Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism
Abstract
1. Introduction
2. Results
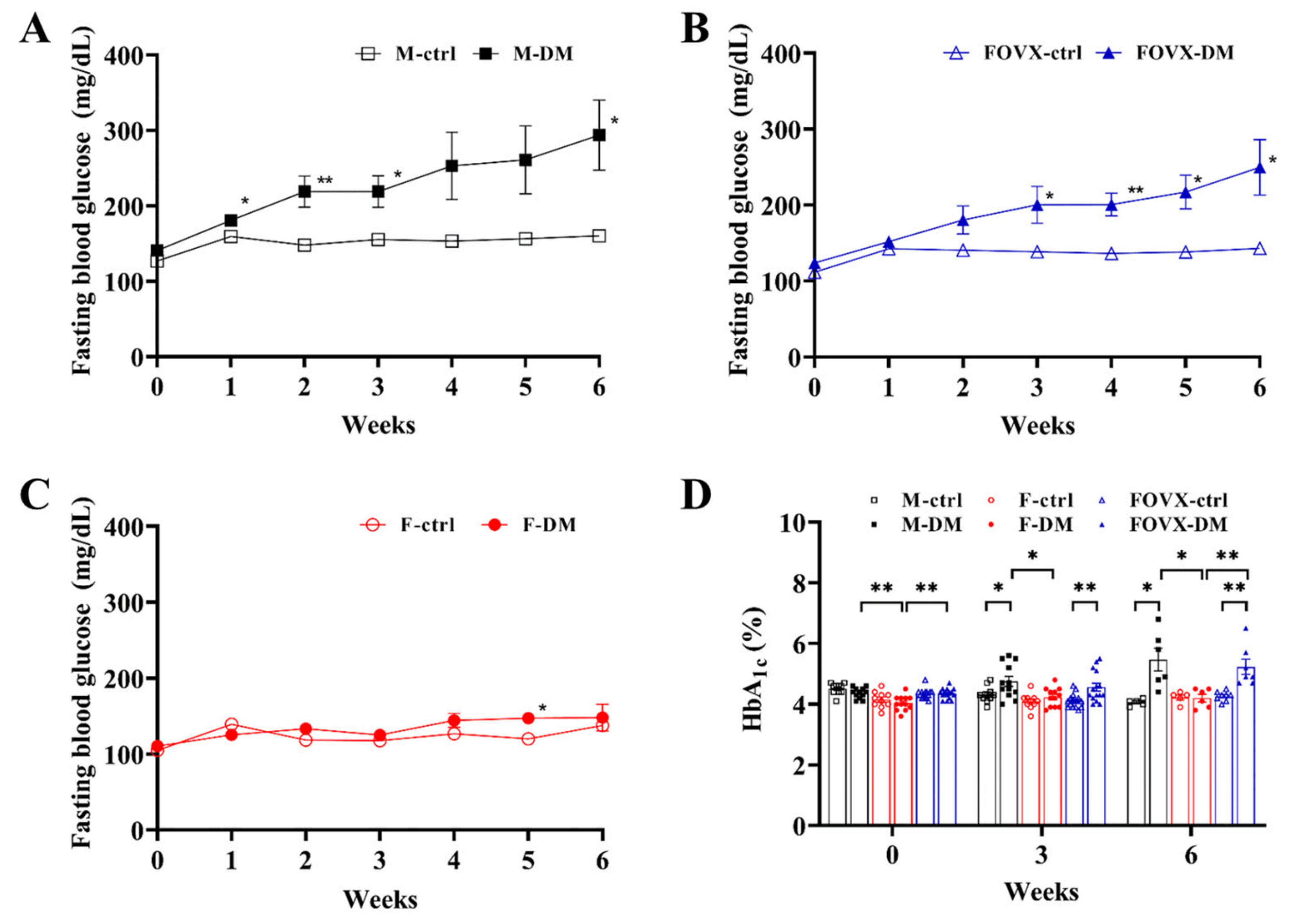
2.1. STZ-Treated Male Mice Rapidly Developed Hyperglycemia and Diabetes
2.2. STZ-Treated Male Mice Showed the Strongest Glucose Tolerance
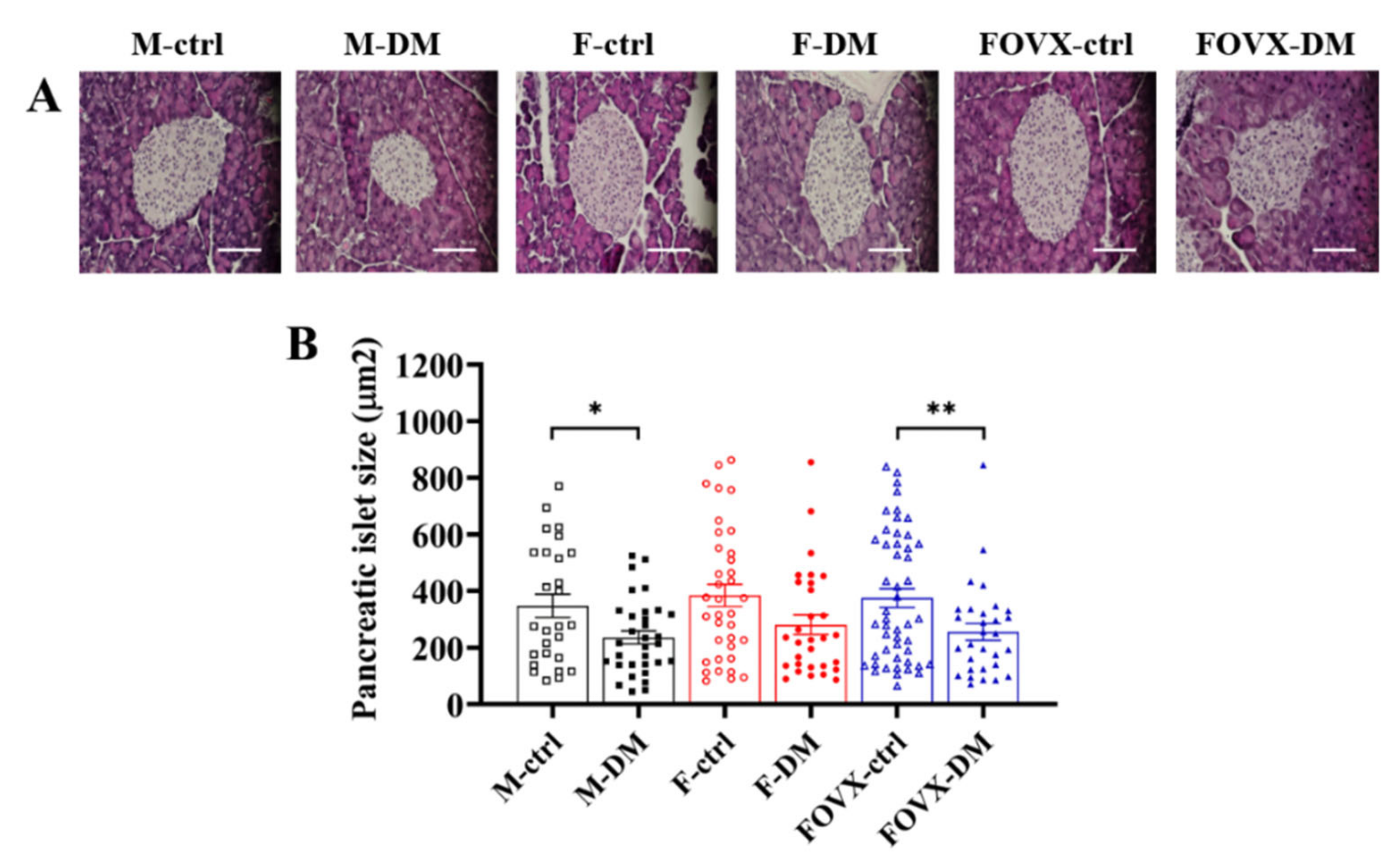
2.3. The Size of Pancreatic Islets in M-DM and FOVX-DM Was Reduced by STZ Treatment
2.4. The Cell Composition of Pancreatic Islets Was Altered in the M-DM and FOVX-DM Groups after STZ Treatment
2.5. Groups M-DM and FOVX-DM Had Impaired Pancreatic Beta-Cell Function
2.6. Insulin Secretion in the M-DM and FOVX-DM Groups Was Inhibited by Urocortin 3 and Somatostatin
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Streptozotocin (STZ) Treatment
4.2. Measurement of FBG and HbA1c
4.3. Oral Glucose Tolerance Test (OGTT) and Area under the Curve (AUC)
4.4. Histological Analysis of the Pancreas
4.5. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Taylor, K.; Gordon, N.; Langley, G.; Higgins, W. Estimates for worldwide laboratory animal use in 2005. Altern. Lab. Anim. 2008, 36, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F. Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research. ILAR J. 2014, 55, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F. Phenotypic variability of inbred and outbred mice. Nature 1976, 263, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, A.H.; Philip, V.M.; Chesler, E.J.; Mogil, J.S. Comparing phenotypic variation between inbred and outbred mice. Nat. Methods 2018, 15, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Wang, Q.; He, Z.; Chen, S.; Zhang, H.; Li, H.; Guo, P.; Li, Q.; Zhang, R.; et al. Strain differences between CD-1 and C57BL/6 mice in expression of metabolic enzymes and DNA methylation modifications of the primary hepatocytes. Toxicology 2019, 412, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Danahy, D.B.; Hartwig, S.M.; Harty, J.T.; Badovinac, V.P. Revealing the Complexity in CD8 T Cell Responses to Infection in Inbred C57B/6 versus Outbred Swiss Mice. Front. Immunol. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, J.; Mims, B.M.D.; Trasti, S.; Furr, K.L.; Grisham, M.B. Genomic, microbial and environmental standardization in animal experimentation limiting immunological discovery. BMC Immunol. 2020, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, Y.Y.; Nguyen, P.T.-T.; Nam, H.; Suh, J.G. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl. Biol. Chem. 2020, 63, 59. [Google Scholar] [CrossRef]
- De Paoli, M.; Werstuck, G.H. Role of Estrogen in Type 1 and Type 2 Diabetes Mellitus: A Review of Clinical and Preclinical Data. Can. J. Diabetes 2020, 44, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Remedi, M.S.; Emfinger, C. Pancreatic beta-cell identity in diabetes. Diabetes Obes. Metab. 2016, 18 (Suppl. 1), 110–116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Vaughan, J.; Blount, A.; Chen, A.; Jamieson, P.M.; Rivier, J.; Smith, M.S.; Vale, W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology 2003, 144, 3216–3224. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, T.; Xie, R.; Kelly, O.G.; Vale, W.W.; Sander, M.; Huising, M.O. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS ONE 2012, 7, e52181. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, T.; Huising, M.O. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J. Mol. Endocrinol. 2015, 54, R103–R117. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.; Roose, A.N.; Barrandon, O.; Maehr, R.; Arvanites, A.C.; Davidow, L.S.; Davis, J.C.; Peterson, Q.P.; Rubin, L.L.; Melton, D.A. Reversal of beta cell de-differentiation by a small molecule inhibitor of the TGFbeta pathway. Elife 2014, 3, e02809. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Vaughan, J.; Lee, K.F.; Vale, W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc. Natl. Acad. Sci. USA 2007, 104, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, T.; Donaldson, C.J.; Caceres, E.; Hunter, A.E.; Cowing-Zitron, C.; Pound, L.D.; Adams, M.W.; Zembrzycki, A.; Grove, K.L.; Huising, M.O. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 2015, 21, 769–776. [Google Scholar] [CrossRef] [PubMed]
- May, C.L.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.; Mauvais-Jarvis, F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; May, C.L.; Tiano, J.P.; Liu, S.; Kilic-Berkman, G.; Kim, J.H. The Role of Estrogens in Pancreatic Islet Physiopathology. Adv. Exp. Med. Biol. 2017, 1043, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, e78. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Park, E.-S.; Lee, J.-S.; Suh, J.-G. Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism. Int. J. Mol. Sci. 2023, 24, 5210. https://doi.org/10.3390/ijms24065210
Kim B, Park E-S, Lee J-S, Suh J-G. Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism. International Journal of Molecular Sciences. 2023; 24(6):5210. https://doi.org/10.3390/ijms24065210
Chicago/Turabian StyleKim, Boyoung, Eun-Sun Park, Jong-Sun Lee, and Jun-Gyo Suh. 2023. "Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism" International Journal of Molecular Sciences 24, no. 6: 5210. https://doi.org/10.3390/ijms24065210
APA StyleKim, B., Park, E.-S., Lee, J.-S., & Suh, J.-G. (2023). Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism. International Journal of Molecular Sciences, 24(6), 5210. https://doi.org/10.3390/ijms24065210





