Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Analysis
3. Results and Discussion
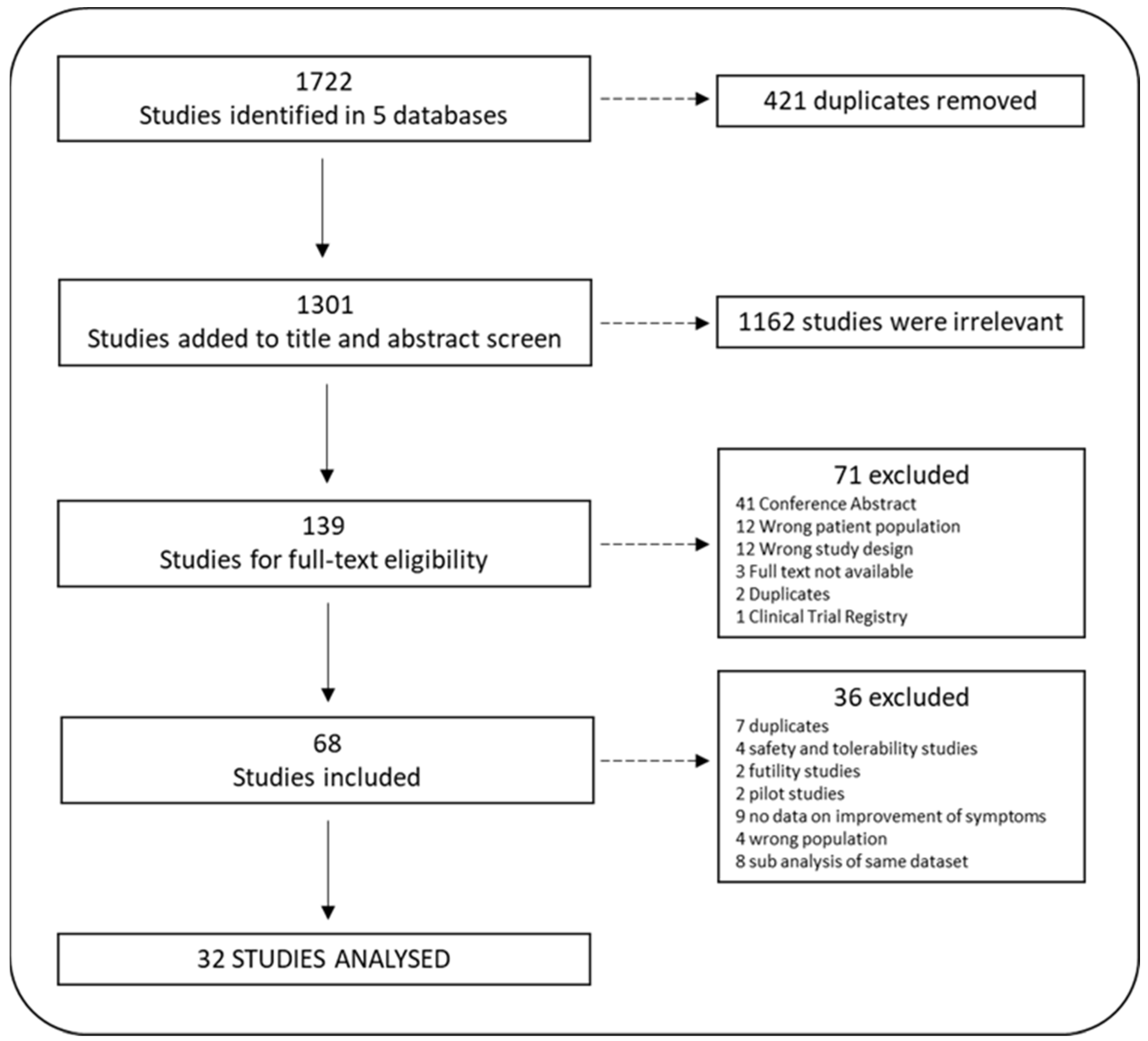
3.1. Search Yield and Study Inclusion
3.2. Psychiatric Disorders
3.2.1. Schizophrenia
3.2.2. Major Depressive Disorder
3.2.3. Bipolar Disorder
3.2.4. Substance Use
3.2.5. OCD
3.3. Neurological Disorders
3.3.1. Stroke
3.3.2. Injury
3.3.3. Other
3.3.4. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, O.M.; Data-Franco, J.; Giorlando, F.; Berk, M. Minocycline: Therapeutic Potential in Psychiatry. CNS Drugs 2012, 26, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fava, M. The Promise and Challenges of Drug Repurposing in Psychiatry. World Psychiatry 2018, 17, 28–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berk, M.; Vieta, E.; Dean, O.M. Anti-Inflammatory Treatment of Bipolar Depression: Promise and Disappointment. Lancet Psychiatry 2020, 7, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kakkar, A.K.; Chauhan, P. Repurposing Minocycline for COVID-19 Management: Mechanisms, Opportunities, and Challenges. Expert Rev. Anti. Infect Ther. 2020, 18, 997–1003. [Google Scholar] [CrossRef]
- Fond, G.; Hamdani, N.; Kapczinski, F.; Boukouaci, W.; Drancourt, N.; Dargel, A.; Oliveira, J.; Le Guen, E.; Marlinge, E.; Tamouza, R.; et al. Effectiveness and Tolerance of Anti-Inflammatory Drugs’ Add-on Therapy in Major Mental Disorders: A Systematic Qualitative Review. Acta Psychiatr. Scand. 2014, 129, 163–179. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, X.-M.; Zhang, Q.-E.; Cheng, G.; Cai, D.-B.; He, J.; Ng, C.H.; Ungvari, G.S.; Peng, X.-J.; Ning, Y.-P.; et al. Adjunctive Minocycline for Major Mental Disorders: A Systematic Review. J. Psychopharmacol. 2019, 33, 1215–1226. [Google Scholar] [CrossRef]
- Oya, K.; Kishi, T.; Iwata, N. Efficacy and Tolerability of Minocycline Augmentation Therapy in Schizophrenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hum. Psychopharmacol. 2014, 29, 483–491. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Thapa, N.; Facchini, S.; Stubbs, B.; Fornaro, M.; Carvalho, A.F.; Correll, C.U. Systematic Review and Meta-Analysis of the Efficacy and Safety of Minocycline in Schizophrenia. CNS Spectr. 2017, 22, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.; Lee, T.Y.; Kwak, Y.B.; Yoon, Y.B.; Kim, M.; Kwon, J.S. Adjunctive Use of Anti-Inflammatory Drugs for Schizophrenia: A Meta-Analytic Investigation of Randomized Controlled Trials. Aust. N. Z. J. Psychiatry 2019, 53, 742–759. [Google Scholar] [CrossRef] [Green Version]
- Ventriglio, A.; Bellomo, A.; Ricci, F.; Magnifico, G.; Rinaldi, A.; Borraccino, L.; Piccininni, C.; Cuoco, F.; Gianfelice, G.; Fornaro, M.; et al. New Pharmacological Targets for the Treatment of Schizophrenia: A Literature Review. Curr. Top Med. Chem. 2021, 21, 1500–1516. [Google Scholar] [CrossRef]
- Minichino, A.; Brondino, N.; Solmi, M.; Del Giovane, C.; Fusar-Poli, P.; Burnet, P.; Cipriani, A.; Lennox, B.R. The Gut-Microbiome as a Target for the Treatment of Schizophrenia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials of Add-on Strategies. Schizophr. Res. 2021, 234, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, R.; Christensen, R.H.B.; Pedersen, E.M.J.; Nordentoft, M.; Hjorthøj, C.; Köhler-Forsberg, O.; Benros, M.E. Efficacy and Safety of Anti-Inflammatory Agents in Treatment of Psychotic Disorders—A Comprehensive Systematic Review and Meta-Analysis. Brain Behav. Immun. 2020, 90, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Kashefinejad, S.; Kamalzadeh, L.; Hoobehfekr, S.; Shalbafan, M. Glutamatergic Medications as Adjunctive Therapy for Moderate to Severe Obsessive-Compulsive Disorder in Adults: A Systematic Review and Meta-Analysis. BMC Pharmacol. Toxicol. 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Chang, J.J.; Khunger, A.; Blacker, D.; Switzer, J.A.; Goyal, N.; Hernandez, A.V.; Pasupuleti, V.; Alexandrov, A.V.; Tsivgoulis, G. Minocycline for Acute Stroke Treatment: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Neurol. 2018, 265, 1871–1879. [Google Scholar] [CrossRef]
- Frontiers|Efficacy of Minocycline in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis of Rodent and Clinical Studies. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2018.01103/full (accessed on 18 August 2022).
- Minocycline and Magnesium as Neuroprotective Agents for Ischemic Stroke: A Systematic Review—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7837630/ (accessed on 18 August 2022).
- Kwon, B.K.; Sekhon, L.H.; Fehlings, M.G. Emerging Repair, Regeneration, and Translational Research Advances for Spinal Cord Injury. Spine 2010, 35, S263–S270. [Google Scholar] [CrossRef]
- Kwon, B.K.; Okon, E.; Hillyer, J.; Mann, C.; Baptiste, D.; Weaver, L.C.; Fehlings, M.G.; Tetzlaff, W. A Systematic Review of Non-Invasive Pharmacologic Neuroprotective Treatments for Acute Spinal Cord Injury. J. Neurotrauma 2011, 28, 1545–1588. [Google Scholar] [CrossRef] [Green Version]
- Kwon, B.K.; Okon, E.B.; Plunet, W.; Baptiste, D.; Fouad, K.; Hillyer, J.; Weaver, L.C.; Fehlings, M.G.; Tetzlaff, W. A Systematic Review of Directly Applied Biologic Therapies for Acute Spinal Cord Injury. J. Neurotrauma 2011, 28, 1589–1610. [Google Scholar] [CrossRef] [Green Version]
- Strickland, B.A.; Bakhsheshian, J.; Emmanuel, B.; Amar, A.; Giannotta, S.L.; Russin, J.J.; Mack, W. Neuroprotective Effect of Minocycline against Acute Brain Injury in Clinical Practice: A Systematic Review. J. Clin. Neurosci. 2021, 86, 50–57. [Google Scholar] [CrossRef]
- Shin, D.A.; Kim, T.U.; Chang, M.C. Minocycline for Controlling Neuropathic Pain: A Systematic Narrative Review of Studies in Humans. J. Pain Res. 2021, 14, 139–145. [Google Scholar] [CrossRef]
- Aly, E.; Masocha, W. Targeting the Endocannabinoid System for Management of HIV-Associated Neuropathic Pain: A Systematic Review. IBRO Neurosci. Rep. 2021, 10, 109–118. [Google Scholar] [CrossRef]
- Selçuk, A.A. A Guide for Systematic Reviews: PRISMA. Turk. Arch. Otorhinolaryngol. 2019, 57, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Bortolasci, C.C.; Marx, W.; Walker, A.J.; Hasebe, K.; Kavanagh, B.E.; Morris, M.J.; Mohebbi, M.; Turner, A.; Gray, L.; Berk, L.; et al. Minocycline for the Treatment of Mental Health and Neurological Conditions: Study Protocol of a Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e035080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 7 February 2023).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandles, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Chochrane Handbook for Systematic Reviews of Interventions; Version 6.0; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Higgins, J.P.; Altman, D.G. Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 187–241. ISBN 978-0-470-71218-4. [Google Scholar]
- Pannucci, C.; Wilkins, E. Identifying and Avoiding Bias in Research. Plast. Reconstr. Surg. 2010, 126, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Levin, K.A. Study Design II. Issues of Chance, Bias, Confounding and Contamination. Evid. Based Dent. 2005, 6, 102–103. [Google Scholar] [CrossRef] [Green Version]
- Krishna, R.; Maithreyi, R.; Surapaneni, K. Research Bias: A Review For Medical Students. J. Clin. Diagn. Res. 2010, 4, 2320–2324. [Google Scholar]
- Henderson, M.; Page, L. Appraising the Evidence: What Is Selection Bias? Evid. Based Ment. Health 2007, 10, 67–68. [Google Scholar] [CrossRef]
- Furnham, A. Response Bias, Social Desirability and Dissimulation. Personal. Individ. Differ. 1986, 7, 385–400. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]; The Cochrane Collaboration: London, UK, 2020. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- August, S.M.; Kiwanuka, J.N.; McMahon, R.P.; Gold, J.M. The MATRICS Consensus Cognitive Battery (MCCB): Clinical and Cognitive Correlates. Schizophr. Res. 2012, 134, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, I.B.; Hallak, J.; Husain, N.; Laganis, C.; Minhas, F.; Stirling, J.; Richardson, P.; Dursun, S.; Dunn, G.; Deakin, J.F.W. Minocycline Benefits Negative Symptoms in Early Schizophrenia; A Randomised Double-Blind Placebo-Controlled Clinical Trial in Patients on a Standard Treatment. Early Interv. Psychiatry 2012, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Guo, X.; Wu, R.; Ou, J.; Zheng, Y.; Zhang, B.; Xie, L.; Zhang, L.; Yang, L.; Yang, S.; et al. Minocycline Supplementation for Treatment of Negative Symptoms in Early-Phase Schizophrenia: A Double Blind, Randomized, Controlled Trial. Schizophr. Res. 2014, 153, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, B.; Xie, L.; Ruan, Y.; Xu, X.; Zeng, Y.; Messina, L.; Zhao, J.; Fan, X. Changes in Plasma Levels of Nitric Oxide Metabolites and Negative Symptoms after 16-Week Minocycline Treatment in Patients with Schizophrenia. Schizophr. Res. 2018, 199, 390–394. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A Double-Blind, Randomized Study of Minocycline for the Treatment of Negative and Cognitive Symptoms in Early-Phase Schizophrenia. J. Clin. Psychiatry 2010, 71, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Khodaie-Ardakani, M.-R.; Mirshafiee, O.; Farokhnia, M.; Tajdini, M.; Hosseini, S.-M.-R.; Modabbernia, A.; Rezaei, F.; Salehi, B.; Yekehtaz, H.; Ashrafi, M.; et al. Minocycline Add-on to Risperidone for Treatment of Negative Symptoms in Patients with Stable Schizophrenia: Randomized Double-Blind Placebo-Controlled Study. Psychiatry Res. 2014, 215, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Sullivan, K.M.; McEvoy, J.P.; McMahon, R.P.; Wehring, H.J.; Gold, J.M.; Liu, F.; Warfel, D.; Vyas, G.; Richardson, C.M.; et al. Adjunctive Minocycline in Clozapine-Treated Schizophrenia Patients with Persistent Symptoms. J. Clin. Psychopharmacol. 2015, 35, 374–381. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, H.; Wu, R.; Zhu, F.; Kosten, T.R.; Zhang, X.-Y.; Zhao, J. Minocycline Adjunctive Treatment to Risperidone for Negative Symptoms in Schizophrenia: Association with pro-Inflammatory Cytokine Levels. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 85, 69–76. [Google Scholar] [CrossRef]
- Deakin, B.; Suckling, J.; Barnes, T.R.E.; Byrne, K.; Chaudhry, I.B.; Dazzan, P.; Drake, R.J.; Giordano, A.; Husain, N.; Jones, P.B.; et al. The Benefit of Minocycline on Negative Symptoms of Schizophrenia in Patients with Recent-Onset Psychosis (BeneMin): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Psychiatry 2018, 5, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Ghoreishi, A.; Granpy, S.; Armani, A. The Role of Minocycline in Neuro-Cognitive Symptoms in the Episodes of Primary Psychosis: A Clinical Trial. Acta Med. Iran. 2020, 58, 194–198. [Google Scholar] [CrossRef]
- Weiser, M.; Levi, L.; Burshtein, S.; Chirita, R.; Cirjaliu, D.; Gonen, I.; Yolken, R.; Davidson, M.; Zamora, D.; Davis, J. The Effect of Minocycline on Symptoms in Schizophrenia: Results from a Randomized Controlled Trial; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Dean, O.M.; Kanchanatawan, B.; Ashton, M.; Mohebbi, M.; Ng, C.H.; Maes, M.; Berk, L.; Sughondhabirom, A.; Tangwongchai, S.; Singh, A.B.; et al. Adjunctive Minocycline Treatment for Major Depressive Disorder: A Proof of Concept Trial. Aust. N. Z. J. Psychiatry 2017, 51, 829–840. [Google Scholar] [CrossRef]
- Husain, M.I.; Chaudhry, I.B.; Husain, N.; Khoso, A.B.; Rahman, R.R.; Hamirani, M.M.; Hodsoll, J.; Qurashi, I.; Deakin, J.F.W.; Young, A.H.; et al. Minocycline as an Adjunct for Treatment-Resistant Depressive Symptoms: A Pilot Randomised Placebo-Controlled Trial. J. Psychopharmacol. 2017, 31, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Nettis, M.A.; Lombardo, G.; Hastings, C.; Zajkowska, Z.; Mariani, N.; Nikkheslat, N.; Worrell, C.; Enache, D.; McLaughlin, A.; Kose, M.; et al. Augmentation Therapy with Minocycline in Treatment-Resistant Depression Patients with Low-Grade Peripheral Inflammation: Results from a Double-Blind Randomised Clinical Trial. Neuropsychopharmacology 2021, 46, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.I.; Chaudhry, I.B.; Khoso, A.B.; Husain, M.O.; Hodsoll, J.; Ansari, M.A.; Naqvi, H.A.; Minhas, F.A.; Carvalho, A.F.; Meyer, J.H.; et al. Minocycline and Celecoxib as Adjunctive Treatments for Bipolar Depression: A Multicentre, Factorial Design Randomised Controlled Trial. Lancet Psychiatry 2020, 7, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.B.; Teague, T.K.; Misaki, M.; Macaluso, M.; Wurfel, B.E.; Meyer, M.; Drevets, D.; Yates, W.; Gleason, O.; Drevets, W.C.; et al. Treatment of Bipolar Depression with Minocycline and/or Aspirin: An Adaptive, 2 × 2 Double-Blind, Randomized, Placebo-Controlled, Phase IIA Clinical Trial. Transl. Psychiatry 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Arout, C.A.; Waters, A.J.; MacLean, R.R.; Compton, P.; Sofuoglu, M. Minocycline Does Not Affect Experimental Pain or Addiction-Related Outcomes in Opioid Maintained Patients. Psychopharmacology 2019, 236, 2857–2866. [Google Scholar] [CrossRef]
- Sofuoglu, M.; Waters, A.J.; Mooney, M.; O’Malley, S.S. Minocycline Reduced Craving for Cigarettes but Did Not Affect Smoking or Intravenous Nicotine Responses in Humans. Pharmacol. Biochem. Behav. 2009, 92, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esalatmanesh, S.; Abrishami, Z.; Zeinoddini, A.; Rahiminejad, F.; Sadeghi, M.; Najarzadegan, M.; Shalbafan, M.; Akhondzadeh, S. Minocycline Combination Therapy with Fluvoxamine in Moderate-to-severe Obsessive–Compulsive Disorder: A Placebo-controlled, Double-blind, Randomized Trial. Psychiatry Clin. Neurosci. 2016, 70, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Lampl, Y.; Boaz, M.; Gilad, R.; Lorberboym, M.; Dabby, R.; Rapoport, A.; Anca-Hershkowitz, M.; Sadeh, M. Minocycline Treatment in Acute Stroke: An Open-Label, Evaluator-Blinded Study. Neurology 2007, 69, 1404–1410. [Google Scholar] [CrossRef]
- Padma Srivastava, M.V.; Bhasin, A.; Bhatia, R.; Garg, A.; Gaikwad, S.; Prasad, K.; Singh, M.B.; Tripathi, M.; Padma Srivastava, M.V.; Bhasin, A.; et al. Efficacy of Minocycline in Acute Ischemic Stroke: A Single-Blinded, Placebo-Controlled Trial. Neurol. India 2012, 60, 23–28. [Google Scholar] [CrossRef]
- Kohler, E.; Prentice, D.A.; Bates, T.R.; Hankey, G.J.; Claxton, A.; van Heerden, J.; Blacker, D. Intravenous Minocycline in Acute Stroke: A Randomized, Controlled Pilot Study and Meta-Analysis. Stroke 2013, 44, 2493–2499. [Google Scholar] [CrossRef] [Green Version]
- Amiri-Nikpour, M.R.; Nazarbaghi, S.; Hamdi-Holasou, M.; Rezaei, Y. An Open-label Evaluator-blinded Clinical Study of Minocycline Neuroprotection in Ischemic Stroke: Gender-dependent Effect. Acta Neurol. Scand. 2015, 131, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Shamsaei, G.; Mohammadi, P. Effect of Oral Minocycline on Clinical Recovery Process in Patients with Acute Ischemic Stroke: A Randomized Clinical Trial. Jundishapur J. Nat. Pharm. Prod. 2017, 12. [Google Scholar] [CrossRef]
- Singh, R.; Augustin, S.J.; Jane, M.; Cheong, J.M.Y.; Haur, L.S.; Choo, T.B. Does Minocycline Improve Recovery After Acute Ischemic Stroke? J. Stroke Med. 2019, 2, 40–46. [Google Scholar] [CrossRef]
- Fouda, A.Y.; Newsome, A.S.; Spellicy, S.; Waller, J.L.; Zhi, W.; Hess, D.C.; Ergul, A.; Edwards, D.J.; Fagan, S.C.; Switzer, J.A. Minocycline in Acute Cerebral Hemorrhage: An Early Phase Randomized Trial. Stroke 2017, 48, 2885–2887. [Google Scholar] [CrossRef] [PubMed]
- Koulaeinejad, N.; Haddadi, K.; Ehteshami, S.; Shafizad, M.; Salehifar, E.; Emadian, O.; Mohammadpour, R.A.; Ala, S. Effects of Minocycline on Neurological Outcomes in Patients with Acute Traumatic Brain Injury: A Pilot Study. Iran. J. Pharm. Res. 2019, 18, 1086–1096. [Google Scholar] [CrossRef]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; John Hurlbert, R. Results of a Phase II Placebo-Controlled Randomized Trial of Minocycline in Acute Spinal Cord Injury. Brain A J. Neurol. 2012, 135, 1224–1236. [Google Scholar] [CrossRef] [Green Version]
- Pontieri, F.E.; Ricci, A.; Pellicano, C.; Benincasa, D.; Buttarelli, F.R. Minocycline in Amyotrophic Lateral Sclerosis: A Pilot Study. Neurol. Sci. 2005, 26, 285–287. [Google Scholar] [CrossRef]
- Howard, R.; Zubko, O.; Bradley, R.; Harper, E.; Pank, L.; O’Brien, J.; Fox, C.; Tabet, N.; Livingston, G.; Bentham, P.; et al. Minocycline at 2 Different Dosages vs Placebo for Patients with Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 164–174. [Google Scholar] [CrossRef]
- Dodel, R.; Spottke, A.; Gerhard, A.; Reuss, A.; Reinecker, S.; Schimke, N.; Trenkwalder, C.; Sixel-Döring, F.; Herting, B.; Kamm, C.; et al. Minocycline 1-Year Therapy in Multiple-System-Atrophy: Effect on Clinical Symptoms and (R)-PK11195 PET (MEMSA-Trial). Mov. Disord. 2010, 25, 97–107. [Google Scholar] [CrossRef]
- Tilley, B.C.; Alarcon, G.S.; Heyse, S.P.; Trentham, D.E.; Neuner, R.; Kaplan, D.A.; Clegg, D.O.; Leisen, J.C.C.; Buckley, L.; Cooper, S.M.; et al. Minocycline in Rheumatoid Arthritis. A 48-Week, Double-Blind, Placebo- Controlled Trial. Ann. Intern. Med. 1995, 122, 81–89. [Google Scholar] [CrossRef]
- Curtin, C.M.; Kenney, D.; Suarez, P.; Hentz, V.R.; Hernandez-Boussard, T.; Mackey, S.; Carroll, I.R. A Double-Blind Placebo Randomized Controlled Trial of Minocycline to Reduce Pain After Carpal Tunnel and Trigger Finger Release. J. Hand Surg. 2017, 42, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Swanepoel, T.; Harvey, B.H. Neurodevelopmental Animal Models Reveal the Convergent Role of Neurotransmitter Systems, Inflammation, and Oxidative Stress as Biomarkers of Schizophrenia: Implications for Novel Drug Development. ACS Chem. Neurosci. 2015, 6, 987–1016. [Google Scholar] [CrossRef] [PubMed]
- Zazula, R.; Ishrat Husain, M.; Mohebbi, M.; Walker, A.J.; Chaudhry, I.B.; Khoso, A.B.; Ashton, M.M.; Agustini, B.; Husain, N.; Deakin, J.F.W.; et al. Minocycline as Adjunctive Treatment for Major Depressive Disorder: Pooled Data from Two Randomized Controlled Trials. Aust. N. Z. J. Psychiatry 2021, 55, 784–798. [Google Scholar] [CrossRef]
- Hasebe, K.; Mohebbi, M.; Gray, L.; Walker, A.J.; Bortolasci, C.C.; Turner, A.; Berk, M.; Walder, K.; Maes, M.; Kanchanatawan, B.; et al. Exploring Interleukin-6, Lipopolysaccharide-Binding Protein and Brain-Derived Neurotrophic Factor Following 12 Weeks of Adjunctive Minocycline Treatment for Depression. Acta Neuropsychiatr. 2022, 34, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Steeger, C.M.; Buckley, P.R.; Pampel, F.C.; Gust, C.J.; Hill, K.G. Common Methodological Problems in Randomized Controlled Trials of Preventive Interventions. Prev. Sci. 2021, 22, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Bird, S.M.; Higgins, J.P.T. The Impact of Study Size on Meta-Analyses: Examination of Underpowered Studies in Cochrane Reviews. PLoS ONE 2013, 8, e59202. [Google Scholar] [CrossRef]






| Search | Query |
|---|---|
| #1 | minocycline[MeSH Terms] |
| #2 | minocycline[Title/Abstract] |
| #3 | #1 OR #2 |
| #4 | neurology [MeSH terms] OR Child Development Disorders, Pervasive [MeSH terms] OR dementia [MeSH terms] OR amyotrophic lateral sclerosis [MeSH terms] OR multiple sclerosis [MeSH terms] OR neurodegenerative diseases [MeSH terms] OR migraine disorders [MeSH terms] OR headache disorders [MeSH terms] OR brain injury, traumatic [MeSH terms] OR cerebrovascular disorders [MeSH terms] OR epilepsy [MeSH terms] OR seizures [MeSH terms] OR Ischemia [MeSH terms] OR psychiatry [MeSH terms] OR mental disorders [MeSH terms] OR anxiety [MeSH terms] OR Disruptive, Impulse Control, and Conduct Disorders [MeSH terms] OR nail biting [MeSH terms] OR cannabis [MeSH terms] OR benzodiazepines [MeSH terms] OR nicotine [MeSH terms] OR analgesics, opioid [MeSH terms] OR cocaine [MeSH terms] OR heroin [MeSH terms] OR methamphetamine [MeSH terms] OR amphetamine [MeSH terms] OR methylphenidate [MeSH terms] OR substance-related disorders [MeSH terms] |
| #5 | (((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((((“neurological disorder”[Title/Abstract]) OR (neurology[Title/Abstract])) OR (autism[Title/Abstract])) OR (“autistic disorder”[Title/Abstract])) OR (ASD[Title/Abstract])) OR (Asperger’s[Title/Abstract])) OR (“Asperger Syndrome”[Title/Abstract])) OR (“pervasive developmental disorder”[Title/Abstract])) OR (“Alzheimer’s disease”[Title/Abstract])) OR (dementia[Title/Abstract])) OR (“amyotrophic lateral sclerosis”[Title/Abstract])) OR (ALS[Title/Abstract])) OR (seizures[Title/Abstract])) OR (epilepsy[Title/Abstract])) OR (“traumatic brain injury”[Title/Abstract])) OR (TB[Title/Abstract])) OR (stroke[Title/Abstract])) OR (ischemia[Title/Abstract])) OR (ischaemia[Title/Abstract])) OR (haemorrhage[Title/Abstract])) OR (neuropathy[Title/Abstract])) OR (“peripheral neuropathy”[Title/Abstract])) OR (neurodegenerative[Title/Abstract])) OR (“Parkinson disease”[Title/Abstract])) OR (“Huntington disease”[Title/Abstract])) OR (“brain injury”[Title/Abstract])) OR (“multiple sclerosis”[Title/Abstract])) OR (“spinal cord injury”[Title/Abstract])) OR (migraine[Title/Abstract])) OR (headache[Title/Abstract])) OR (“psychiatric disorder”[Title/Abstract])) OR (“mental illness”[Title/Abstract])) OR (“mental disorders”[Title/Abstract])) OR (anxiety[Title/Abstract])) OR (addiction[Title/Abstract])) OR (“major depressive disorder”[Title/Abstract])) OR (“major depression”[Title/Abstract])) OR (“depressive disorder”[Title/Abstract])) OR (MDD[Title/Abstract])) OR (“bipolar I disorder”[Title/Abstract])) OR (“bipolar II disorder”[Title/Abstract])) OR (“bipolar disorder”[Title/Abstract])) OR (“bipolar depression”[Title/Abstract])) OR (mania[Title/Abstract])) OR (“manic disorder”[Title/Abstract])) OR (“manic state”[Title/Abstract])) OR (hypo-mania[Title/Abstract])) OR (psychosis[Title/Abstract])) OR (“psychotic disorders”[Title/Abstract])) OR (schizophrenia[Title/Abstract])) OR (“schizoaffective disorder”[Title/Abstract])) OR (“panic disorder”[Title/Abstract])) OR (“social anxiety disorder”[Title/Abstract])) OR (PTSD[Title/Abstract])) OR (“posttraumatic stress disorder”[Title/Abstract])) OR (“generalised anxiety disorder”[Title/Abstract])) OR (OCD[Title/Abstract])) OR (“personality disorder”[Title/Abstract])) OR (“obsessive compulsive disorder”[Title/Abstract])) OR (“obsessive-compulsive neurosis”[Title/Abstract])) OR (“obsessive-compulsive neuroses”[Title/Abstract])) OR (“attention deficit hyperactivity disorder”[Title/Abstract])) OR (ADHD[Title/Abstract])) OR (trauma[Title/Abstract])) OR (“stress disorders”[Title/Abstract])) OR (“post traumatic”[Title/Abstract])) OR (“feeding disorder”[Title/Abstract])) OR (“appetite disorder”[Title/Abstract])) OR (disruptive, impulse control, conduct disorders[Title/Abstract])) OR (“eating disorder”[Title/Abstract])) OR (“binge eating disorder”[Title/Abstract])) OR (“bulimia nervosa”[Title/Abstract])) OR (“anorexia nervosa”[Title/Abstract])) OR “other specified feeding and eating disorder”[Title/Abstract])) OR (methylphenidate[Title/Abstract])) OR (amphetamine[Title/Abstract])) OR (methamphetamine[Title/Abstract])) OR (cocaine[Title/Abstract])) OR (cannabis[Title/Abstract])) OR (marijuana[Title/Abstract])) OR (heroin[Title/Abstract])) OR (“prescription pills”[Title/Abstract])) OR (opioids[Title/Abstract])) OR (benzodiazepine[Title/Abstract])) OR (nicotine[Title/Abstract])) OR (tobacco[Title/Abstract])) OR (“pathological gambling”[Title/Abstract])) OR (“skin picking”[Title/Abstract])) OR (“impulse control disorder”[Title/Abstract])) OR (kleptomania[Title/Abstract]) |
| #6 | #4 OR #5 |
| #7 | (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti] |
| #8 | animals [mh] NOT humans [mh] |
| #9 | #7 NOT #8 |
| #10 | #3 AND #6 AND #9 |
| #11 | #3 AND #6 AND #9 Filters: English; Humans |
| Author | Country | Disease | N Minocycline | N Control Group | Control Group (Placebo) | Primary Outcome | Treatment | Treatment Length | Conclusion | RoB | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chaundhry 2012 [37] | Pakistan and Brazil | SZ—within 5 years of onset | 71 | 73 | Y | PANSS | 50 mg increments until 200 mg/daily | 12 months | The addition of minocycline to TAU early in the illness course of schizophrenia improves negative symptoms without a detectable effect on cognition. | Some concerns |
| 2 | Liu 2014 [38] | China | SZ—early stage | 39 | 40 | Y | SANS/PANSS | 200 mg/daily | 16 weeks | The addition of minocycline to atypical antipsychotic drugs in early schizophrenia had significant efficacy on negative symptoms but had a slight effect on the attention domains of patients with schizophrenia. | High Risk |
| 3 | Liu 2018 [39] | China | SZ | 27 | 28 | Y | SANS/PANSS | 200 mg/daily | 16 weeks | 16-week adjunctive minocycline treatment significantly improved schizophrenia symptoms, in particular the negative symptoms. | High Risk |
| 4 | Levkovitz 2010 [40] | Israel | SZ | 13 | 8 | Y | SANS/PANSS | 200 mg/daily | 24 weeks—2 weeks single-blind placebo lead-in phase | Minocycline treatment was associated with improvement in negative symptoms and executive function, both related to frontal lobe activity. Supports benefits of minocycline for schizophrenia. | High Risk |
| 5 | Khodaie-Ardakani 2014 [41] | Iran | SZ—with a duration of >2 years | 20 | 18 | Y | PANSS | 100 mg/daily for 1 week then increase to 200 mg/daily | 8 weeks | Minocycline seems to be beneficial and tolerable as a short-term add-on to risperidone for the treatment of negative and general psychopathology symptoms of schizophrenia. | Some concerns |
| 6 | Kelly 2015 [42] | USA | SZ | 28 | 23 | Y | BPRS/SANS | 100 mg/daily increase to 200 mg/daily | 13 weeks: 3 weeks screening and stabilization + 10 weeks treatment | Minocycline’s effect on the MCCB composite score and positive symptoms was not statistically significant. Significant improvements with minocycline were seen in working memory, avolition, and anxiety/depressive symptoms in a chronic population with persistent symptoms. | Low risk |
| 7 | Zhang 2018 [43] | China | SZ | 25 LD and 25 HD | 25 | Y | PANSS/SANS | 100 mg/daily = low dose; 200 mg/daily = high dose | 3 months | The addition of high-dose minocycline to risperidone reduced the negative symptoms of patients with schizophrenia. The improvement in negative symptoms correlated with the reduction in serum levels of pro-inflammatory cytokines. Participants receiving high-dose minocycline had a greater reduction in SANS and PANSS negative subscale scores than those receiving low-dose minocycline or placebo. | High risk |
| 8 | Deakin 2018 [44] | UK | SZ—first episode | 103 | 104 | Y | PANSS | 200 mg/daily for 2 weeks, then 300 mg/daily for the remainder of the 12-month period | 12 months | 12 months’ treatment with minocycline does not improve the symptomatic or functional status of people within 5 years of a diagnosis of schizophrenia. | Low risk |
| 9 | Ghoreishi 2020 [45] | Iran | SZ—<10 years diagnosis | 14 | 16 | Y | PANSS | 200 mg/daily | 8 weeks | Minocycline could correct positive and negative symptoms of schizophrenia. | High risk |
| 10 | Weiser 2018 [46] | Romania and the Republic of Moldova | SZ | 100 | 100 | Y | PANSS | 200 mg/daily | 16 weeks | Minocycline was not efficacious in treating negative symptoms or cognition. | High risk |
| 11 | Dean 2017 [47] | Australia and Thailand | MDD | 36 | 35 | Y | MADRS | 200 mg/daily | 16 weeks of treatment with a follow-up visit at week 16. | The four-point difference between minocycline and placebo in the MADRS scores (primary outcome measure) was not significant, this is on par with the magnitude of change seen with conventional antidepressants. There were significant improvements in several important outcomes including global impression, functioning, and quality of life. | Low risk |
| 12 | Husain 2017 [48] | Pakistan | MDD | 21 | 20 | Y | HAMD-16 | 100 mg/daily for 2 weeks then 200 mg/daily for 10 weeks | 12 weeks | Minocycline has a potential role as an augmentation strategy in patients with treatment-resistant depression. | Some concerns |
| 13 | Nettis 2021 [49] | England | MDD | 18 | 21 | Y | HAMD-17 | 200 mg/daily | 4 weeks | Participants selected for elevated C-reactive protein (≥1 mg/L) and found no clear difference between minocycline and placebo in improving depressive symptoms at week 4. | Some concerns |
| 14 | Husain 2020 [50] | Pakistan | BD I and II on the current MD episode | 66 | 66 | Y, with extra arms for celecoxib and Mino + Calecoxib | HAMD-24 | 100 mg/daily for 2 weeks then 200 mg/daily | 12 weeks | Neither minocycline nor celecoxib were associated with an antidepressant effect for bipolar depression. | Low risk |
| 15 | Savitz 2018 [51] | USA | BD I, II and NOS | 19 | 20 | Y, with extra arms for Apirin and Mino + Aspirin | MADRS | 200 mg/daily | 6 weeks | No significant main effect of aspirin or minocycline on the mean change in MADRS score across visits. Participants receiving minocycline plus aspirin are twice as likely to show sustained response (OR = 2.93). | High risk |
| 16 | Arout 2019 [52] | USA | Opioid dependence | 10 | 10 | Y | CTP and BPI-SF | 200 mg/daily | 15 days | Neither pain threshold nor tolerance for pain in the CPT was affected by minocycline treatment. There was no evidence that minocycline had an effect on BPI, SOWS, POMS, or McGill measures assessed in the lab, or pain, craving, and opioid withdrawal assessed during EMA. | High risk |
| 17 | Sofuoglu 2009 [53] | USA | Smoking | 12 | - | Y | Choice procedure | 200 mg/daily | 4 days | There was no treatment effect on smoking self-administration. No differences between minocycline and placebo on most of the measures of behavioral, biochemical, physiological, subjective, and cognitive domains. | High risk |
| 18 | Esalatmanesh 2016 [54] | Iran | OCD | 47 | 47 | Y | Y-BOCS | 200 mg/daily | 10 weeks | Minocycline significantly reduced OCD symptoms as an adjuvant agent to fluvoxamine in moderate-to-severe OCD patients compared to a placebo. | High risk |
| 19 | Lampl 2007 [55] | Israel | Acute ischemic stroke | 74 | 77 | Y | NIHSS/BI/mRS | 200 mg/daily | 5 days | Patients with acute stroke had a significantly better outcome with minocycline treatment compared with a placebo in all outcomes. | High risk |
| 20 | PadmaSrivatava 2012 [56] | India | Acute ischemic stroke | 23 | 27 | Vit B | NIHSS/BI/mRS | 200 mg/daily | 5 days | Minocycline was observed to be beneficial in patients with acute ischemic stroke and was associated with better clinical and functional outcomes. | High risk |
| 21 | Kohler 2013 [57] | Australia | Acute stroke (ischemic or hemorrhagic) | 47 | 48 | Routine care | NIHSS/mRS | 100 mg/daily | 12 hourly for 5 doses | In this pilot study of a small sample of acute stroke patients, intravenous minocycline was safe but not efficacious. The study was not powered to identify reliably or exclude a modest but clinically important treatment effect of minocycline. | High risk |
| 22 | Amiri-Nikpour 2015 [58] | Iran | Acute ischemic stroke | 26 | 27 | Routine care | NIHSS | 200 mg/daily | 5 days | Patients who received minocycline had significantly better neurological outcomes on day 90 than controls. However, female patients showed no significant clinical improvement compared with males. | High risk |
| 23 | Shamsaei 2017 [59] | Iran | Acute ischemic stroke | 18 | 18 | Y | NIHSS | 200 mg/daily | 5 days | Both groups improved significantly over time, but neither group demonstrated superiority at 90-day follow-ups. | High risk |
| 24 | Singh 2019 [60] | Singapore | Acute ischemic stroke | 69 | 70 | Y | NIHSS/BI/mRS | 200 mg/daily | 5 days | At day 90, no significant difference was noted in the proportion of subjects given minocycline for any of the outcomes. | Low risk |
| 25 | Fouda 2017 [61] | USA | Acute ICH | 8 | 8 | TAU | mRS | 400 mg/daily | 5 days | No significant differences between groups reported at day 90. | High risk |
| 26 | Koulaeinejad 2019 [62] | Iran | TBI (moderate to severe) | 14 | 20 | GCS | 200 mg/daily | 7 days | Conclude as a result of this pilot study that minocycline treatment was associated with improvement in neurological outcomes of acute TBI compared with a placebo, warranting further clinical trials. | High risk | |
| 27 | Casha 2012 [63] | Canada | SCI—acute traumatic | 27 | 25 | Y | ASIA | 200 mg/daily, increased to 800 mg | 7 days | In a randomized, double-blind manner, the treatment was associated with an apparent improvement in neurological and functional outcomes compared with a placebo, warranting further formal investigations of efficacy. | Some concerns |
| 28 | Pontieri 2005 [64] | Italy | ALS | 10 | 10 | TAU | ALS-FRS | 100 mg/daily | 6 months | The study was not powered to assess the efficacy of treatment, thus, the lack of effect of combined riluzole/minocycline treatment on ALS-FRS score or pulmonary score is not negative for efficacy, but inconclusive. | High risk |
| 29 | Howard 2020 [65] | UK | AD (SMMSE > 23) | HD: 184; LD: 181 | 179 | Y | sMMSE and BADLS | 400 md or 200 mg/daily | 24 months | Minocycline did not delay the progress of cognitive or functional impairment in people with mild AD during a 2-year period. This study also found that 400 mg minocycline is poorly tolerated in this population. | Low risk |
| 30 | Dodel 2010 [66] | Germany and Austria | MSA—Parkinsonian symptoms | 32 | 31 | Y | UMSARS/UPDRSII | 100 mg/daily | 48 weeks | No beneficial effect of minocycline, either symptomatic or neuroprotective. Treatment with minocycline failed to improve global ratings of motor function, such as the UMSARSII (primary endpoint) and UPDRSIII (secondary endpoint) over the 48-week observation period. | High risk |
| 31 | Tilley 1995 [67] | USA | Rheumatoid Arthritis | 109 | 110 | Y | MHAQ | 200 mg/daily | 48 weeks | The MIRA trial showed that minocycline is both effective and safe for treating patients with mild to moderately active rheumatoid arthritis. Benefit became evident after 12 weeks of therapy, and the proportion of patients treated with minocycline showing improvement continued to increase through week 48 of the study. | Low risk |
| 32 | Curtin 2017 [68] | USA | Carpal tunnel syndrome (pain) | 66 | 65 | Cross-over | The Brief Pain Inventory | 200 mg 2 h prior to procedure, then 100 mg twice a day (200 mg/day) for 5 days | 5 days | This is a negative study with the principal result failing to identify a clinically meaningful overall change in TPR following treatment with minocycline (used at common antibiotic doses) during the perioperative period surrounding CTR surgery and TFR surgery. | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panizzutti, B.; Skvarc, D.; Lin, S.; Croce, S.; Meehan, A.; Bortolasci, C.C.; Marx, W.; Walker, A.J.; Hasebe, K.; Kavanagh, B.E.; et al. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5250. https://doi.org/10.3390/ijms24065250
Panizzutti B, Skvarc D, Lin S, Croce S, Meehan A, Bortolasci CC, Marx W, Walker AJ, Hasebe K, Kavanagh BE, et al. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(6):5250. https://doi.org/10.3390/ijms24065250
Chicago/Turabian StylePanizzutti, Bruna, David Skvarc, Sylvia Lin, Sarah Croce, Alcy Meehan, Chiara Cristina Bortolasci, Wolfgang Marx, Adam J. Walker, Kyoko Hasebe, Bianca E. Kavanagh, and et al. 2023. "Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 6: 5250. https://doi.org/10.3390/ijms24065250







