C-Terminal Truncated HBx Facilitates Oncogenesis by Modulating Cell Cycle and Glucose Metabolism in FXR-Deficient Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
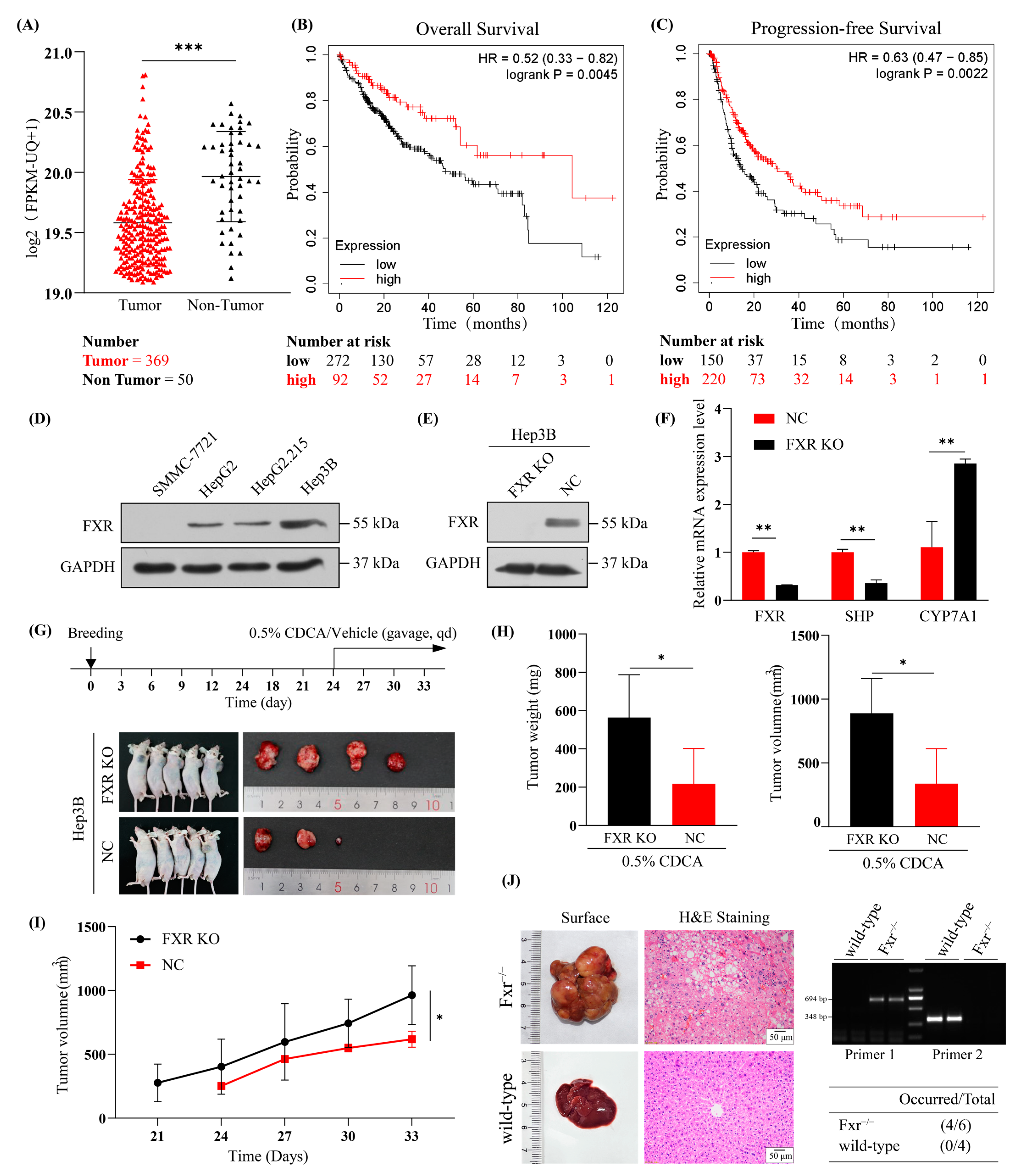
2.1. Underexpressed FXR in HBV-Related HCC
2.2. HBx C40 Promotes Cell Proliferation and Migration in FXR KO Hep3B Cells In Vitro
2.3. The Impact of HBx C40 on Cell Cycling and Apoptosis in FXR KO Hep3B Cells
2.4. The Biological Effect of HBx C40 in Transiently Overexpressed Mouse Models
2.5. HBx C40 Promotes Tumorigenesis in FXR KO Cells In Vivo
2.6. RNA Sequencing Identifies the Glucose Metabolism Signaling Pathway and Corresponding Crucial Genes in HBx C40-Expressing FXR KO Hep3B Cells
2.7. HBx/HBx C40-HSPB8-HK2-Glucose Metabolism Axis in FXR-Deficient HCC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids
4.3. Transient Transfection
4.4. Stable Transfection, Lentiviral Production, and Cell Transduction
4.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Cell Viability and Colony Formation Assay
4.7. Cell Migration Assay
4.8. Cell Cycle Analysis
4.9. Cell Apoptosis Analysis
4.10. Western Blotting
4.11. Subcutaneous Nude Murine Xenograft Models
4.12. Transgenic Fxr−/− Mice and Hydrodynamic Liver Transfection and Blood Glucose Assay
4.13. Gene Expression Profiling and Functional Analysis
4.14. Hematoxylin and Eosin (H&E) Staining
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chimed, T.; Sandagdorj, T.; Znaor, A.; Laversanne, M.; Tseveen, B.; Genden, P.; Bray, F. Cancer incidence and cancer control in Mongolia: Results from the National Cancer Registry 2008-12. Int. J. Cancer 2017, 140, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, W.; Wang, Y.; Qiao, L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014, 345, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Buendia, M.A.; Neuveut, C. Hepatocellular carcinoma. Cold Spring Harb. Perspect. Med. 2015, 5, a021444. [Google Scholar] [CrossRef]
- Song, X.; Tan, S.; Wu, Z.; Xu, L.; Wang, Z.; Xu, Y.; Wang, T.; Gao, C.; Gong, Y.; Liang, X.; et al. HBV suppresses ZHX2 expression to promote proliferation of HCC through miR-155 activation. Int. J. Cancer 2018, 143, 3120–3130. [Google Scholar] [CrossRef]
- Wang, Y.; Lau, S.H.; Sham, J.S.-T.; Wu, M.-C.; Wang, T.; Guan, X.-Y. Characterization of HBV integrants in 14 hepatocellular carcinomas: Association of truncated X gene and hepatocellular carcinogenesis. Oncogene 2004, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Amaddeo, G.; Cao, Q.; Ladeiro, Y.; Imbeaud, S.; Nault, J.C.; Jaoui, D.; Gaston Mathe, Y.; Laurent, C.; Laurent, A.; Bioulac-Sage, P.; et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut 2015, 64, 820–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Q.; Gong, L.; Xu, H.; Liu, B.; Fang, X.; Yu, D.; Li, L.; Wei, T.; Wang, Y.; et al. C-terminal truncated HBx initiates hepatocarcinogenesis by downregulating TXNIP and reprogramming glucose metabolism. Oncogene 2021, 40, 1147–1161. [Google Scholar] [CrossRef]
- Sze, K.M.; Chu, G.K.; Lee, J.M.; Ng, I.O. C-terminal truncated hepatitis B virus x protein is associated with metastasis and enhances invasiveness by c-jun/matrix metalloproteinase protein 10 activation in hepatocellular carcinoma. Hepatology 2013, 57, 131–139. [Google Scholar] [CrossRef]
- Ma, N.-F.; Lau, S.H.; Hu, L.; Xie, D.; Wu, J.; Yang, J.; Wang, Y.; Wu, M.-C.; Fung, J.; Bai, X.; et al. COOH-Terminal Truncated HBV X Protein Plays Key Role in Hepatocarcinogenesis. Clin. Cancer Res. 2008, 14, 5061–5068. [Google Scholar] [CrossRef]
- Tu, H.; Bonura, C.; Giannini, C.; Mouly, H.; Soussan, P.; Kew, M.; Paterlini-Brechot, P.; Brechot, C.; Kremsdorf, D. Biological impact of natural COOH-terminal deletions of hepatitis B virus X protein in hepatocellular carcinoma tissues. Cancer Res. 2001, 61, 7803–7810. [Google Scholar] [PubMed]
- Iavarone, M.; Trabut, J.-B.; Delpuech, O.; Carnot, F.; Colombo, M.; Kremsdorf, D.; Bréchot, C.; Thiers, V. Characterisation of hepatitis B virus X protein mutants in tumour and non-tumour liver cells using laser capture microdissection. J. Hepatol. 2003, 39, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Rashid, A.; Wang, Y.; Jain, A.; Li, D.; Behari, A.; Kapoor, V.K.; Koay, E.J.; Chang, P.; Vauthey, J.N.; et al. RNA sequencing-based analysis of gallbladder cancer reveals the importance of the liver X receptor and lipid metabolism in gallbladder cancer. Oncotarget 2016, 7, 35302–35312. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, W.D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef]
- Su, H.; Ma, C.; Liu, J.; Li, N.; Gao, M.; Huang, A.; Wang, X.; Huang, W.; Huang, X. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1245–G1253. [Google Scholar] [CrossRef]
- Yang, F.; Huang, X.; Yi, T.; Yen, Y.; Moore, D.D.; Huang, W. Spontaneous Development of Liver Tumors in the Absence of the Bile Acid Receptor Farnesoid X Receptor. Cancer Res. 2007, 67, 863–867. [Google Scholar] [CrossRef]
- Kim, I.; Morimura, K.; Shah, Y.; Yang, Q.; Ward, J.M.; Gonzalez, F.J. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 2007, 28, 940–946. [Google Scholar] [CrossRef]
- Degirolamo, C.; Modica, S.; Vacca, M.; Di Tullio, G.; Morgano, A.; D’Orazio, A.; Kannisto, K.; Parini, P.; Moschetta, A. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor–null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 2015, 61, 161–170. [Google Scholar] [CrossRef]
- Wolfe, A.; Thomas, A.; Edwards, G.; Jaseja, R.; Guo, G.L.; Apte, U. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J. Pharmacol. Exp. Ther. 2011, 338, 12–21. [Google Scholar] [CrossRef]
- Niu, Y.; Xu, M.; Slagle, B.L.; Huang, H.; Li, S.; Guo, G.L.; Shi, G.; Qin, W.; Xie, W. Farnesoid X receptor ablation sensitizes mice to hepatitis b virus X protein–induced hepatocarcinogenesis. Hepatology 2017, 65, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiao, H.; Hu, Z.; Zhou, F.; Yang, B. Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur. J. Cell Biol. 2018, 97, 216–229. [Google Scholar] [CrossRef]
- Yu, L.L.; Wang, Y.; Xiao, Z.K.; Chen, S.S. Heat shock protein B8 promotes proliferation and migration in lung adeno-carcinoma A549 cells by maintaining mitochondrial function. Mol. Cell. Biochem. 2021, 476, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Matsushima-Nishiwaki, R.; Kuroyanagi, G.; Suzuki, N.; Takamatsu, R.; Furui, T.; Yoshimi, N.; Kozawa, O.; Morishige, K.-I. Regulation by heat shock protein 22 (HSPB8) of transforming growth factor-α-induced ovary cancer cell migration. Arch. Biochem. Biophys. 2015, 571, 40–49. [Google Scholar] [CrossRef]
- Modem, S.; Chinnakannu, K.; Bai, U.; Reddy, G.P.; Reddy, T.R. Hsp22 (HspB8/H11) knockdown induces Sam68 expression and stimulates proliferation of glioblastoma cells. J. Cell. Physiol. 2011, 226, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- Cristofani, R.; Piccolella, M.; Crippa, V.; Tedesco, B.; Marelli, M.M.; Poletti, A.; Moretti, R.M. The Role of HSPB8, a Component of the Chaperone-Assisted Selective Autophagy Machinery, in Cancer. Cells 2021, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, L.; Wu, M.; Huang, W.; Wu, X.; Huang, D.; Xie, Y.; Shi, G. Partial abrogation of FXR-KNG1 signaling by carboxyl-terminal truncated HBx-C30 in hepatitis B virus-associated hepatocellular carcinoma. Virus Res. 2021, 293, 198264. [Google Scholar] [CrossRef]
- Shamay, M.; Agami, R.; Shaul, Y. HBV integrants of hepatocellular carcinoma cell lines contain an active enhancer. Oncogene 2001, 20, 6811–6819. [Google Scholar] [CrossRef]
- Kennedy, L.; Francis, H. Defining the Relationship between Farsenoid X Receptor, Hepatitis B Virus X Protein and Hepatocellular Carcinoma: It’s Complicated. Hepatology 2017, 65, 774–776. [Google Scholar] [CrossRef]
- Muroyama, R.; Nakagawa, R.; Matsubara, Y.; Hirata, Y.; Omata, M.; Shirasawa, H.; Kato, N. Fusion HBx from HBV integrant affects hepatocarcinogenesis through deregulation of ER stress response. Virus Res. 2022, 315, 198787. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, C.-L.; Li, N.; Zhang, X.; Zhang, X.-Z.; Xu, F.-Q.; Zhang, S.; Qiu, L.-Y.; Ye, L.-H.; Zhang, X.-D. Identification of a natural mutant of HBV X protein truncated 27 amino acids at the COOH terminal and its effect on liver cell proliferation. Acta Pharmacol. Sin. 2008, 29, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.-Y.; Zang, S.-F.; Wang, L.; Luo, Y.; Shi, J.-P.; Lou, G.-Q. Isocitrate Dehydrogenase 2 Suppresses the Invasion of Hepatocellular Carcinoma Cells via Matrix Metalloproteinase 9. Cell. Physiol. Biochem. 2015, 37, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Kon, N.; Zhao, W.; Jiang, L.; Knight, C.M.; Welch, C.; Pajvani, U.; Gu, W.; Accili, D. Hepatic SirT1-Dependent Gain of Function of Stearoyl-CoA Desaturase-1 Conveys Dysmetabolic and Tumor Progression Functions. Cell Rep. 2015, 11, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipo-protein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef]
- Huang, G.-M.; Jiang, Q.-H.; Cai, C.; Qu, M.; Shen, W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015, 358, 180–190. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Soto, J.; Park, K.; Viswanath, G.; Kuwada, S.; Abel, E.D.; Wang, L. Nuclear Receptor SHP, a Death Receptor That Targets Mitochondria, Induces Apoptosis and Inhibits Tumor Growth. Mol. Cell. Biol. 2010, 30, 1341–1356. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A Regulatory Cascade of the Nuclear Receptors FXR, SHP-1, and LRH-1 Represses Bile Acid Biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Nesbit, C.E.; Tersak, J.M.; Prochownik, E.V. MYC oncogenes and human neoplastic disease. Oncogene 1999, 18, 3004–3016. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Guo, J.; Zheng, J.; Sun, X.; Yu, J. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J. Cell. Mol. Med. 2020, 24, 14549–14560. [Google Scholar] [CrossRef]
- Lis, P.; Dyląg, M.; Niedźwiecka, K.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. The HK2 Dependent “Warburg Effect” and Mitochondrial Oxidative Phosphorylation in Cancer: Targets for Effective Therapy with 3-Bromopyruvate. Molecules 2016, 21, 1730. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 446. [Google Scholar] [CrossRef]
- Xu, S.; Herschman, H.R. A Tumor Agnostic Therapeutic Strategy for Hexokinase 1–Null/Hexokinase 2–Positive Cancers. Cancer Res. 2019, 79, 5907–5914. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, Z.; Shen, Q.; Song, J.; Luo, Q.; You, H.; Shi, G.; Qin, W. Hepatitis B virus X protein co-activates pregnane X receptor to induce the cytochrome P450 3A4 enzyme, a potential implication in hepatocarcinogenesis. Dig. Liver Dis. 2013, 45, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ni, Z.; Feng, H.; Xing, Y.; Wu, X.; Huang, D.; Chen, L.; Niu, Y.; Shi, G. Combination of curcumin with N-n-butyl haloperidol iodide inhibits hepatocellular carcinoma malignant proliferation by downregulating enhancer of zeste homolog 2 (EZH2)-lncRNA H19 to silence Wnt/β-catenin signaling. Phytomedicine 2021, 91, 153706. [Google Scholar] [CrossRef] [PubMed]






| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| hFXR | GCATTACCAAAAACGCTGTG | CAGCCAACATTCCCATCTCT |
| hSHP | GAATATGCCTGCCTGAAAGG | TCCAGGACTTCACACAGCAC |
| hCYP7A1 | GAGAAGGCAAACGGGTGAAC | GCACAACACCTTATGGTATGACA |
| hCyclophilin | TGGTGTTTGGCAAAGTGAAA | TCGAGTTGTCCACAGTCAGC |
| hTGF-β1 | CTAATGGTGGAAACCCACAAGG | TATCGCCAGGAATTGTTGCT |
| hc-Myc | CACTAACATCCCACGCTCTGA | AAACCGCATCCTTGTCCTGT |
| hCyclin D1 | GATCAAGTGTGACCCGGACTG | CCTTGGGGTCCATGTTCTGC |
| hP21 | AGTCAGTTCCTTGTGGAGCC | CATTAGCGCATCACAGTCGC |
| hHSPB8 | AAGCCAGAGGAGTTGATGGTG | CTCTGCAGGAAGCTGGATTTT |
| hHK2 | TCGCCGGTAGCCTTCTTTGT | AGAGATACTGGTCAACCTTCTGC |
| hPFKL | CGGTGGACCTGGAGAAGCTG | TCCAGGCGGAGTCAATGTG |
| hPKM2 | ATGCAGCACCTGATAGCTCG | GTGGAGTGACTTGAGGCTCG |
| hLDHA | TTGTCTCTGGCAAAGTGGATATCTT | CCACTCCATACAGGCACACT |
| mCyclophilin | GGCTGAGAACGGGAAGCTTGTCAT | CAGCCTTCTCCATGGTGGTGAAGA |
| mFxr | TCGTTCGGCGGAGATTTTCA | TGTAGCACATCAAGCAGGGG |
| mShp | TCCTCTTCAACCCAGATGTGC | TCTCCCATGATAGGGCGGAA |
| mc-Myc | GTTGGAAACCCCGCAGACAG | ATAGGGCTGTACGGAGTCGT |
| mcyclin D1 | AGAACAAGCAGACCATCCGC | GTCCTTGTTTAGCCAGAGGC |
| mp21 | GCAGAATAAAAGGTGCCACAGG | AAAGTTCCACCGTTCTCGGG |
| mMad2l2 | TCCACTGCGTCAAACCTCTC | TAAATGTGCAGCCTGGAGGG |
| mHk2 | AAGCTTCTTTGTGTGGCTCCT | AGAGATACTGGTCAACCTTCTGC |
| mPfkl | GGCTCTCGGCTGAACATCAT | TGTGGTTCTGGAGGCATCCTT |
| mPgk1 | CCTCTCCTTCCTTTTAGACGCC | AGTCTTCCTCCAGTTCGCTCA |
| mPkm | GCAGCGACTCGTCTTCACT | GCATGGTTCCTGAAGTCCTCG |
| mLdha | AACTTGGCGCTCTACTTGCT | GGACTTTGAATCTTTTGAGACCTTG |
| mHspb8 | CAATTGCCTTTCCCGTGCTC | TCTGGAAAAGGGTCCATGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Ni, Z.; Song, T.; Lv, W.; Chen, Y.; Huang, D.; Xie, Y.; Huang, W.; Niu, Y. C-Terminal Truncated HBx Facilitates Oncogenesis by Modulating Cell Cycle and Glucose Metabolism in FXR-Deficient Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 5174. https://doi.org/10.3390/ijms24065174
Wu X, Ni Z, Song T, Lv W, Chen Y, Huang D, Xie Y, Huang W, Niu Y. C-Terminal Truncated HBx Facilitates Oncogenesis by Modulating Cell Cycle and Glucose Metabolism in FXR-Deficient Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(6):5174. https://doi.org/10.3390/ijms24065174
Chicago/Turabian StyleWu, Xuejun, Zhengzhong Ni, Tiantian Song, Wenya Lv, Yan Chen, Danmei Huang, Yangmin Xie, Weiyi Huang, and Yongdong Niu. 2023. "C-Terminal Truncated HBx Facilitates Oncogenesis by Modulating Cell Cycle and Glucose Metabolism in FXR-Deficient Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 6: 5174. https://doi.org/10.3390/ijms24065174
APA StyleWu, X., Ni, Z., Song, T., Lv, W., Chen, Y., Huang, D., Xie, Y., Huang, W., & Niu, Y. (2023). C-Terminal Truncated HBx Facilitates Oncogenesis by Modulating Cell Cycle and Glucose Metabolism in FXR-Deficient Hepatocellular Carcinoma. International Journal of Molecular Sciences, 24(6), 5174. https://doi.org/10.3390/ijms24065174





