FTIR Analysis of Renal Tissue for the Assessment of Hypertensive Organ Damage and proANP31–67 Treatment
Abstract
1. Introduction
2. Results
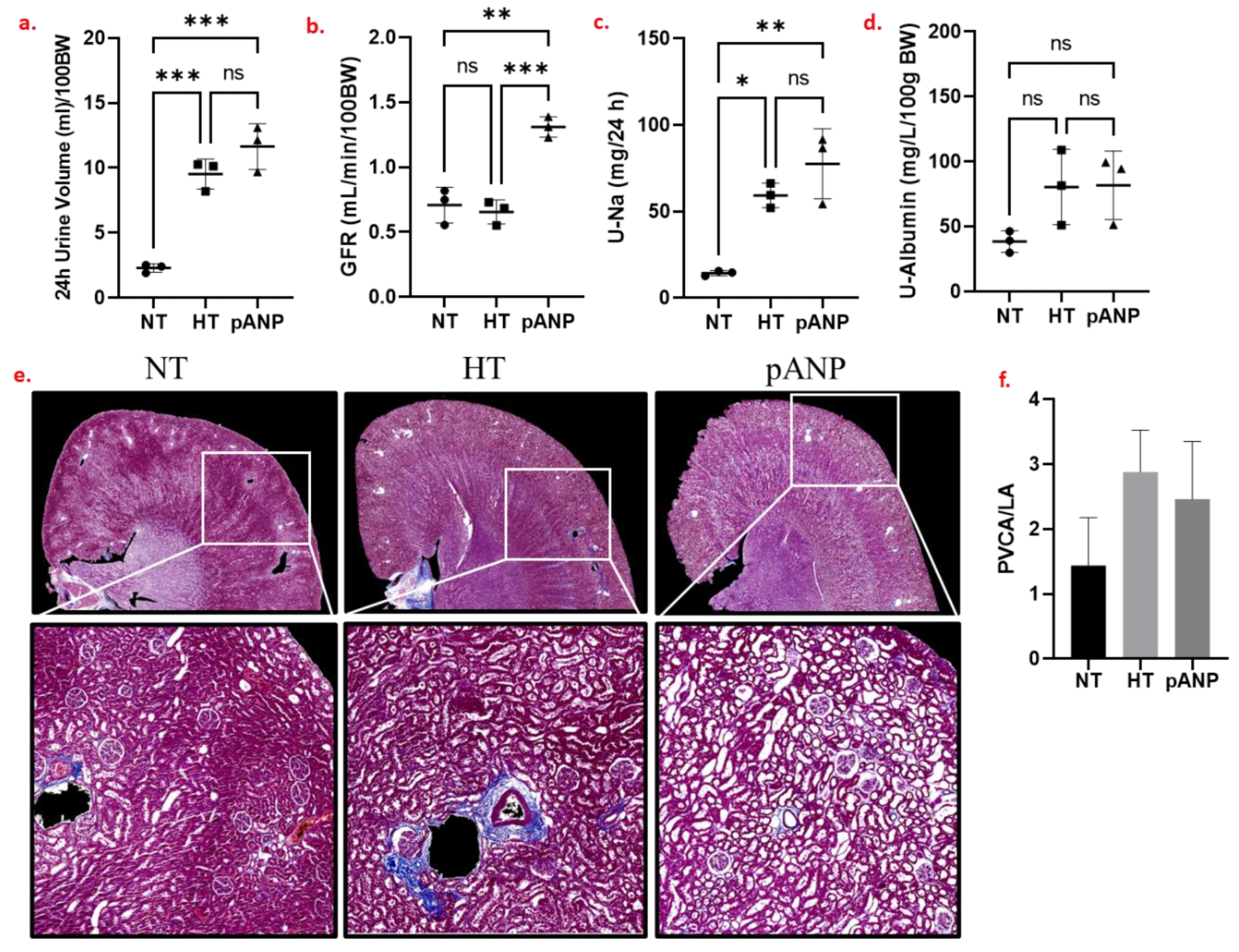
2.1. Renal Functional and Structural Assessment
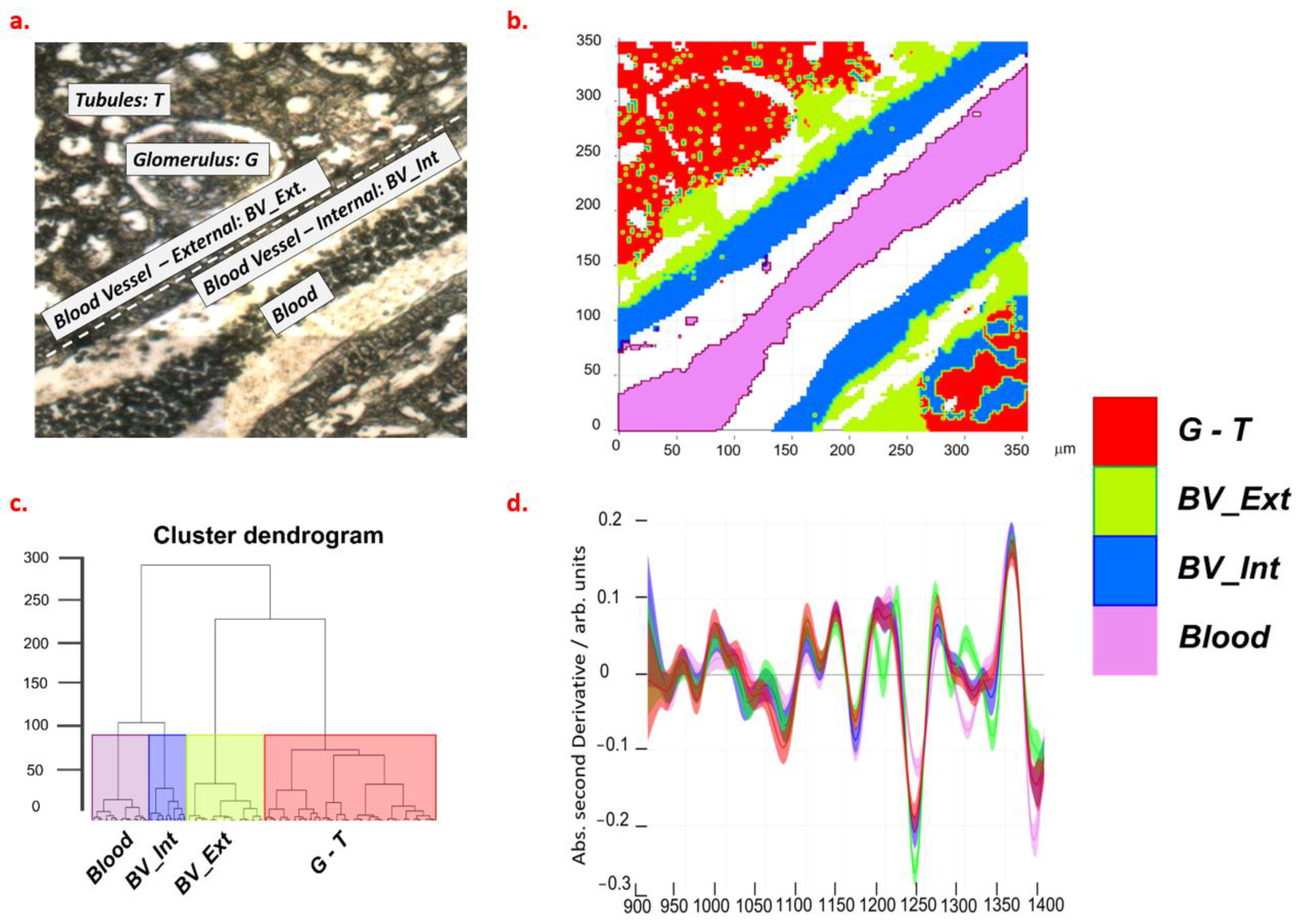
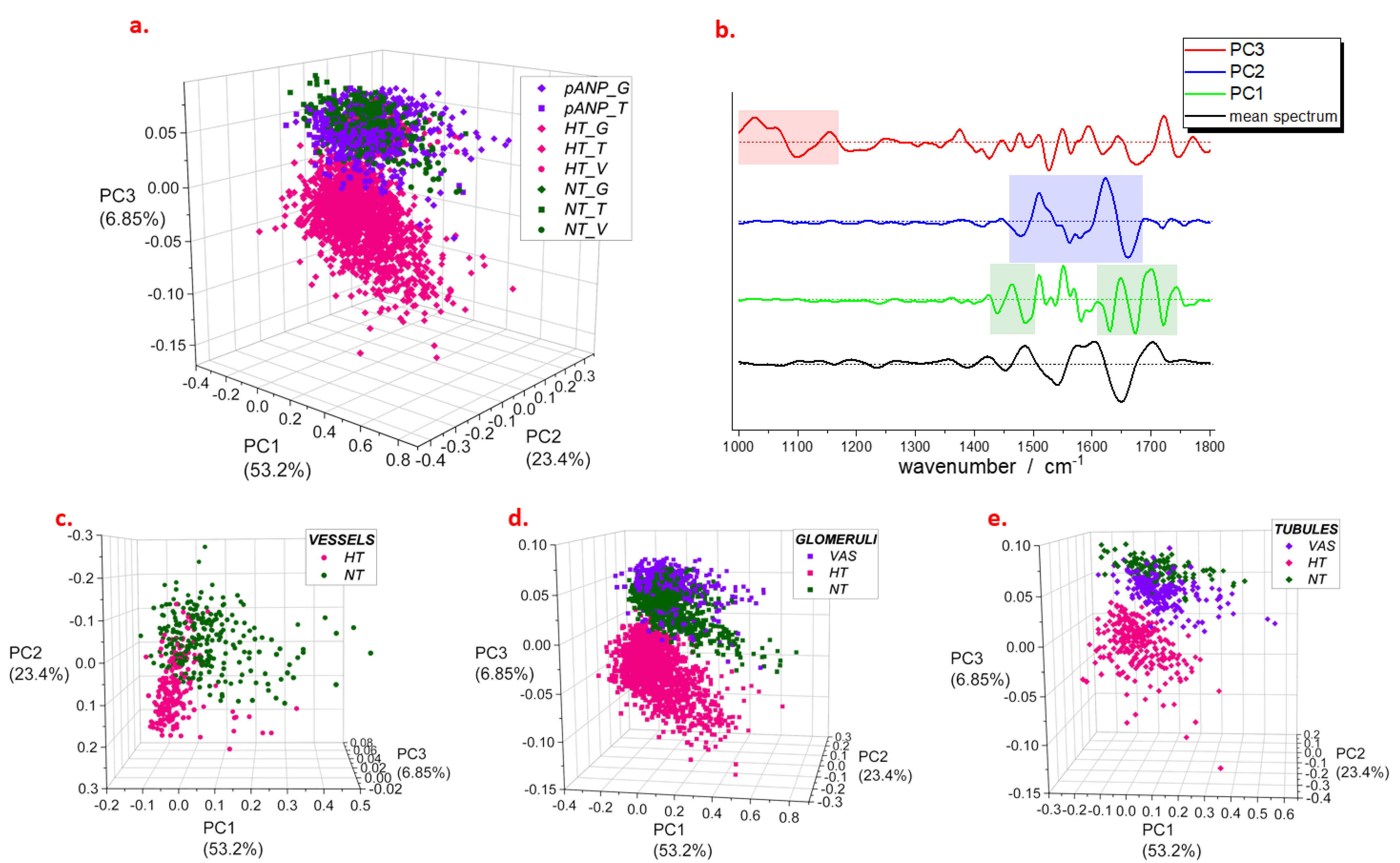
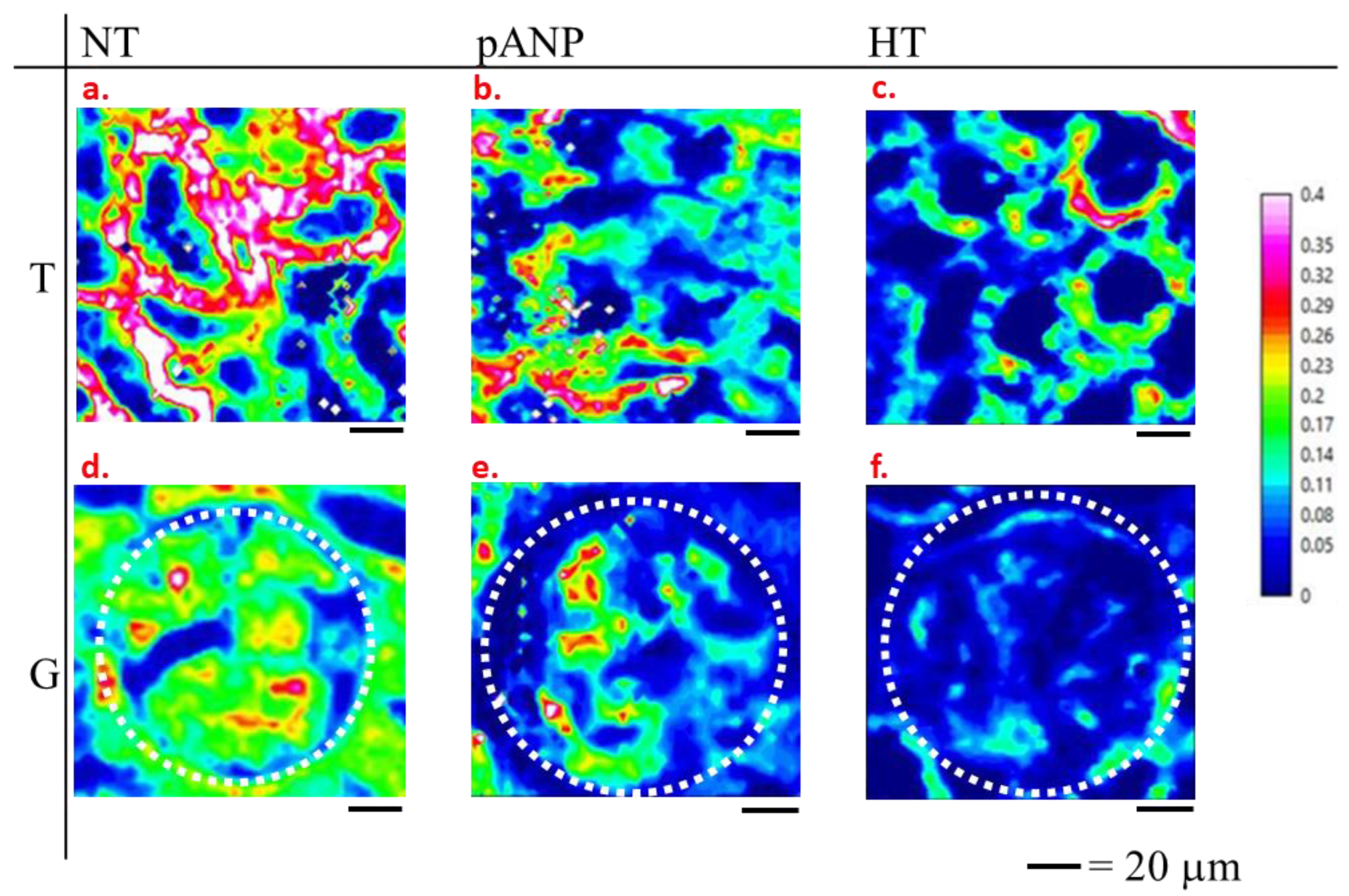
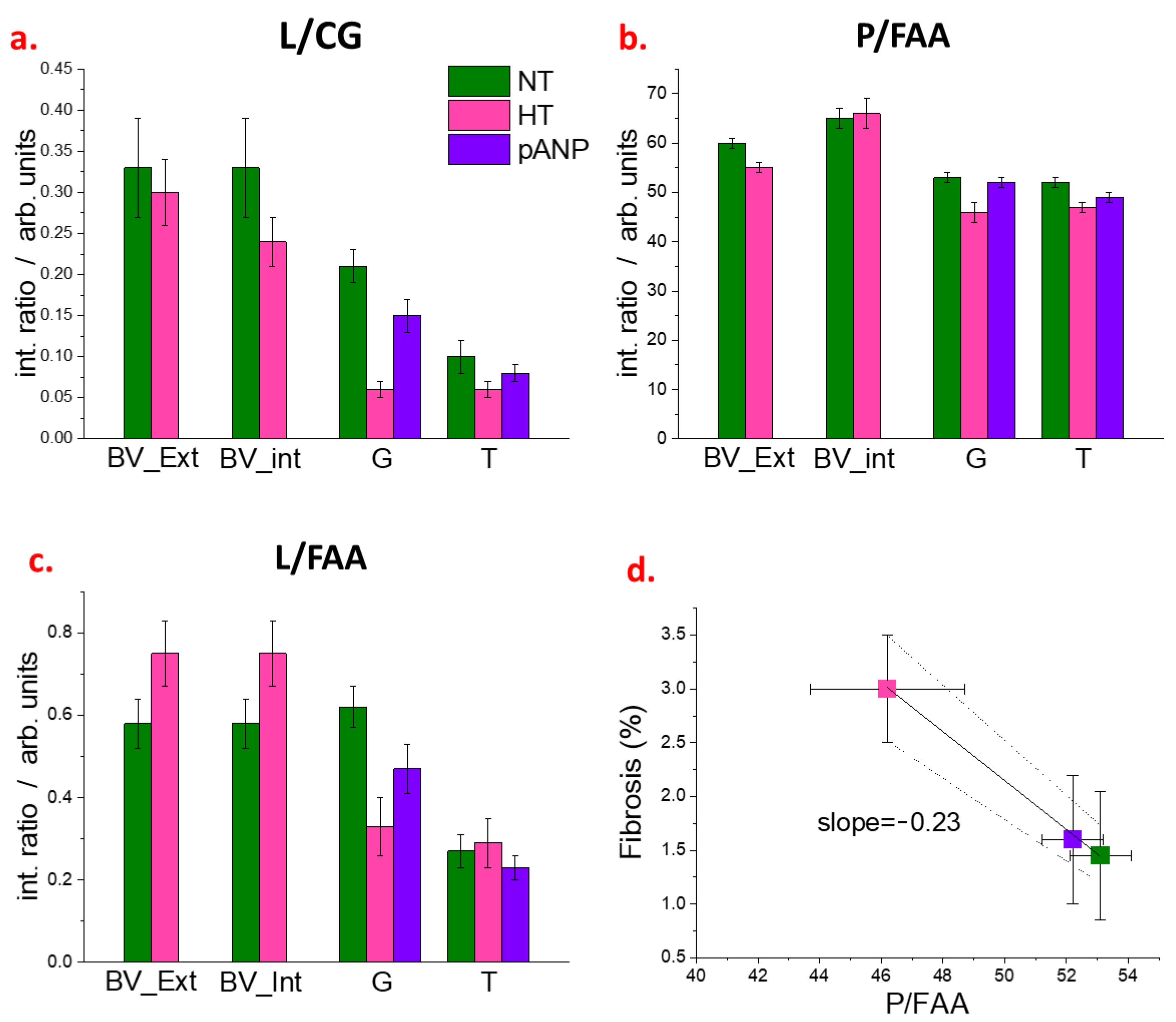
2.2. Spectroscopic Characterization of Renal Tissue
3. Discussion
4. Materials and Methods
4.1. Experimental Group and Study Protocol
4.2. Histochemistry
4.3. FTIR Measurements
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lager, D. Heptinstall’s Pathology of the Kidney. Am. J. Surg. Pathol. 2008, 32, 344. [Google Scholar] [CrossRef]
- Folkow, B.; Göthberg, G.; Lundin, S.; Ricksten, S.-E. Structural “Resetting” of the Renal Vascular Bed in Spontaneously Hypertensive Rats (SHR). Acta Physiol. Scand. 1977, 100, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Textor, S.C. The Role of the Kidney in Regulating Arterial Blood Pressure. Nat. Rev. Nephrol. 2012, 8, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and Kidneys: Unraveling Complex Molecular Mechanisms Underlying Hypertensive Renal Damage. J. Hum. Hypertens 2014, 28, 74–79. [Google Scholar] [CrossRef]
- Shankland, S.J. The Podocyte’s Response to Injury: Role in Proteinuria and Glomerulosclerosis. Kidney Int. 2006, 69, 2131–2147. [Google Scholar] [CrossRef]
- Weldegiorgis, M.; Woodward, M. The Impact of Hypertension on Chronic Kidney Disease and End-Stage Renal Disease Is Greater in Men than Women: A Systematic Review and Meta-Analysis. BMC Nephrol. 2020, 21, 506. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Tummalapalli, S.L.; Boulware, L.E.; Grams, M.E.; Ix, J.H.; Jha, V.; Kengne, A.P.; Madero, M.; Mihaylova, B.; Tangri, N.; et al. The Case for Early Identification and Intervention of Chronic Kidney Disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021, 99, 34–47. [Google Scholar] [CrossRef]
- Walker, P.D. The Renal Biopsy. Arch. Pathol. Lab. Med. 2009, 133, 181–188. [Google Scholar] [CrossRef]
- Esposito, V.; Mazzon, G.; Baiardi, P.; Torreggiani, M.; Semeraro, L.; Catucci, D.; Colucci, M.; Mariotto, A.; Grosjean, F.; Bovio, G.; et al. Safety and Adequacy of Percutaneous Kidney Biopsy Performed by Nephrology Trainees. BMC Nephrol. 2018, 19, 14. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Mengel, M. The Rise of Renal Pathology in Nephrology: Structure Illuminates Function. Am. J. Kidney Dis. 2013, 61, 1016–1025. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Šablinskas, V.; Urbonienė, V.; Ceponkus, J.; Laurinavicius, A.; Dasevicius, D.; Jankevičius, F.; Hendrixson, V.; Koch, E.; Steiner, G. Infrared Spectroscopic Imaging of Renal Tumor Tissue. J. Biomed. Opt. 2011, 16, 096006. [Google Scholar] [CrossRef]
- Pucetaite, M.; Velicka, M.; Urboniene, V.; Ceponkus, J.; Bandzeviciute, R.; Jankevicius, F.; Zelvys, A.; Sablinskas, V.; Steiner, G. Rapid Intra-Operative Diagnosis of Kidney Cancer by Attenuated Total Reflection Infrared Spectroscopy of Tissue Smears. J. Biophotonics 2018, 11, e201700260. [Google Scholar] [CrossRef]
- Palaniappan, P.L.R.M.; Vijayasundaram, V. Arsenic-Induced Biochemical Changes in Labeo Rohita Kidney: An FTIR Study. Spectrosc. Lett. 2009, 42, 213–218. [Google Scholar] [CrossRef]
- Esteve, E.; Luque, Y.; Waeytens, J.; Bazin, D.; Mesnard, L.; Jouanneau, C.; Ronco, P.; Dazzi, A.; Daudon, M.; Deniset-Besseau, A. Nanometric Chemical Speciation of Abnormal Deposits in Kidney Biopsy: Infrared-Nanospectroscopy Reveals Heterogeneities within Vancomycin Casts. Anal. Chem. 2020, 92, 7388–7392. [Google Scholar] [CrossRef]
- Tombolesi, N.; Altara, R.; da Silva, G.J.J.; Tannous, C.; Zouein, F.A.; Stensløkken, K.O.; Morresi, A.; Paolantoni, M.; Booz, G.W.; Cataliotti, A.; et al. Early Cardiac-Chamber-Specific Fingerprints in Heart Failure with Preserved Ejection Fraction Detected by FTIR and Raman Spectroscopic Techniques. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- da Silva, G.J.J.; Altara, R.; Booz, G.W.; Cataliotti, A. Atrial Natriuretic Peptide31-67: A Novel Therapeutic Factor for Cardiovascular Diseases. Front. Physiol. 2021, 12, 691407. [Google Scholar] [CrossRef]
- Altara, R.; da Silva, G.J.J.; Frisk, M.; Spelta, F.; Zouein, F.A.; Louch, W.E.; Booz, G.W.; Cataliotti, A. Cardioprotective Effects of the Novel Compound Vastiras in a Preclinical Model of End-Organ Damage. Hypertension 2020, 75, 1195–1204. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Jain, M. Hypertensive Renal Disease: Histological Aspects. Clin. Queries Nephrol. 2013, 2, 23–28. [Google Scholar] [CrossRef]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Zscherp, C. What Vibrations Tell Us about Proteins. Q Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef] [PubMed]
- Rüger, B.M.; Hasan, Q.; Greenfaill, N.S.; Davis, E.F.; Dunbar, P.R.; Neaie, T.J. Mast Cells and Type VIII Collagen in Human Diabetic Nephropathy. Diabetologia 1996, 39, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Strupler, M.; Hernest, M.; Fligny, C.; Martin, J.-L.; Tharaux, P.-L.; Schanne-Klein, M.-C. Second Harmonic Microscopy to Quantify Renal Interstitial Fibrosis and Arterial Remodeling. J. Biomed. Opt. 2008, 13, 054041. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Palygin, O.; Guijas, C.; Palermo, A.; Palacio-Escat, N.; Domingo-Almenara, X.; Montenegro-Burke, R.; Saez-Rodriguez, J.; Staruschenko, A.; Siuzdak, G. Metabolic Rewiring of the Hypertensive Kidney. Sci. Signal. 2019, 12, 9760. [Google Scholar] [CrossRef]
- Domondon, M.; Polina, I.; Nikiforova, A.B.; Sultanova, R.F.; Kruger, C.; Vasileva, V.Y.; Fomin, M.V.; Beeson, G.C.; Nieminen, A.L.; Smythe, N.; et al. Renal Glomerular Mitochondria Function in Salt-Sensitive Hypertension. Front. Physiol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Liang, M.; Sun, Q.; Sun, N.; Tian, Z. Mitochondrial Dysfunction and Altered Renal Metabolism in Dahl Salt-Sensitive Rats. Kidney Blood. Press Res 2017, 42, 587–597. [Google Scholar] [CrossRef]
- Varma, V.K.; Kajdacsy-Balla, A.; Akkina, S.K.; Setty, S.; Walsh, M.J. A Label-Free Approach by Infrared Spectroscopic Imaging for Interrogating the Biochemistry of Diabetic Nephropathy Progression. Kidney Int. 2016, 89, 1153–1159. [Google Scholar] [CrossRef]
- Tombolesi, N. Combined FTIR and Raman Spectroscopic Study for the Characterization of Biological Tissues. Ph.D. Thesis, University of Perugia, Perugia, Italy, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pioppi, L.; Tombolesi, N.; Parvan, R.; da Silva, G.J.J.; Altara, R.; Paolantoni, M.; Morresi, A.; Sassi, P.; Cataliotti, A. FTIR Analysis of Renal Tissue for the Assessment of Hypertensive Organ Damage and proANP31–67 Treatment. Int. J. Mol. Sci. 2023, 24, 5196. https://doi.org/10.3390/ijms24065196
Pioppi L, Tombolesi N, Parvan R, da Silva GJJ, Altara R, Paolantoni M, Morresi A, Sassi P, Cataliotti A. FTIR Analysis of Renal Tissue for the Assessment of Hypertensive Organ Damage and proANP31–67 Treatment. International Journal of Molecular Sciences. 2023; 24(6):5196. https://doi.org/10.3390/ijms24065196
Chicago/Turabian StylePioppi, Leonardo, Niki Tombolesi, Reza Parvan, Gustavo Jose Justo da Silva, Raffaele Altara, Marco Paolantoni, Assunta Morresi, Paola Sassi, and Alessandro Cataliotti. 2023. "FTIR Analysis of Renal Tissue for the Assessment of Hypertensive Organ Damage and proANP31–67 Treatment" International Journal of Molecular Sciences 24, no. 6: 5196. https://doi.org/10.3390/ijms24065196
APA StylePioppi, L., Tombolesi, N., Parvan, R., da Silva, G. J. J., Altara, R., Paolantoni, M., Morresi, A., Sassi, P., & Cataliotti, A. (2023). FTIR Analysis of Renal Tissue for the Assessment of Hypertensive Organ Damage and proANP31–67 Treatment. International Journal of Molecular Sciences, 24(6), 5196. https://doi.org/10.3390/ijms24065196




