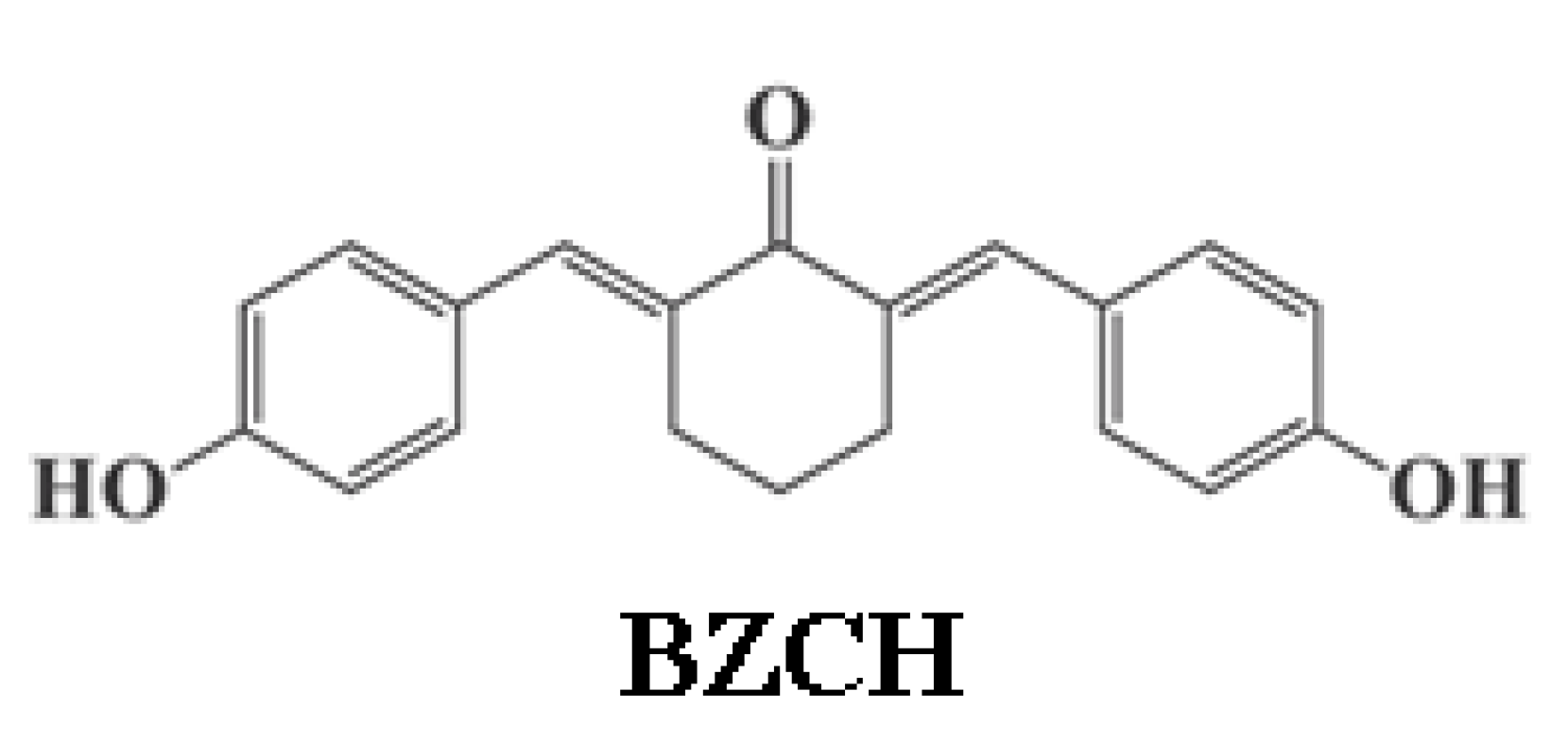
Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solvatochromic Analysis
2.2. Basichromism of 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone
2.3. Fluorescence Intensity Modulation by Addition of NaOH and Followed by UV Light Irradiation
2.4. Effects of UV Light Irradiation on the Optical Spectra
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corici, L.; Caschera, D.; Cseh, L.; De Luca, G.; Szerb, E.I.; Calandra, P. Amphiphiles as novel solvents for photochromics: Stability and photophysical properties. Mol. Cryst. Liq. Cryst. 2019, 684, 24–36. [Google Scholar] [CrossRef]
- Homocianu, M. Optical properties of solute molecules: Environmental effects, challenges, and their practical implications. Microchem. J. 2021, 161, 105797. [Google Scholar] [CrossRef]
- Asaoka, M.; Shima, K.; Takei, H. (R)-and (S)-5-trimethylsilyl-2-cyclohexenone: A versatile chiral source for the synthesis of optically active cyclohexanone derivatives. Tetrahedron Lett. 1987, 28, 5669–5672. [Google Scholar] [CrossRef]
- Mittal, A.; Devi, S.P.; Kakkar, R. A DFT study of the conformational and electronic properties of echinatin, a retrochalcone, and its anion in the gas phase and aqueous solution. Struct. Chem. 2020, 31, 2513–2524. [Google Scholar] [CrossRef]
- Kagatikar, S.; Sunil, D. Aggregation induced emission of chalcones. Chem. Pap. 2021, 75, 6147–6156. [Google Scholar] [CrossRef]
- Wangngae, S.; Chansaenpak, K.; Nootem, J.; Ngivprom, U.; Aryamueang, S.; Lai, R.-Y.; Kamkaew, A. Photophysical study and biological applications of synthetic chalcone-based fluorescent dyes. Molecules 2021, 26, 2979. [Google Scholar] [CrossRef] [PubMed]
- Serbezeanu, D.; Homocianu, M.; Macsim, A.M.; Enache, A.A.; Tăchiță, V.-B. Flexible thin films based on poly(ester imide) materials for optoelectronic applications. Polym. Int. 2022, 71, 98–106. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kim, S.; Yu, N.H.; Park, A.R. Antimicrobial activities of an oxygenated cyclohexanone derivative isolated from Amphirosellinia nigrospora JS-1675 against various plant pathogenic bacteria and fungi. J. Appl. Microbiol. 2019, 126, 894–904. [Google Scholar] [CrossRef]
- El-Zahar, M.I.; Abd El-Karim, S.S.; Anwar, M.M.; Danial, E.M. Synthesis, antimicrobial and antioxidant activities of some novel cyclized naphthyl cyclohexanone derivatives. Der Pharma Chem. 2010, 2, 118–134. [Google Scholar]
- Kocharov, S.L.; Panosyan, H.A.; Jaghatspanyan, I.A. Synthesis and anticonvulsant activity of some cyclohexanone derivatives–knoevenagel condensation products. Pharm. Chem. J. 2020, 54, 897–903. [Google Scholar] [CrossRef]
- Alotaibi, N.H.; Alharbi, K.S.; Alzarea, A.I.; Alruwaili, N.K.; Alotaibi, M.R.; Alotaibi, N.M.; Alotaibi, B.S.; Bukhari, S.N.A. Pharmacological appraisal of ligustrazine based cyclohexanone analogs as inhibitors of inflammatory markers. Eur. J. Pharm. Sci. 2020, 147, 105299. [Google Scholar] [CrossRef]
- Üstündağ, G.C.; Kara, E.M. Synthesis, characterization, antibacterial and antifungal evaluation of novel cyclohexanone benzoylhydrazones. Istanbul. J. Pharm. 2019, 49, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Thoume, A.; Elmakssoudi, A.; Left, D.B.; Achagar, R.; Irahal, I.N.; Dakir, M.; Azzi, M.; Zertoubi, M. Dibenzylidenecyclohexanone as a new corrosion inhibitor of carbon steel in 1 M HCl. J. Bio. Tribo. Corros. 2021, 7, 130. [Google Scholar] [CrossRef]
- Khan, J.; Sadia, M.; Shah, S.W.A.; Zahoor, M.; Alsharif, K.F.; Al-Joufi, F.A. Development of [(2E,6E)-2,6-bis(4-(dimethylamino)benzylidene)cyclohexanone] as fluorescence-on probe for Hg2+ ion detection: Computational aided experimental studies. Arab. J. Chem. 2022, 15, 103710. [Google Scholar] [CrossRef]
- Khan, J.; Sadia, M.; Shah, S.W.A.; Naz, R.; Ali, F. 2,6-bis(E)-4-methylbenzylidine)-cyclohexan-1-one as a fluorescent-on sensor for ultra selective detection of chromium ion in aqueous media. J. Fluoresc. 2021, 31, 1759–1770. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Corici, L.; Buta, I.; Cseh, L.; Moro, A.J.; Parola, A.J.; Lima, J.C.; Pina, F. Multistate of chemical species of 2,6-Bis(arylidene)cyclohexanones. On the role of chalcone and spiro species. Dyes Pigm. 2020, 174, 108013. [Google Scholar] [CrossRef]
- Xiang, D.; Meng, Q.; Liu, H.; Lan, M.; Wei, G. The study of a curcumin-resembling molecular probe for the pH-responsive fluorometric assay and application in cell imaging. Talanta 2016, 146, 851–856. [Google Scholar] [CrossRef]
- Badal, M.M.R.; Islam, H.M.A.; Maniruzzaman, M.; Yousuf, M.A. Acidochromic behavior of dibenzylidene cyclohexanone-based bischalcone: Experimental and theoretical study. ACS Omega 2020, 5, 22978–22983. [Google Scholar] [CrossRef]
- Homocianu, M.; Airinei, A. Intra-/inter-molecular interactions–Identification and evaluation by optical spectral data in solution. J. Mol. Liq. 2017, 225, 869–876. [Google Scholar] [CrossRef]
- Reichardt, C. Empirical parameters of solvent polarity and chemical reactivity. In Molecular Interactions; Ratajczak, H., Orville-Thomas, W.J., Eds.; Wiley: Chichester, UK, 1982; Volume 3. [Google Scholar]
- Byli´nska, I.; Wierzbicka, M.; Czaplewski, C.; Wiczk, W. Solvatochromic studies of pull–push molecules containing dimethylaniline and aromatic hydrocarbon linked by an acetylene unit. RSC Adv. 2014, 4, 48783. [Google Scholar] [CrossRef]
- Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Abdel Aziz, Y.M.; Soliman, S.M.; Barakat, A. Synthesis, structure combined with conformational analysis, biological activities and docking studies of bis benzylidene cyclohexanone derivatives. J. Saudi Chem. Soc. 2017, 21, 619–632. [Google Scholar] [CrossRef]
- Homocianu, M.; Serbezeanu, D.; Macsim, A.M.; Bubulac, T.V. From cyclohexanone to photosensitive polyesters: Synthetic pathway, basic characterization, and photo-/halochromic properties. J. Mol. Liq. 2020, 316, 113888. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, K.T.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalán, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- Laurence, C.; Legros, J.; Chantzis, A.; Planchat, A.; Jacquemin, D. A database of dispersion-induction DI, Electrostatic ES, and hydrogen bonding α1 and β1 solvent parameters and some applications to the multiparameter correlation analysis of solvent effects. J. Phys. Chem. B 2015, 119, 3174–3184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, H.; Jin, B.; Liu, X.; Fu, H.; Shangguan, D. A guanidine derivative of naphthalimide with excited-state deprotonation coupled intramolecular charge transfer properties and its application. J. Mat. Chem. C 2013, 1, 4427. [Google Scholar] [CrossRef]
- Aygün, E.N.; Çoşkun, M. Poly[4-pyridinyl-4 ’-(2-methacryloyloxyethoxy)styryl ketone-co-2-hydroxypropyl methacrylate]: Synthesis, characterization, thermal and electrical properties, and photocrosslinking behavior. El-Cezerî 2018, 5, 24–34. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Emelyanenko, A.V.; Liu, J.-H. Fabrication of resonance core assisted self-assembling gelators derived from cyclohexanone. J. Taiwan Inst. Chem. Eng. 2016, 65, 444–451. [Google Scholar] [CrossRef]
- Aly, K.I.; Abdel-Rahmana, M.A.; EI-Aal, A.; Gaber, M.; Qutai, M.M. Photoactive poly(hydroxyl-amino ether)s based on 4-methyl-cyclohexanone and 4-tert-butylcyclohexanone moieties in the main chain: Synthetic methodology and characterization. Assiut Univ. J. Multidiscip. Sci. Res. 2018, 47, 81–99. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Kathuria, I.; Kumar, S. The structural arrangement of the ligand-metal complex with centered zinc and nickel atoms and their optical features. J. Mol. Str. 2022, 1262, 133010. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Kumar, A.; Kumar, A.; Kumar, S. Synthesis and optical properties of copper(II) and nickel(II) complexes of a highly fluorescent morpholine-derivative. Polyhedron 2019, 171, 559–570. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Prakash, K.; Kumar, S. Synthesis of an oxadiazole through an indole mediated single step procedure for selective optical recognition of Cu2+ ions. Sens. Actuators B Chem. 2017, 242, 299–304. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Kumar, S. Photochromic spirooxazine as highly sensitive and selective probe for optical detection of Fe3+ in aqueous solution, Sens. Actuators B Chem. 2016, 226, 548–552. [Google Scholar] [CrossRef]







| No. | Solvents | λmax (nm) | ET a (exp) | α | β | π* | |
|---|---|---|---|---|---|---|---|
| 1. | Dioxane | 356 | 28,089.88 | 336.02 | 0.00 | 0.37 | 0.55 |
| 2. | Toluene | 351 | 28,490.02 | 340.81 | 0.00 | 0.11 | 0.54 |
| 3. | EtAc | 356 | 28,089.88 | 336.02 | 0.00 | 0.45 | 0.54 |
| 4. | THF | 358 | 27,932.96 | 334.14 | 0.00 | 0.55 | 0.58 |
| 5. | DCE | 354 | 28,248.58 | 337.92 | 0.00 | 0.00 | 0.807 |
| 6. | 1-Butanol | 246.5; 376 | 26,595.74 | 318.15 | 0.79 | 0.88 | 0.47 |
| 7. | 2-Propanol | 374 | 26,737.96 | 319.85 | 0.76 | 0.95 | 0.48 |
| 8. | 1-Propanol | 246; 374 | 26,737.96 | 319.85 | 0.78 | 0.85 | 0.52 |
| 9. | Methanol | 245; 369.5 | 27,063.59 | 323.74 | 0.93 | 0.62 | 0.60 |
| 10. | Acetone | 356.5 | 28,050.49 | 335.55 | 0.08 | 0.48 | 0.71 |
| 11. | DMF | 367.0 | 27,247.95 | 325.95 | 0.00 | 0.69 | 0.87 |
| 12. | ACN | 241; 354 | 28,248.58 | 337.92 | 0.19 | 0.31 | 0.75 |
| 13. | DMSO | 369 | 27,173.91 | 325.06 | 0.00 | 0.76 | 1.00 |
| 14. | NMP | 369 | 27,100.27 | 324.18 | 0.00 | 0.00 | 0.92 |
| 15. | DMAc | 368.5 | 28,089.88 | 324.18 | 0.00 | 0.76 | 0.88 |
| No. | Solvent | SA | SB | SP | SdP | DI | ES | α1 | β1 |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Dioxane | 0 | 0.444 | 0.737 | 0.312 | 0.77 | 0.36 | 0.00 | 0.44 |
| 2. | Toluene | 0 | 0.128 | 0.782 | 0.284 | 0.86 | 0.20 | 0.00 | 0.15 |
| 3. | EtAc | 0 | 0.542 | 0.656 | 0.603 | 0.71 | 0.51 | 0.00 | 0.52 |
| 4. | THF | 0 | 0.591 | 0.714 | 0.634 | 0.75 | 0.47 | 0.00 | 0.58 |
| 5. | DCE | 0.030 | 0.126 | 0.771 | 0.742 | 0.80 | 0.74 | 0.00 | 0.00 |
| 6. | 1-Butanol | 0.341 | 0.809 | 0.674 | 0.655 | 0.74 | 0.75 | 0.65 | 0.67 |
| 7. | 2-Propanol | 0.283 | 0.830 | 0.633 | 0.808 | 0.72 | 0.77 | 0.68 | 0.65 |
| 8. | 1-Propanol | 0.367 | 0.782 | 0.658 | 0.748 | 0.71 | 0.77 | 0.53 | 0.68 |
| 9. | Methanol | 0.605 | 0.545 | 0.608 | 0.904 | 0.64 | 0.84 | 1.00 | 0.54 |
| 10. | Acetone | 0.000 | 0.475 | 0.651 | 0.907 | 0.69 | 0.78 | 0.04 | 0.49 |
| 11. | DMF | 0.031 | 0.613 | 0.759 | 0.977 | 0.78 | 0.87 | 0.00 | 0.69 |
| 12. | ACN | 0.044 | 0.286 | 0.645 | 0.974 | 0.67 | 0.84 | 0.23 | 0.37 |
| 13. | DMSO | 0.072 | 0.647 | 0.830 | 1.000 | 0.84 | 1.00 | 0.00 | 0.71 |
| 14. | NMP | 0.024 | 0.613 | 0.812 | 0.959 | 0.83 | 0.80 | 0.00 | 0.76 |
| 15. | DMAc | 0.028 | 0.650 | 0.763 | 0.987 | 0.79 | 0.85 | 0.00 | 0.75 |
| KAT | Y0 | a1 (α) | b1 (β) | c1 (π*) | a R2 | |
|---|---|---|---|---|---|---|
| 29.50 | −1.19 | −0.943 | −1.786 | 0.775 | ||
| ET | 352.96 | −14.08 | −11.39 | −21.21 | 0.767 | |
| Catalán | Y0 | a2 (SA) | b2 (SB) | c2 (SP) | d2 (SdP) | R2 |
| 31.32 | −1.858 | −1.976 | −3.062 | −0.434 | 0.961 | |
| ET | 375.44 | −22.01 | −23.91 | −37.55 | −4.97 | 0.959 |
| Laurence | Y0 | a3 (DI) | b3 (ES) | c3 (α1) | d3 (β1) | R2 |
| 31.96 | −3.948 | −0.490 | −1.503 | −1.393 | 0.843 | |
| ET | 387.20 | −50.83 | −7.78 | −16.00 | −20.24 | 0.944 |
| Parameter Sets | % α | % β | % π* | |
|---|---|---|---|---|
| KAT | 30.36 | 24.06 | 45.57 | |
| Catalán | % SA | % SB | % SP | % SdP |
| 25.34 | 26.95 | 41.77 | 5.92 | |
| Laurence | % α1 | % β1 | % DI | % ES |
| 7.75 | 22.03 | 62.45 | 7.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homocianu, M.; Serbezeanu, D.; Tachita, V.B. Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative. Int. J. Mol. Sci. 2023, 24, 5286. https://doi.org/10.3390/ijms24065286
Homocianu M, Serbezeanu D, Tachita VB. Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative. International Journal of Molecular Sciences. 2023; 24(6):5286. https://doi.org/10.3390/ijms24065286
Chicago/Turabian StyleHomocianu, Mihaela, Diana Serbezeanu, and Vlad Bubulac Tachita. 2023. "Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative" International Journal of Molecular Sciences 24, no. 6: 5286. https://doi.org/10.3390/ijms24065286
APA StyleHomocianu, M., Serbezeanu, D., & Tachita, V. B. (2023). Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative. International Journal of Molecular Sciences, 24(6), 5286. https://doi.org/10.3390/ijms24065286







