Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese
Abstract
:1. Introduction
2. Results
2.1. Anthropometric and Blood Profiles
2.2. Liver, Kidney, and Inflammatory Parameters
2.3. Lipid and Lipoprotein Profiles
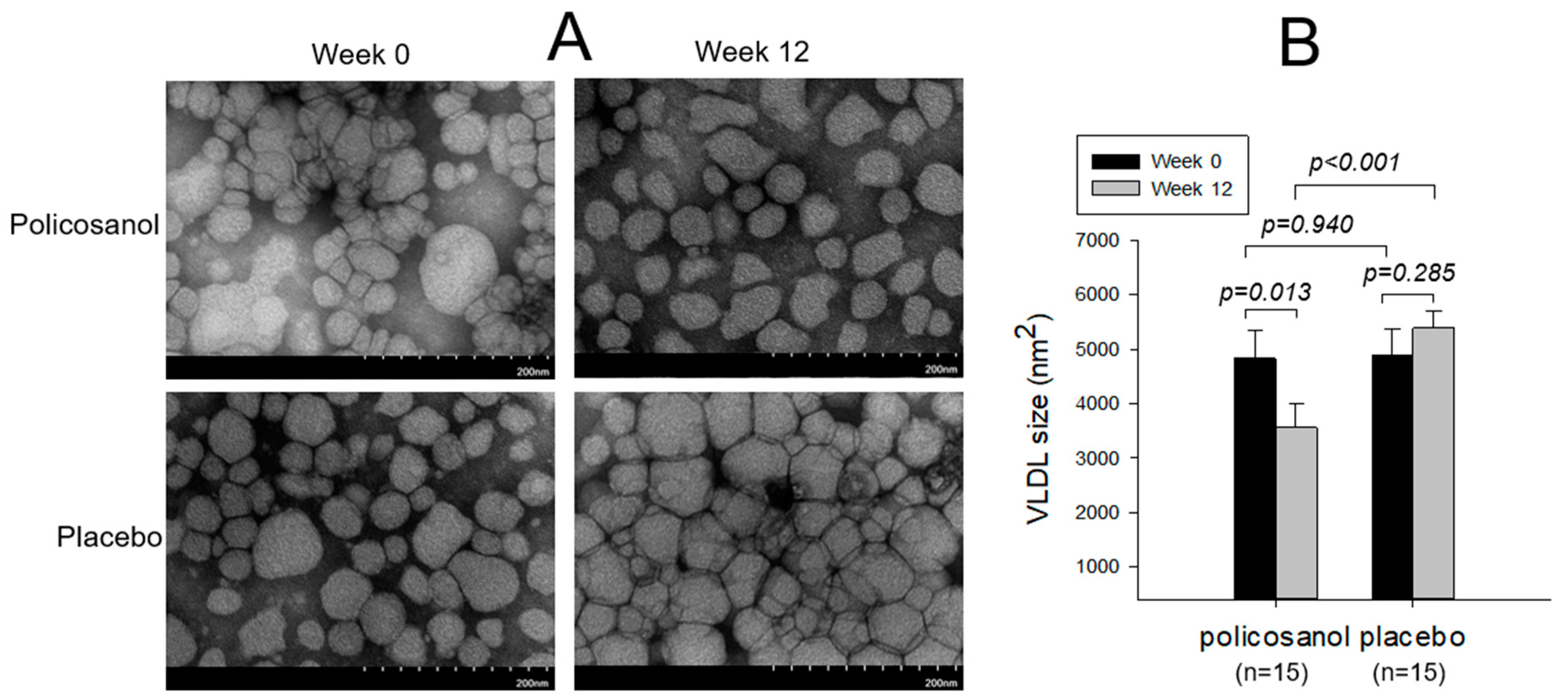
2.4. VLDL Particle Observation and Composition Analysis
2.5. LDL Particle Observation and Composition Analysis
2.6. Electromobility of LDL and Oxidation Extent
2.7. Change in apoA-I Contents in HDL2 and HDL3
2.8. Paraoxonase Activities in HDL2 and HDL3
2.9. Ferric Ion Reduction Ability of HDL2 and HDL3
2.10. Embryo Survivability
3. Discussion
4. Materials and Methods
4.1. Policosanol
4.2. Participants
4.3. Study Design
4.4. Anthropometric Analysis
4.5. Blood Analysis
4.6. Isolation of Lipoproteins and Quantification
4.7. Quantification of Oxidation Extent in VLDL and LDL
4.8. Oxidation of VLDL and LDL
4.9. Agarose Electrophoresis
4.10. Electron Microscopy
4.11. Paraoxonase Assay
4.12. Ferric Ion Reduction Ability Assay
4.13. Electrophoretic Patterns of HDL
4.14. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arruzazabala, M.d.L.; Carbajal, D.; Mas, R.; Garcia, M.; Fraga, V. Effects of policosanol on platelet aggregation in rats. Thromb. Res. 1993, 69, 321–327. [Google Scholar] [CrossRef]
- Batista, J.; Stüsser, R.; Saez, F.; Perez, B. Effect of policosanol on hyperlipidemia and coronary heart disease in middle-aged patients. A 14-month pilot study. Int. J. Clin. Pharmacol. Ther. 1996, 34, 134–137. [Google Scholar] [PubMed]
- Valdes, S.; Arruzazabala, M.; Fernandez, L.; Más, R.; Carbajal, D.; Aleman, C.; Molina, V. Effect of policosanol on platelet aggregation in healthy volunteers. Int. J. Clin. Pharmacol. Res. 1996, 16, 67–72. [Google Scholar] [PubMed]
- Lee, H.-G.; Woo, S.-Y.; Ahn, H.-J.; Yang, J.-Y.; Lee, M.-J.; Kim, H.-Y.; Song, S.-Y.; Lee, J.-H.; Seo, W.-D. comparative analysis of policosanols related to growth times from the seedlings of various Korean oat (Avena sativa L.) cultivars and screening for adenosine 5′-monophosphate-activated protein kinase (AMPK) activation. Plants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Muthusamy, M.; Kim, J.H.; Kim, S.H.; Kim, J.Y.; Heo, J.W.; Lee, H.; Lee, K.-S.; Seo, W.D.; Park, S.; Kim, J.A. Changes in beneficial C-glycosylflavones and policosanol content in wheat and barley sprouts subjected to differential LED light conditions. Plants 2020, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, X.; Ma, C.; He, Z.; Zhang, X.; Wang, C.; Zhao, M.; Gan, J.; Feng, Y. Improving effect of the policosanol from Ericerus pela wax on learning and memory impairment caused by scopolamine in mice. Foods 2022, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, C.; Sun, L.; He, Z.; Feng, Y.; Li, X.; Gan, J.; Chen, X. Effect of policosanol from insect wax on amyloid β-peptide-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. BMC Complement. Med. Ther. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, A.; Brighenti, V.; Mascolo, D.; Pellati, F. A new strategy based on microwave-assisted technology for the extraction and purification of beeswax policosanols for pharmaceutical purposes and beyond. J. Pharm. Biomed. Anal. 2019, 172, 200–205. [Google Scholar] [CrossRef]
- Wong, W.-T.; Ismail, M.; Tohit, E.R.M.; Abdullah, R.; Zhang, Y.-D. Attenuation of thrombosis by crude rice (Oryza sativa) bran policosanol extract: Ex vivo platelet aggregation and serum levels of arachidonic acid metabolites. Evid.-Based Complement. Altern. Med. 2016, 2016, 7343942. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ding, Y.; Si, Q.; Li, K.; Xu, K. Multiple functions of policosanol in elderly patients with dyslipidemia. J. Int. Med. Res. 2020, 48, 0300060520936082. [Google Scholar] [CrossRef]
- Kaup, R.M.; Khayyal, M.T.; Verspohl, E.J. Antidiabetic effects of a standardized Egyptian rice bran extract. Phytother. Res. 2013, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef] [Green Version]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; Symonds, M.E.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lim, D.-K.; Suh, Y.-H.; Chang, K.-A. long-term treatment of Cuban policosanol attenuates abnormal oxidative stress and inflammatory response via amyloid plaques reduction in 5xFAD Mice. Antioxidants 2021, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Mirazi, N.; Ahmadi, N.; Asadbegi, M.; Nourian, A.; Ghaderi, S.; Rashno, M.; Komaki, A. The protective effects of policosanol on learning and memory impairments in a male rat model of Alzheimer’s Disease. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Yoo, J.-A.; Lee, E.-Y.; Cho, K.-H. Enhancement of high-density lipoprotein cholesterol functions by encapsulation of policosanol exerts anti-senescence and tissue regeneration effects via improvement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of Cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy Korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of Cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef] [Green Version]
- Hui, N.; Barter, P.J.; Ong, K.-L.; Rye, K.-A. Altered HDL metabolism in metabolic disorders: Insights into the therapeutic potential of HDL. Clin. Sci. 2019, 133, 2221–2235. [Google Scholar] [CrossRef]
- Davidson, M.H. Update on CETP inhibition. J. Clin. Lipidol. 2010, 4, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R. Blood pressure lowering effect of Cuban policosanol is accompanied by improvement of hepatic inflammation, lipoprotein profile, and HDL quality in spontaneously hypertensive rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, S. Unique features of high-density lipoproteins in the Japanese: In population and in genetic factors. Nutrients 2015, 7, 2359–2381. [Google Scholar] [CrossRef] [Green Version]
- Carroll, M.D.; Lacher, D.A.; Sorlie, P.D.; Cleeman, J.I.; Gordon, D.J.; Wolz, M.; Grundy, S.M.; Johnson, C.L. Trends in serum lipids and lipoproteins of adults, 1960–2002. J. Am. Med. Assoc. 2005, 294, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, H.; Yamamoto, A.; Matsuzawa, Y.; Saito, Y.; Yamada, N.; Oikawa, S.; Mabuchi, H.; Teramoto, T.; Sasaki, J.; Nakaya, N. Serum lipid survey and its recent trend in the general Japanese population in 2000. J. Atheroscler. Thromb. 2005, 12, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-J.; Park, S.-H.; Park, H.-Y. Increased prevalence of low high-density lipoprotein cholesterol (HDL-C) levels in Korean adults: Analysis of the three Korean national health and nutrition examination surveys (KNHANES 1998–2005). Osong Public Health Res. Perspect. 2011, 2, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Han, J.H.; Park, H.S. Prevalence of low HDL-cholesterol levels and associated factors among Koreans. Circ. J. 2006, 70, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, Y.; Zheng, L.; Wang, P.; Wu, Y.; Gong, Z. The neurotoxicity of Nε-(carboxymethyl) lysine in food processing by a study based on animal and organotypic cell culture. Ecotoxicol. Environ. Saf. 2020, 190, 110077. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoa, B.; Bowman, T.; Zon, L.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish. Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Trede, N.S.; Zapata, A.; Zon, L.I. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K. Policosanol: Clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am. Heart J. 2002, 143, 356–365. [Google Scholar] [CrossRef]
- Berthold, H.K.; Unverdorben, S.; Degenhardt, R.; Bulitta, M.; Gouni-Berthold, I. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: A randomized controlled trial. J. Am. Med. Assoc. 2006, 295, 2262–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illnait, J.; Castaño, G.; Alvarez, E.; Fernández, L.; Mas, R.; Mendoza, S.; Gamez, R. Effects of policosanol (10 mg/d) versus aspirin (100 mg/d) in patients with intermittent claudication: A 10-week, randomized, comparative study. Angiology 2008, 59, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Castaño, G.; Fernández, L.; Mas, R.; Illnait, J.; Mesa, M.; Fernandez, J. Comparison of the effects of policosanol and atorvastatin on lipid profile and platelet aggregation in patients with dyslipidaemia and type 2 diabetes mellitus. Clin. Drug Investig. 2003, 23, 639–650. [Google Scholar] [CrossRef]
- Guo, Y.L.; Xu, R.X.; Zhu, C.G.; Wu, N.Q.; Cui, Z.P.; Li, J.J. Policosanol attenuates statin-induced increases in serum proprotein convertase subtilisin/kexin type 9 when combined with atorvastatin. Evid. Based Complement. Altern. Med. 2014, 2014, 926087. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Liu, X.; Li, Y.; Wang, Y.; Zang, H.; Guo, L.; Wang, Y.; Zhao, W.; Wang, X.; Han, Y. Safety and efficacy of policosanol in patients with high on-treatment platelet reactivity after drug-eluting stent implantation: Two-year follow-up results. Cardiovasc. Ther. 2016, 34, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Osadnik, T.; Goławski, M.; Lewandowski, P.; Morze, J.; Osadnik, K.; Pawlas, N.; Lejawa, M.; Jakubiak, G.K.; Mazur, A.; Schwingschackl, L. A network meta-analysis on the comparative effect of nutraceuticals on lipid profile in adults. Pharmacol. Res. 2022, 183, 106402. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Gill, V.; Singal, P. Role of advanced glycation end products in hypertension and atherosclerosis: Therapeutic implications. Cell Biochem. Biophys. 2007, 49, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Bae, M.; Kim, J.-R. Cuban sugar cane wax acid and policosanol showed similar atheroprotective effects with inhibition of LDL oxidation and cholesteryl ester transfer via enhancement of high-density lipoproteins functionality. Cardiovasc. Ther. 2019, 2019, 8496409. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1700280. [Google Scholar] [CrossRef] [PubMed]
- Gaens, K.H.; Niessen, P.M.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Driessen, A.; Wolfs, M.G.; Hofker, M.H.; Bloemen, J.G.; Dejong, C.H. Endogenous formation of Nε-(carboxymethyl) lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J. Hepatol. 2012, 56, 647–655. [Google Scholar] [CrossRef]
- Yagmur, E.; Tacke, F.; Weiss, C.; Lahme, B.; Manns, M.P.; Kiefer, P.; Trautwein, C.; Gressner, A.M. Elevation of Nε-(carboxymethyl) lysine-modified advanced glycation end products in chronic liver disease is an indicator of liver cirrhosis. Clin. Biochem. 2006, 39, 39–45. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Na, H.-J. Anti-Inflammatory Activity of CIGB-258 against Acute Toxicity of Carboxymethyllysine in Paralyzed Zebrafish via Enhancement of High-Density Lipoproteins Stability and Functionality. Int. J. Mol. Sci. 2022, 23, 10130. [Google Scholar] [CrossRef]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef]
- Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Químicas 2007, 38, 207–213. [Google Scholar]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Zee, S.; Park, M.-H. Enhancement of high-density lipoprotein (HDL) quantity and quality by regular and habitual exercise in middle-aged women with improvements in lipid and apolipoprotein profiles: Larger particle size and higher antioxidant ability of HDL. Int. J. Mol. Sci. 2023, 24, 1151. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Blatter Garin, M.-C.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]










| Policosanol 20 mg (n = 30) | Week 0 vs. 12 (p †) | Placebo (n = 35) | Week 0 vs. 12 p † | p ‡ | ||
|---|---|---|---|---|---|---|
| Age (year) (min., max.) | Week 0 | 51.2 ± 1.7 | 1.000 | 50.9 ± 1.7 | 1.000 | 0.915 |
| Week 12 | 51.2 ± 1.7 (38, 62) | 50.9 ± 1.7 (40, 63) | ||||
| SBP (mmHg) | Week 0 | 114.0 ± 0.8 | <0.001 | 115.7 ± 2.4 | 0.118 | 0.026 |
| Week 12 | 106.1 ± 2.6 | 112.7 ± 2.2 | ||||
| DBP (mmHg) | Week 0 | 70.6 ± 1.8 | 0.034 | 70.8 ± 1.8 | 0.461 | 0.195 |
| Week 12 | 67.8 ± 2.12 | 70.0 ± 1.6 | ||||
| Heart pulse rate (BPM) | Week 0 | 72.4 ± 1.8 | 0.528 | 70.2 ± 1.8 | 0.938 | 0.487 |
| Week 12 | 73.5 ± 2.5 | 70.1 ± 1.9 | ||||
| Height (cm) | Week 0 | 163.7 ± 1.6 | 1.000 | 164.6 ± 1.5 | 1.000 | 0.093 |
| Week 12 | 163.7 ± 1.6 | 164.6 ± 1.5 | ||||
| Body weight (kg) | Week 0 | 58.6 ± 1.7 | 0.531 | 60.7 ± 1.5 | 0.006 | 0.242 |
| Week 12 | 58.5 ± 1.7 | 60.2 ± 1.4 | ||||
| BMI (kg/m2) | Week 0 | 21.8 ± 0.4 | 0.459 | 22.4 ± 0.4 | 0.006 | 0.307 |
| Week 12 | 21.8 ± 0.4 | 22.2 ± 0.4 | ||||
| Total protein (g/dL) | Week 0 | 7.1 ± 0.1 | 0.874 | 7.0 ± 0.1 | 0.159 | 0.386 |
| Week 12 | 7.1 ± 0.1 | 7.1 ± 0.1 | ||||
| Albumin (g/dL) | Week 0 | 4.4 ± 0.1 | 0.500 | 4.3 ± 0.0 | 0.456 | 0.448 |
| Week 12 | 4.4 ± 0.1 | 4.4 ± 0.0 | ||||
| A/G (ratio) | Week 0 | 1.64 ± 0.03 | 0.243 | 1.66 ± 0.04 | 0.239 | 0.911 |
| Week 12 | 1.61 ± 0.03 | 1.63 ± 0.03 | ||||
| AST (IU/L) | Week 0 | 20.8 ± 1.4 | 0.022 | 20.8 ± 0.9 | 0.422 | 0.017 |
| Week 12 | 19.0 ± 0.8 | 21.3 ± 1.0 | ||||
| ALT (IU/L) | Week 0 | 21.5 ± 2.8 | 0.013 | 21.5 ± 2.4 | 0.971 | 0.032 |
| Week 12 | 17.9 ± 1.5 | 21.4 ± 2.5 | ||||
| γ-GTP (IU/L) | Week 0 | 30.0 ± 4.4 | 0.016 | 27.9 ± 3.4 | 0.775 | 0.016 |
| Week 12 | 25.4 ± 3.0 | 28.3 ± 3.6 | ||||
| Creatinine (mg/dL) | Week 0 | 0.73 ± 0.02 | 0.883 | 0.75 ± 0.03 | 0.608 | 0.675 |
| Week 12 | 0.73 ± 0.03 | 0.74 ± 0.03 | ||||
| Glucose (mg/dL) | Week 0 | 90.0 ± 1.7 | 0.652 | 91.2 ± 1.4 | 0.890 | 0.968 |
| Week 12 | 90.5 ± 1.5 | 91.3 ± 1.4 | ||||
| Uric acid (mg/dL) | Week 0 | 5.1 ± 0.2 | 0.891 | 5.2 ± 0.2 | 0.887 | 0.979 |
| Week 12 | 5.1 ± 0.2 | 5.2 ± 0.2 | ||||
| BUN (mg/dL) | Week 0 Week 12 | 13.4 ± 0.4 | 0.052 | 13.5 ± 0.5 | <0.001 | 0.001 |
| 12.6 ± 0.6 | 14.6 ± 0.5 | |||||
| LDH (IU/L) | Week 0 | 158.3 ± 4.6 | 0.827 | 165.5 ± 4.2 | 0.874 | 0.663 |
| Week 12 | 157.9 ± 4.2 | 165.2 ± 4.1 | ||||
| Total bilirubin (mg/dL) | Week 0 Week 12 | 0.82 ± 0.05 0.86 ± 0.05 | 0.294 | 0.76 ± 0.04 0.85 ± 0.05 | 0.030 | 0.419 |
| HbA1c (%) | Week 0 | 5.5 ± 0.0 | 0.009 | 5.5 ± 0.1 | 0.212 | 0.024 |
| Week 12 | 5.3 ± 0.1 | 5.4 ± 0.1 | ||||
| hsCRP (mg/dL) | Week 0 | 0.05 ± 0.02 | 0.422 | 0.11 ± 0.06 | 0.278 | 0.540 |
| Week 12 | 0.03 ± 0.78 | 0.05 ± 0.01 | ||||
| apoA-I (mg/dL) (n = 15) | Week 0 | 165.5 ± 2.3 | 0.045 | 164.6 ± 5.5 | 0.347 | 0.028 |
| Week 12 | 182.8 ± 8.1 | 160.5 ± 2.8 | ||||
| apo-B (mg/dL) (n = 15) | Week 0 | 97.9 ± 2.7 | 0.465 | 101.3 ± 1.8 | 0.333 | 0.182 |
| Week 12 | 100.1 ± 5.5 | 98.2 ± 1.5 | ||||
| Groups | Week 0 | Week 4 | Week 8 | Week 12 | Sources | F | p ‡ | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |||||
| TC (mg/dL) | placebo (n = 17) | 222.1 ± 5.0 | 222.5 ± 4.9 | 219.4 ± 5.2 | 214.8 ± 3.9 | Time × group | 1.407 | 0.246 |
| PCO 20 mg (n = 15) | 217.9 ± 3.8 | 218.5 ± 4.5 | 212.7 ± 4.1 | 218.5 ± 3.7 | ||||
| p † | 0.521 | 0.859 | 0.456 | 0.280 | ||||
| TG (mg/dL) | placebo (n = 17) | 74.6 ± 6.5 | 84.6 ± 8.9 | 86.4 ± 10.3 | 100.2 ± 16.6 | Time × group | 1.586 | 0.215 |
| PCO 20 mg (n = 15) | 97.7 ± 12.3 | 94.1 ± 8.1 | 125.9 ± 21.0 | 101.7 ± 14.9 | ||||
| p † | 0.095 | 0.952 | 0.236 | 0.063 | ||||
| HDL-C (mg/dL) | placebo (n = 17) | 66.1 ± 2.4 | 65.2 ± 3.3 | 63.8 ± 3.2 | 61.8 ± 2.4 | Time × group | 10.583 | <0.001 |
| PCO 20 mg (n = 15) | 63.7 ± 2.9 | 66.1 ± 3.1 | 63.9 ± 2.9 | 67.7 ± 3.6 | ||||
| p † | 0.532 | 0.158 | 0.329 | <0.001 | ||||
| LDL-C (mg/dL) | placebo (n = 17) | 141.0 ± 3.2 | 140.3 ± 3.2 | 138.4 ± 3.0 | 133.0 ± 3.1 | Time × group | 1.944 | 0.128 |
| PCO 20 mg (n = 15) | 134.5 ± 3.5 | 133.5 ± 3.5 | 123.7 ± 3.7 | 130.4 ± 3.6 | ||||
| p † | 0.186 | 0.404 | 0.013 | 0.956 | ||||
| TG /HDL-C (ratio) | placebo (n = 17) | 1.16 ± 0.11 | 1.42 ± 0.21 | 1.46 ± 0.2 | 1.73 ± 0.31 | Time × group | 2.571 | 0.074 |
| PCO 20 mg (n = 15) | 1.66 ± 0.27 | 1.5 ± 0.16 | 2.07 ± 0.36 | 1.68 ± 0.31 | ||||
| p † | 0.099 | 0.524 | 0.355 | 0.018 | ||||
| LDL-C /HDL-C (ratio) | placebo (n = 17) | 2.17 ± 0.08 | 2.23 ± 0.11 | 2.23 ± 0.09 | 2.2 ± 0.09 | Time × group | 2.176 | 0.096 |
| PCO 20 mg (n = 15) | 2.16 ± 0.09 | 2.08 ± 0.1 | 2.00 ± 0.12 | 1.99 ± 0.1 | ||||
| p † | 0.850 | 0.239 | 0.018 | 0.054 | ||||
| HDL-C/TC (%) | placebo (n = 17) | 29.7 ± 0.8 | 29.1 ± 1.1 | 28.9 ± 1.0 | 28.8 ± 1.0 | Time × group | 3.033 | 0.033 |
| PCO 20 mg (n = 15) | 29.2 ± 1.1 | 30.2 ± 1.2 | 30.1 ± 1.4 | 30.9 ± 1.4 | ||||
| p † | 0.671 | 0.085 | 0.086 | 0.003 | ||||
| RC (mg/dL) | placebo (n = 17) | 14.9 ± 1.3 | 16.9 ± 1.8 | 17.2 ± 2.1 | 20.0 ± 3.3 | Time × group | 1.582 | 0.216 |
| PCO 20 mg (n = 15) | 19.6 ± 2.4 | 18.9 ± 1.6 | 25.1 ± 4.2 | 20.3 ± 3.0 | ||||
| p † | 0.090 | 0.945 | 0.244 | 0.057 |
| Policosanol 20 mg | p | Placebo | p | ||||
|---|---|---|---|---|---|---|---|
| Week 0 n = 15 (Mean ± SEM) | Week 12 n = 15 (Mean ± SEM) | Week 0 n = 15 (Mean ± SEM) | Week 12 n = 15 (Mean ± SEM) | ||||
| VLDL | FI (Glycated) | 3851 ± 144 | 3572 ± 143 | 0.067 | 4074 ± 230 | 4379 ± 274 | 0.223 |
| MDA (μM) | 14.9 ± 5.1 | 5.5 ± 1.60 | 0.041 | 12.1 ± 4.7 | 10.6 ± 4.2 | 0.790 | |
| Size (nm2) | 4827 ± 529 | 3562 ± 426 | 0.013 | 4882 ± 496 | 5389 ± 309 | 0.285 | |
| Diameter (nm) | 75.3 ± 3.1 | 65.3 ± 2.3 | 0.016 | 74.1 ± 3.7 | 78.8 ± 3.8 | 0.381 | |
| TC (mg/dL) | 50.4 ± 0.9 | 73.3 ± 8.5 | 0.134 | 57.0 ± 5.3 | 53.0 ± 5.7 | 0.207 | |
| TG (mg/dL) | 95.0 ± 7.2 | 81.2 ± 5.3 | 0.051 | 79.9 ± 13.8 | 84.0 ± 12.1 | 0.459 | |
| LDL | FI (Glycated) | 4958 ± 266 | 4416 ± 121 | 0.082 | 4934 ± 622 | 5109 ± 900 | 0.640 |
| MDA (μM) | 0.53 ± 0.14 | 0.33 ± 0.14 | 0.004 | 0.45 ± 0.17 | 0.38 ± 0.15 | 0.779 | |
| Size (nm2) | 504.5 ± 3.4 | 531.7 ± 8.2 | 0.034 | 508.6 ± 3.3 | 492.7 ± 10.1 | 0.268 | |
| Diameter (nm) | 23.9 ± 0.4 | 25.5 ± 0.5 | 0.064 | 24.6 ± 0.8 | 24.3 ± 0.4 | 0.547 | |
| TC (mg/dL) | 117.6 ± 9.4 | 131.7 ± 6.6 | 0.159 | 133.7 ± 4.5 | 107.6 ± 4.1 | 0.591 | |
| TG (mg/dL) | 19.3 ± 1.3 | 17.6 ± 0.4 | 0.240 | 17.3 ± 0.8 | 17.7 ± 1.0 | 0.750 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. https://doi.org/10.3390/ijms24065185
Cho K-H, Nam H-S, Baek S-H, Kang D-J, Na H, Komatsu T, Uehara Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. International Journal of Molecular Sciences. 2023; 24(6):5185. https://doi.org/10.3390/ijms24065185
Chicago/Turabian StyleCho, Kyung-Hyun, Hyo-Seon Nam, Seung-Hee Baek, Dae-Jin Kang, Hyejee Na, Tomohiro Komatsu, and Yoshinari Uehara. 2023. "Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese" International Journal of Molecular Sciences 24, no. 6: 5185. https://doi.org/10.3390/ijms24065185
APA StyleCho, K.-H., Nam, H.-S., Baek, S.-H., Kang, D.-J., Na, H., Komatsu, T., & Uehara, Y. (2023). Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. International Journal of Molecular Sciences, 24(6), 5185. https://doi.org/10.3390/ijms24065185








