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef]
- Jiménez, L.; Angulo, V.; Ramos, E.; De la Torre, M.J.; Ferrer, J.L. Comparison of various pulping processes for producing pulp from vine shoots. Ind. Crops Prod. 2006, 23, 122–130. [Google Scholar] [CrossRef]
- Ntalos, G.A.; Grigoriou, A.H. Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Ind. Crops Prod. 2002, 16, 59–68. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Pereira, A.R.; de Freitas, V.; Oliveira, J. Chapter 7—Development of lignin-based nanoparticles: Fabrication methods and functionalization approaches. In Lignin-Based Materials for Biomedical Applications; Santos, H., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 227–270. [Google Scholar]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H.; Zaidi, A.A. Natural fiber reinforced composites: Sustainable materials for emerging applications. Results Eng. 2021, 11, 100263. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of Natural Fiber Reinforced Polymer Composites in Sandwich Structures: A Review on Its Mechanical Properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sain, M.M. Carbon storage potential in natural fiber composites. Resour. Conserv. Recycl. 2003, 39, 325–340. [Google Scholar] [CrossRef]
- Huda, M.K.; Widiastuti, I. Natural Fiber Reinforced Polymer in Automotive Application: A Systematic Literature Review. In Journal of Physics: Conference Series, Proceedings of Annual Engineering and Vocational Education Conference (AEVEC), Surakarta, Indonesia, 18–19 September 2020; IOP Publishing: Bristol, UK, 2021; Volume 1808, p. 012015. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pinto, P.C.d.R.; Barreiro, M.F.; da Costa, C.A.E.; da Mota, M.I.F.; Fernandes, I. An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Erdocia, X.; Hernández-Ramos, F.; Morales, A.; Izaguirre, N.; de Hoyos-Martínez, P.L.; Labidi, J. Chapter 3—Lignin extraction and isolation methods. In Lignin-Based Materials for Biomedical Applications, Santos, H., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 61–104. [Google Scholar]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Radar Tool for Lignin Classification on the Perspective of Its Valorization. Ind. Eng. Chem. Res. 2015, 54, 7580–7590. [Google Scholar] [CrossRef]
- Esteves Costa, C.A.; Coleman, W.; Dube, M.; Rodrigues, A.E.; Rodrigues Pinto, P.C. Assessment of key features of lignin from lignocellulosic crops: Stalks and roots of corn, cotton, sugarcane, and tobacco. Ind. Crops Prod. 2016, 92, 136–148. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.D.; Duc Vu, C.; Viet Nguyen, H. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar] [CrossRef]
- Prozil, S.; Evtuguin, D.; Silva, A.; Cruz-Lopes, L. Structural Characterization of Lignin from Grape Stalks (Vitis vinifera L.). J. Agric. Food Chem. 2014, 62, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Dorosh, O.; Rodrigues, F.; Delerue-Matos, C.; Moreira, M.M. Increasing the added value of vine-canes as a sustainable source of phenolic compounds: A review. Sci. Total Environ. 2022, 830, 154600. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. 4—Reuse of Vine-Shoots Wastes for Agricultural Purposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 79–104. [Google Scholar]
- Escobar-Avello, D.; Olmo-Cunillera, A.; Lozano-Castellón, J.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A. A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins. Molecules 2020, 25, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, D.H.; Saldanha, L.A.; Catharino, R.R.; Sawaya, A.C.; Cunha, I.B.; Carvalho, P.O.; Eberlin, M.N. Phenolic antioxidants identified by ESI-MS from Yerba maté (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules 2007, 12, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Schmetz, Q.; Teramura, H.; Morita, K.; Oshima, T.; Richel, A.; Ogino, C.; Kondo, A. Versatility of a Dilute Acid/Butanol Pretreatment Investigated on Various Lignocellulosic Biomasses to Produce Lignin, Monosaccharides and Cellulose in Distinct Phases. ACS Sustain. Chem. Eng. 2019, 7, 11069–11079. [Google Scholar] [CrossRef] [Green Version]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, Á.; Sánchez, Á.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind. Crops Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Rivas, S.; Vila, C.; Alonso, J.L.; Santos, V.; Parajó, J.C.; Leahy, J.J. Biorefinery processes for the valorization of Miscanthus polysaccharides: From constituent sugars to platform chemicals. Ind. Crops Prod. 2019, 134, 309–317. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine shoots as new source for the manufacture of prebiotic oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy; Walter de Gruyter: Berlin, Germany, 1991. [Google Scholar]
- Faix, O. Fourier Transform Infrared Spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 83–109. [Google Scholar]
- Abreu, H.d.S. Estimativa por Infravermelho da Concentração da Unidade Estrutural β-O-4 em Ligninas de Angiospermas Tropicais. Química Nova 1997, 20, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Wahyono, T.; Astuti, D.; Wiryawan, K.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in The Panicle of Sorghum Mutant Lines. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 546, p. 042045. [Google Scholar] [CrossRef]
- Pantoja-Castro, M.A.; González-Rodríguez, H. Study by infrared spectroscopy and thermogravimetric analysis of Tannins and Tannic acid. Rev. Latinoam. Química 2011, 39, 107–112. [Google Scholar]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K. Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym. Sci. 2015, 293, 2585–2592. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Mild Acetosolv Process To Fractionate Bamboo for the Biorefinery: Structural and Antioxidant Properties of the Dissolved Lignin. J. Agric. Food Chem. 2012, 60, 1703–1712. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, J.; Qin, L.; Ge, Y. Enhancing Antioxidant Performance of Lignin by Enzymatic Treatment with Laccase. ACS Sustain. Chem. Eng. 2018, 6, 2591–2595. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin. Ind. Crops Prod. 2014, 61, 479–491. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Yang, P.; Li, H.; Wang, H.; Han, F.; Jing, S.; Yuan, C.; Guo, A.; Zhang, Y.; Xu, Z. Dispersive Liquid-Liquid Microextraction Method for HPLC Determination of Phenolic Compounds in Wine. Food Anal. Methods 2017, 10, 2383–2397. [Google Scholar] [CrossRef]
- Podolec, P.; Szabó, A.H.; Blaško, J.; Kubinec, R.; Górová, R.; Višňovský, J.; Gnipová, A.; Horváth, A.; Bierhanzl, V.; Hložek, T.; et al. Direct silylation of Trypanosoma brucei metabolites in aqueous samples and their GC-MS/MS analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 967, 134–138. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Costa, C.E.; Rodrigues, A.E. Oxidation of Lignin from Eucalyptus globulus Pulping Liquors to Produce Syringaldehyde and Vanillin. Ind. Eng. Chem. Res. 2013, 52, 4421–4428. [Google Scholar] [CrossRef]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Borges da Silva, E.A.; Reis, R.L.; Rodrigues, A.E. Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Sameni, J.; Krigstin, S.; Sain, M. Characterization of Lignins Isolated from Industrial Residues and their Beneficial Uses. Bioresources 2016, 11, 8435–8456. [Google Scholar] [CrossRef] [Green Version]
- Araújo, P.; Costa, A.; Fernandes, I.; Mateus, N.; de Freitas, V.; Sarmento, B.; Oliveira, J. Stabilization of bluish pyranoanthocyanin pigments in aqueous systems using lignin nanoparticles. Dye. Pigment. 2019, 166, 367–374. [Google Scholar] [CrossRef]
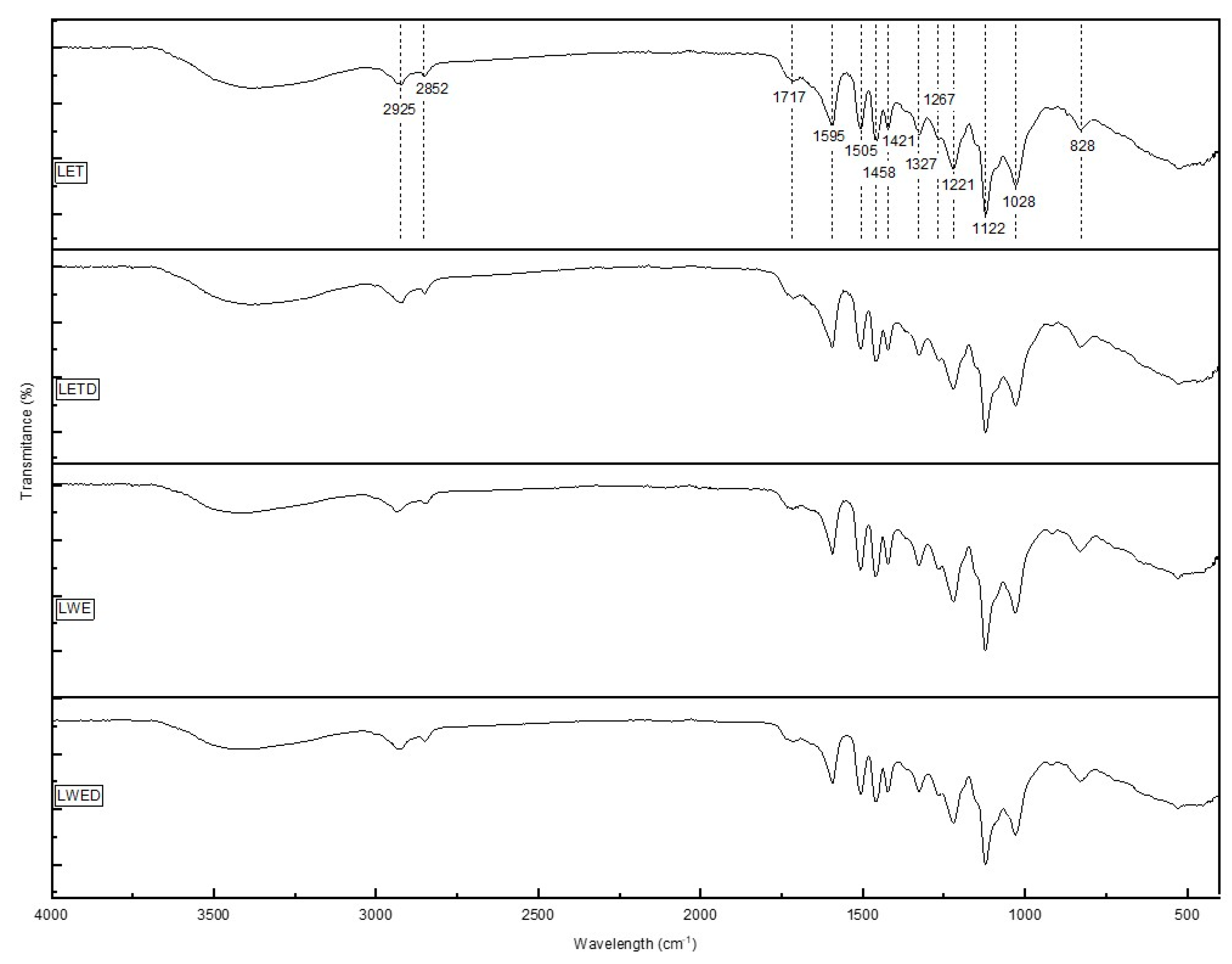

| Lignin | Ashes, %w/w lignin | Carbohydrates, %w/w lignin | Proanthocyanidins, % w/w lignin |
|---|---|---|---|
| LET | 0.12 | 5.4 | 11.3 ± 0.02 |
| LETD | 0.19 | 4.2 | 9.95 ± 0.03 |
| LWE | 0.30 | 6.9 | 4.65 ± 0.01 |
| LWED | 1.17 | 5.1 | 3.38 ± 0.01 |
| Assignments (Spectroscopic Range) | Amount (Number per Ar) | |||
|---|---|---|---|---|
| LET | LETD | LWE | LWED | |
| Cβ in β-5 and β-β structures (δ 51.0–53.8 ppm) | 0.11 | 0.12 | 0.14 | 0.12 |
| Aromatic OCH3 (δ 54.3–57.3 ppm) | 1.29 | 1.21 | 1.50 | 1.43 |
| Cγ in β-O-4 structures without Cα=O (δ 59.3–60.8 ppm) | 0.39 | 0.36 | 0.42 | 0.39 |
| Cγ in β-5, β-O-4 structures with Cα=O and β-1 (δ 62.5–63.8 ppm) | 0.12 | 0.11 | 0.13 | 0.12 |
| Cα in β-O-4 structures; Cγ in pinoresinol/syringaresinol and β-β structures (δ 70.0–76.0 ppm) | 0.82 | 0.77 | 0.79 | 0.76 |
| Cβ in β-O-4 structures; Cα in β-5 and β-β structures (δ 80.0–90.0 ppm) | 0.71 | 0.69 | 0.77 | 0.77 |
| Aromatic CAr-H (δ 103.0–123.0 ppm) | 2.28 | 2.25 | 2.16 | 2.16 |
| Aromatic CAr-C (δ 123.0–137.0 ppm) | 1.19 | 1.30 | 1.27 | 1.31 |
| Aromatic CAr-O (δ 137.0–156.0 ppm) | 2.42 | 2.37 | 1.47 | 2.44 |
| C4 in H units (δ 157.0–162.0 ppm) | 0.11 | 0.08 | 0.10 | 0.09 |
| CHO in benzaldehyde structures (δ 191.0–192.0 ppm) | 0.02 | 0.02 | 0.03 | 0.02 |
| CHO in cinnamaldehyde structures (δ 193.5–194.5 ppm) | 0.02 | 0.03 | 0.04 | 0.03 |
| CO in aldehydes and ketones (δ 195.0–210.0 ppm) | 0.39 | 0.65 | 0.50 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.R.; Costa, C.; Mateus, N.; de Freitas, V.; Rodrigues, A.; Oliveira, J. Exploring the Potential of Vine Shoots as a Source of Valuable Extracts and Stable Lignin Nanoparticles for Multiple Applications. Int. J. Mol. Sci. 2023, 24, 5165. https://doi.org/10.3390/ijms24065165
Pereira AR, Costa C, Mateus N, de Freitas V, Rodrigues A, Oliveira J. Exploring the Potential of Vine Shoots as a Source of Valuable Extracts and Stable Lignin Nanoparticles for Multiple Applications. International Journal of Molecular Sciences. 2023; 24(6):5165. https://doi.org/10.3390/ijms24065165
Chicago/Turabian StylePereira, Ana Rita, Carina Costa, Nuno Mateus, Victor de Freitas, Alírio Rodrigues, and Joana Oliveira. 2023. "Exploring the Potential of Vine Shoots as a Source of Valuable Extracts and Stable Lignin Nanoparticles for Multiple Applications" International Journal of Molecular Sciences 24, no. 6: 5165. https://doi.org/10.3390/ijms24065165






