The Role of Light-Regulated Auxin Signaling in Root Development
Abstract
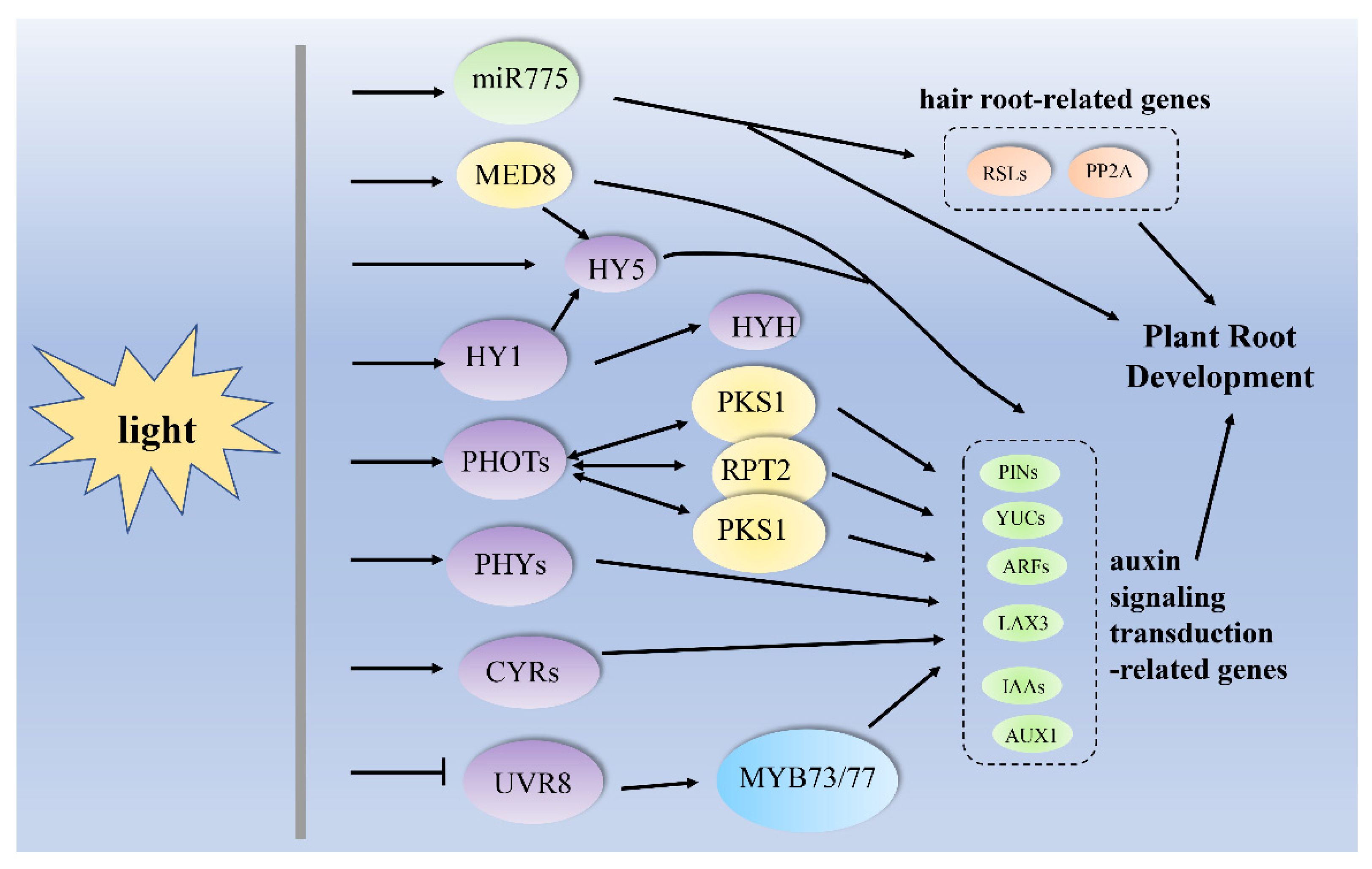
:1. Introduction
2. Light Signaling in Root Development
2.1. Light Perception
2.2. Photoreceptors Involved in Root Development
2.3. Key Components in Response to Light in Root Development
| Photoreceptors | Genes | Response to Light | Species | Function | References |
|---|---|---|---|---|---|
| phyA | - | far red (FR) | Arabidopsis thaliana | root hair formation | [24] |
| phyA and phyB | - | red light (RL) | A. thaliana | root hair formation | [24] |
| phyA and phyB | - | - | A. thaliana | root elongation and irregular root hair formation | [20] |
| phyA, phyB, and phyE | - | - | A. thaliana | lateral root formation | [28] |
| phyD | - | - | A. thaliana | lateral root formation | [25] |
| PhyA, PhyB1, and PhyB2 | NaPhyA, NaPhyB1, and NaPhyB2 | - | Nicotiana attenuata | shoot-root development | [26] |
| phyA and phyB | - | - | A. thaliana | lateral root formation | [25] |
| PhyB | - | - | Lotus japonicus | root nodule formation | [27] |
| PhyB | - | - | A. thaliana | root growth | [28] |
| PhyB | IAA14, ARF7 and ARF19 | A. thaliana | adventitious root formation | [29] | |
| CRY1 and CRY2 | - | blue light (BL) | A. thaliana | primary root elongation | [30] |
| CRY1 and CRY2 | - | white light | A. thaliana | primary root elongation | [34] |
| CRY1 and CRY2 | - | white light | Solanum lycopersicum L. | primary root elongation | [35] |
| CRY1 | - | BL | A. thaliana | lateral root formation | [36] |
| CRY1 | - | BL | Glycine max L. Merr. | root nodulation | [37] |
| PHOT1 | - | BL | A. thaliana | root phototropism | [40] |
| PHOT1 and PHOT2 | RPT2 and JAC1 | BL | A. thaliana | hypocotyl phototropism | [4] |
| UVR8 | - | low-fluence UV-B | A. thaliana | hypocotyl development | [44] |
| UVR8 | MYB73/MYB77 | UV-B-dependent manner | A. thaliana | lateral root development and hypocotyl elongation | [44] |
| PIF3 | - | white light | A. thaliana | primary root development | [46] |
| PIF4 | - | - | A. thaliana | primary root growth | [47] |
| PIF5 | - | BL | A. thaliana | hypocotyl elongation | [48] |
| PIF1 | HB1 | - | A. thaliana | hypocotyl elongation | [50] |
| PIF1, PIF2, PIF3, PIF4 and PIF5 | - | darkness | A. thaliana | adventitious root formation | [56] |
| COP1 | - | darkness | A. thaliana | primary root length | [55] |
| HY5 | HY5 and NRT2.1 | - | A. thaliana | root growth | [57] |
| HY5 | HY5 | white light | A. thaliana | root growth | [56] |
3. Light Regulates Root Growth and Development via the Auxin-Signaling Transduction Pathway
3.1. Primary Root, Root Hair and Growth and Development
3.2. Lateral Root and Adventitious Root Growth and Development
3.3. Rhizoid, Seminal and Crown Root Development
| Light Treatment | Genes/Proteins | Species | Function | References |
|---|---|---|---|---|
| darkness to light | miR775, PIN1, PIN2, AUXR1, YUC1 and YUC4, HY5 | Arabidopsis thaliana, (A. thaliana) | primary root growth | [58] |
| light | MED18 | A. thaliana | primary root elongation | [59] |
| direct light | PIN2 | A. thaliana | root hair formation | [60] |
| light | miR775, RSL2, RSL4, PP2A, HY5, PIN1, PIN2, AUXR1, YUC1 and YUC4 | A. thaliana | root hair formation | [58] |
| red light (RL) | PIN3 | A. thaliana | lateral root development | [62] |
| blue light (BL) | PIN1, PIN3 and PIN4 | A. thaliana | lateral root development | [62] |
| white light with FR light | - | A. thaliana | decreased lateral root density | [10] |
| far red (FR) light | HY5, ARF19, PIN3 and LAX3 | A. thaliana | lateral root development | [10] |
| UV-B | HAT2, SUAR23, MYB73/MYB77, UVR8, IAA29, SAUR28, SAUR68, SAUR-like and SAUR-like-3 | A. thaliana | lateral root growth | [16] |
| BL | PIN3, PHOT1 and PHOT2 | A. thaliana | adventitious root formation | [63] |
| - | CRYs | Physcomitrella patens | rhizoid development | [67] |
| white light | PHYA and PHYB | Oryza sativa L. | seminal root development | [68] |
| BL | CRY1, PHOT2, PIN3 | A. thaliana | root negative phototropism | [45] |
| BL | PHOT1, PIN1 and PIN2 | A. thaliana | root negative phototropism | [46] |
| BL | PIN2 | A. thaliana | root negative phototropism | [70] |
| white light | ZM2G141383 | Zea mays | root gravitropism | [71] |
| - | HY5, GLK2, IAA14, ARF7 and ARF19 | A. thaliana | root greening | [72] |
| - | HY1, HY5, HYH, AUX1, PIN1, PIN2, PIN3 and PIN7, | A. thaliana | lateral root branching | [73] |
| - | HY5, AXR2/IAA7 and SLR/IAA14 | A. thaliana | lateral root branching | [74] |
4. Light-Regulated Tropic Movement, Root Greening and Root Branching through Auxin Signaling
4.1. Root Negative Phototropism
4.2. Gravitropism/U-Turn Formation at Root Apex
4.3. Root Greening and Root Branching
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, J.; Sheng, Y. Phototropin2-mediated hypocotyl phototropism is negatively regulated by JAC1 and RPT2 in Arabidopsis. Plant Physiol. Biochem. 2021, 164, 289–298. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Arena, C.; Pesce, G. The role of light quality of photoperiodic lighting on photosynthesis, flowering and metabolic profiling in Ranunculus asiaticus L. Physiol. Plant 2020, 170, 187–201. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, J.; Wang, R.; Wang, L.; Zhang, C.; Xu, W.; Wang, S.; Jiu, S. The Role of Strigolactones in the Regulation of Root System Architecture in Grapevine (Vitis vinifera L.) in Response to Root-Restriction Cultivation. Int. J. Mol. Sci. 2021, 22, 8799. [Google Scholar] [CrossRef]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.; Fragoso, V.; Shin, K.; et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef] [PubMed]
- van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell. 2018, 30, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, L.; Chen, P.; Liang, T.; Li, X.; Liu, H. UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 2020, 39, e101928. [Google Scholar] [CrossRef] [PubMed]
- van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yan, X.; Xu, M.; Qi, W.; Shi, C.; Li, X.; Ma, J.; Tian, D.; Shou, J.; Wu, H.; et al. Transmembrane kinase 1-mediated auxin signal regulates membrane-associated clathrin in Arabidopsis roots. J. Integr. Plant Biol. 2023, 65, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Halliday, K.J.; Martínez-García, J.F.; Josse, E.M. Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, H. Coordinated Shoot and Root Responses to Light Signaling in Arabidopsis. Plant Commun. 2020, 1, 100026. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Rai, N.; O’Hara, A.; Farkas, D.; Safronov, O.; Ratanasopa, K.; Wang, F.; Lindfors, A.V.; Jenkins, G.I.; Lehto, T.; Salojärvi, J.; et al. The photoreceptor UVR8 mediates the perception of both UV-B and UV-A wavelengths up to 350 nm of sunlight with responsivity moderated by cryptochromes. Plant Cell Environ. 2020, 43, 1513–1527. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Shin, D.H.; Cho, M.H.; Kim, T.L.; Yoo, J.; Kim, J.I.; Han, Y.J.; Song, P.S.; Jeon, J.S.; Bhoo, S.H.; Hahn, T.R. A small GTPase activator protein interacts with cytoplasmic phytochromes in regulating root development. J. Biol. Chem. 2010, 285, 32151–32159. [Google Scholar] [CrossRef] [Green Version]
- Schafer, E.; Bowle, C. Phytochrome-mediated photoperception and signal transduction in higher plants. EMBO Rep. 2002, 3, 1042–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sineshchekov, V.A. Phytochrome A: Functional diversity and polymorphism. Photochem. Photobiol. Sci. 2004, 3, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z.; Mullen, J.L.; Correll, M.J.; Hangarter, R.P. Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 2003, 131, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Warnasooriya, S.N.; Montgomery, B.L. Spatial-specific regulation of root development by phytochromes in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 2047–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef]
- Oh, Y.; Fragoso, V.; Guzzonato, F.; Kim, S.G.; Park, C.M.; Baldwin, I.T. Root-expressed phytochromes B1 and B2, but not PhyA and Cry2, regulate shoot growth in nature. Plant Cell Environ. 2018, 41, 2577–2588. [Google Scholar] [CrossRef]
- Shigeyama, T.; Tominaga, A.; Arima, S.; Sakai, T.; Inada, S.; Jikumaru, Y.; Kamiya, Y.; Uchiumi, T.; Abe, M.; Hashiguchi, M.; et al. Additional cause for reduced JA-Ile in the root of a Lotus japonicus phyB mutant. Plant Signal. Behav. 2012, 7, 746–748. [Google Scholar] [CrossRef] [Green Version]
- Gil, K.E.; Ha, J.H.; Park, C.M. Abscisic acid-mediated phytochrome B signaling promotes primary root growth in Arabidopsis. Plant Signal. Behav. 2018, 13, e1473684. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, Z.; Wang, Y.L.; Zhong, Y.; Chao, Z.F.; Gao, Y.Q.; Han, M.L.; Xu, L.; Chao, D.Y. Phytochrome B inhibits darkness-induced hypocotyl adventitious root formation by stabilizing IAA14 and suppressing ARF7 and ARF19. Plant J. 2021, 105, 1689–1702. [Google Scholar] [CrossRef]
- Canamero, R.C.; Bakrim, N.; Bouly, J.P.; Garay, A.; Dudkin, E.E.; Habricot, Y.; Ahmad, M. Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 2006, 224, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida-Mayama, T.; Saka, T.; Hanada, A.; Uehara, Y.; Asami, T.; Yamaguchi, S. Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 2010, 62, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantini, E.; Sulli, M.; Zhang, L.; Aprea, G.; Jiménez-Gómez, J.M.; Bendahmane, A.; Perrotta, G.; Giuliano, G.; Facella, P. Pivotal Roles of Cryptochromes 1a and 2 in Tomato Development and Physiology. Plant Physiol. 2019, 179, 732–748. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, J.; Matsushita, T.; Watanabe, Y. DWARF4 accumulation in root tips is enhanced via blue light perception by cryptochromes. Plant Cell Environ. 2019, 42, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, Q.; Lin, J.; Deng, K.; Zhao, X.; Tang, D.; Liu, X. Arabidopsis cryptochrome-1 restrains lateral roots growth by inhibiting auxin transport. J. Plant Physiol. 2010, 167, 670–673. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, R.; Lyu, X.; Chen, J.; Zhang, X.; Wang, Z.; Deng, Z.; Wang, Y.; Wang, H.; Li, R.; et al. Differential light-dependent regulation of soybean nodulation by papilionoid-specific HY5 homologs. Curr. Biol. 2022, 32, 783–795.e5. [Google Scholar] [CrossRef]
- Fankhauser, C.; Christie, J.M. Plant phototropic growth. Curr. Biol. 2015, 25, R384–R389. [Google Scholar] [CrossRef] [Green Version]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 2001, 98, 6969–6974. [Google Scholar] [CrossRef] [Green Version]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant flavoprotein photoreceptors. Plant Cell Physiol. 2015, 56, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Lariguet, P.; Fankhauser, C. Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J. 2004, 40, 826–834. [Google Scholar] [CrossRef]
- Liang, T.; Yang, Y.; Liu, H. Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol. 2019, 221, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhu, Z. UV-B Response: When UVR8 Meets MYBs. Trends Plant Sci. 2020, 25, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Fasano, R.; Gonzalez, N.; Tosco, A.; Dal Piaz, F.; Docimo, T.; Serrano, R.; Grillo, S.; Leone, A.; Inzé, D. Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol. Plant 2014, 7, 773–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mayba, O.; Pfeiffer, A.; Shi, H.; Tepperman, J.M.; Speed, T.P.; Quail, P.H. A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 2013, 9, e1003244. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Yao, T.; Li, M.; Guo, X.; Zhang, Y.; Zhu, S.; He, Y. PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol. Plant 2014, 7, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Gao, S.; Tian, H.; Wu, W.; Robert, H.S.; Ding, Z. Local Transcriptional Control of YUCCA Regulates Auxin Promoted Root-Growth Inhibition in Response to Aluminium Stress in Arabidopsis. PLoS Genet. 2016, 12, e1006360. [Google Scholar] [CrossRef] [Green Version]
- Soy, J.; Leivar, P.; Monte, E. PIF1 promotes phytochrome-regulated growth under photoperiodic conditions in Arabidopsis together with PIF3, PIF4, and PIF5. J. Exp. Bot. 2014, 65, 2925–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunihiro, A.; Yamashino, T.; Mizuno, T. PHYTOCHROME-INTERACTING FACTORS PIF4 and PIF5 are implicated in the regulation of hypocotyl elongation in response to blue light in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2010, 74, 2538–2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capella, M.; Ribone, P.A.; Arce, A.L.; Chan, R.L. Arabidopsis thaliana HomeoBox 1 (AtHB1), a Homedomain-Leucine Zipper I (HD-Zip I) transcription factor, is regulated by PHYTOCHROME-INTERACTING FACTOR 1 to promote hypocotyl elongation. New Phytol. 2015, 207, 669–682. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhang, Z.; Zhang, C.X.; Wang, Y.L.; Liu, C.B.; Wu, J.C.; Han, M.L.; Wang, Q.X.; Chao, D.Y. Phytochrome-interacting factors orchestrate hypocotyl adventitious root initiation in Arabidopsis. Development 2022, 149, dev200362. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Liu, R.; Hao, H.; Wang, Z.; Bi, Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 2011, 168, 1771–1779. [Google Scholar] [CrossRef]
- Bours, R.; Kohlen, W.; Bouwmeester, H.J.; van der Krol, A. Thermoperiodic control of hypocotyl elongation depends on auxin-induced ethylene signaling that controls downstream PHYTOCHROME INTERACTING FACTOR3 activity. Plant Physiol. 2015, 167, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ouyang, X.; Deng, X.W. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 2014, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, C.; Xu, H.; Shi, X.; Zhen, W.; Hu, Z.; Huang, J.; Zheng, Y.; Huang, P.; Zhang, K.X.; et al. HY5 Contributes to Light-Regulated Root System Architecture under a Root-Covered Culture System. Front. Plant Sci. 2019, 10, 1490. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddam, S.R.; Bhatia, C.; Sharma, A.; Badola, P.K.; Saxena, G.; Trivedi, P.K. miR775 integrates light, sucrose and auxin associated pathways to regulate root growth in Arabidopsis thaliana. Plant Sci. 2021, 313, 111073. [Google Scholar] [CrossRef]
- Raya-González, J.; Oropeza-Aburto, A.; López-Bucio, J.S.; Guevara-García, Á.A.; de Veylder, L.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR18 influences Arabidopsis root architecture, represses auxin signaling and is a critical factor for cell viability in root meristems. Plant J. 2018, 96, 895–909. [Google Scholar] [CrossRef] [Green Version]
- García-González, J.; Lacek, J.; Retzer, K. Dissecting Hierarchies between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth. Plants 2021, 10, 111. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. The plant hormone auxin beats the time for oscillating light-regulated lateral root induction. Development 2018, 145, dev169839. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Wenjing, S.; Shangjun, L.; Jianxin, D.; Yali, Z.; Chengdong, W.; Xu, Y.; Wang, S. Light Quality Regulates Lateral Root Development in Tobacco Seedlings by Shifting Auxin Distributions. J. Plant Growth Regul. 2015, 34, 574–583. [Google Scholar] [CrossRef]
- Zhai, S.; Cai, W.; Xiang, Z.X.; Chen, C.Y.; Lu, Y.T.; Yuan, T.T. PIN3-mediated auxin transport contributes to blue light-induced adventitious root formation in Arabidopsis. Plant Sci. 2021, 312, 111044. [Google Scholar] [CrossRef] [PubMed]
- Fett-Neto, A.G.; Fett, J.P.; Veira Goulart, L.W.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Jang, G.; Yi, K.; Pires, N.D.; Menand, B.; Dolan, L. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 2011, 138, 2273–2281. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, T.; Kadota, A.; Hasebe, M.; Wada, M. Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell. 2002, 14, 373–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakibara, K.; Nishiyama, T.; Sumikawa, N.; Kofuji, R.; Murata, T.; Hasebe, M. Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 2003, 130, 4835–4846. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Tanabata, T.; Xie, X.; Inagaki, N.; Takano, M.; Shinomura, T.; Yamamoto, K.T. Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol. Plant 2009, 137, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Ho, C.H.; Chen, H.W. Rice develop wavy seminal roots in response to light stimulus. Plant Cell Rep. 2011, 30, 1747–1758. [Google Scholar] [CrossRef]
- Wan, Y.; Jasik, J.; Wang, L.; Hao, H.; Volkmann, D.; Menzel, D.; Mancuso, S.; Baluška, F.; Lin, J. The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell. 2012, 24, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Yokawa, K.; Nakano, S.; Yoshida, Y.; Fabrissin, I.; Okamoto, T.; Baluška, F.; Koshiba, T. Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex. J. Exp. Bot. 2016, 67, 4581–4591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M.; Aro, E.M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell. 2012, 24, 1081–1095. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Xu, S.; Xie, Y.; Li, L.; Qi, W.; Parizot, B.; Zhang, Y.; Chen, T.; Han, Y.; Van Breusegem, F.; et al. Periodic root branching is influenced by light through an HY1-HY5-auxin pathway. Curr. Biol. 2021, 31, 3834–3847.e5. [Google Scholar] [CrossRef]
- Cluis, C.P.; Mouchel, C.F.; Hardtke, C.S. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 2004, 38, 332–347. [Google Scholar] [CrossRef]
- Zhang, K.X.; Xu, H.H.; Yuan, T.T.; Zhang, L.; Lu, Y.T. Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 2013, 76, 308–321. [Google Scholar]
- Inada, S.; Ohgishi, M.; Mayama, T.; Okada, K.; Sakai, T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell. 2004, 16, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lariguet, P.; Schepens, I.; Hodgson, D.; Pedmale, U.V.; Trevisan, M.; Kami, C.; de Carbonnel, M.; Alonso, J.M.; Ecker, J.R.; Liscum, E.; et al. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 2006, 103, 10134–10139. [Google Scholar] [CrossRef] [Green Version]
- Boccalandro, H.E.; De Simone, S.N.; Bergmann-Honsberger, A.; Schepens, I.; Fankhauser, C.; Casal, J.J. PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol. 2008, 146, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Blakeslee, J.J.; Bandyopadhyay, A.; Peer, W.A.; Makam, S.N.; Murphy, A.S. Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiol. 2004, 134, 28–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.X.; Xu, H.H.; Gong, W.; Jin, Y.; Shi, Y.Y.; Yuan, T.T.; Li, J.; Lu, Y.T. Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PLoS ONE 2014, 9, e85720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The Role of Light-Regulated Auxin Signaling in Root Development. Int. J. Mol. Sci. 2023, 24, 5253. https://doi.org/10.3390/ijms24065253
Yun F, Liu H, Deng Y, Hou X, Liao W. The Role of Light-Regulated Auxin Signaling in Root Development. International Journal of Molecular Sciences. 2023; 24(6):5253. https://doi.org/10.3390/ijms24065253
Chicago/Turabian StyleYun, Fahong, Huwei Liu, Yuzheng Deng, Xuemei Hou, and Weibiao Liao. 2023. "The Role of Light-Regulated Auxin Signaling in Root Development" International Journal of Molecular Sciences 24, no. 6: 5253. https://doi.org/10.3390/ijms24065253
APA StyleYun, F., Liu, H., Deng, Y., Hou, X., & Liao, W. (2023). The Role of Light-Regulated Auxin Signaling in Root Development. International Journal of Molecular Sciences, 24(6), 5253. https://doi.org/10.3390/ijms24065253








