Contrasting Metabolisms in Green and White Leaf Sectors of Variegated Pelargonium zonale—An Integrative Transcriptomic and Metabolomic Study
Abstract
:1. Introduction
2. Results
2.1. De Novo Transcriptome Assembly of P. zonale GL and WL
2.2. Gene Functional Analysis
2.3. Quantification
2.4. Differentially Expressed Genes of P. zonale GL and WL
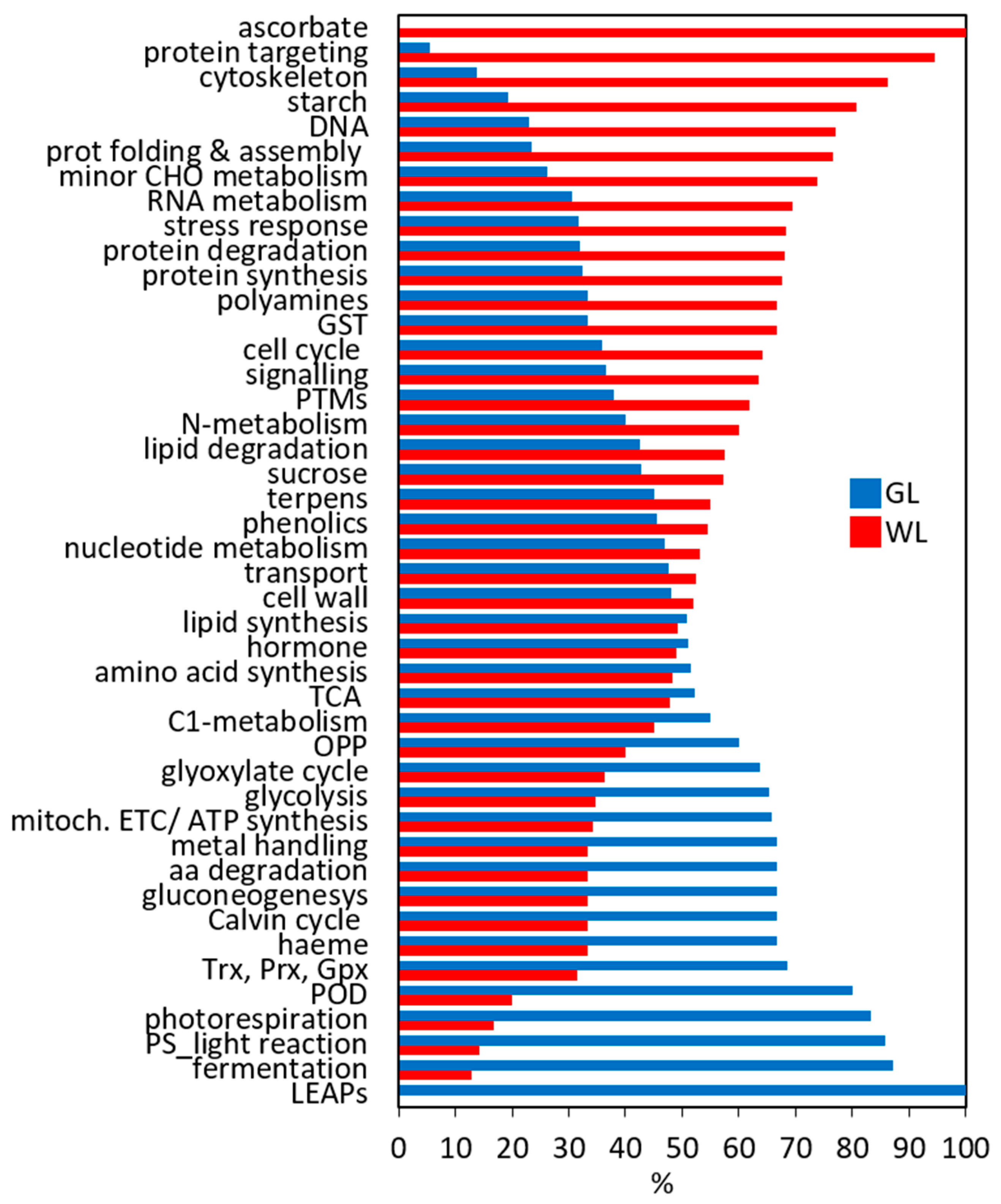
2.5. Functional Classification of DEGs in P. zonale Leaves
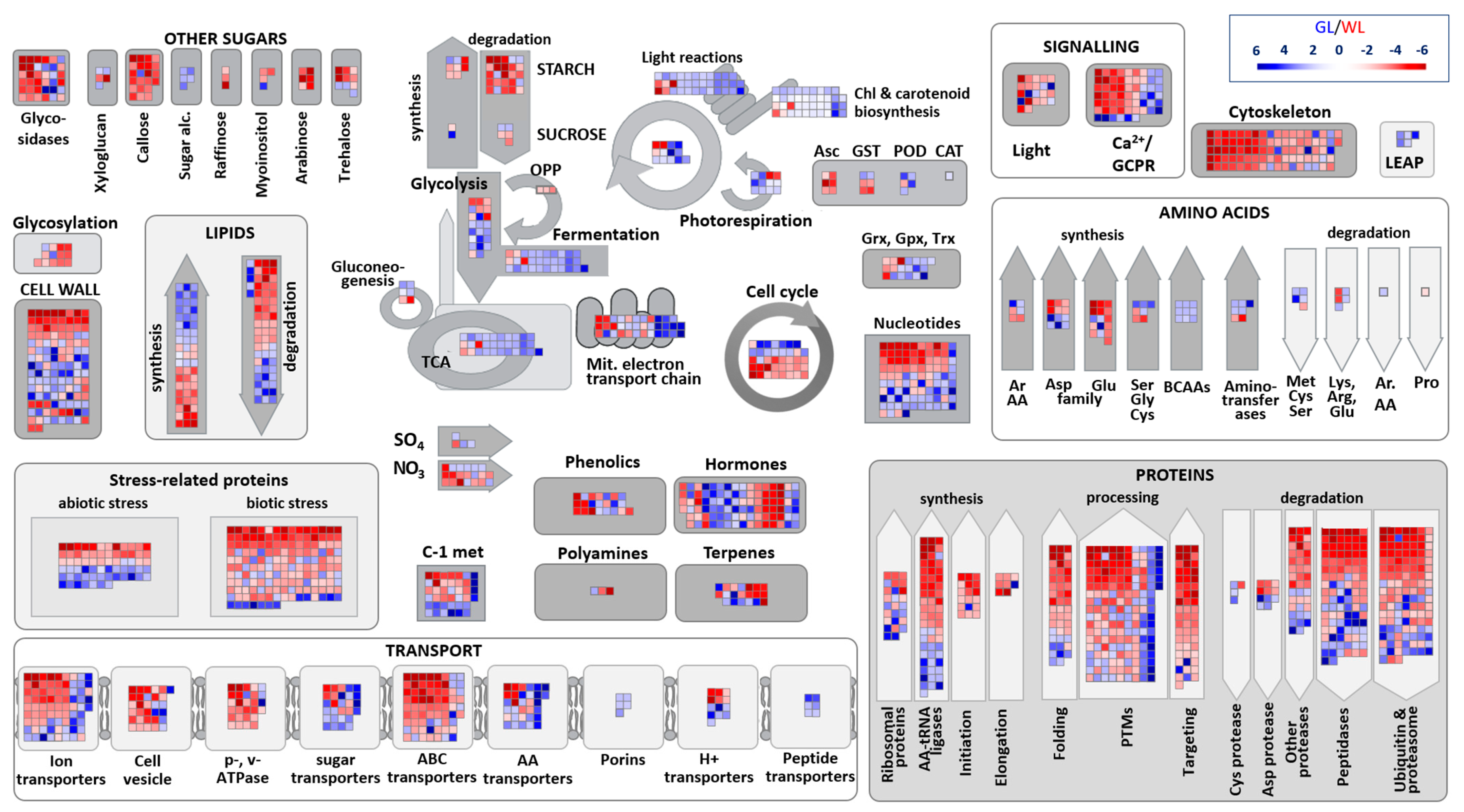
2.6. Major Metabolic Pathways in P. zonale Leaves
2.6.1. Photosynthesis
2.6.2. Carbohydrate Metabolism
2.6.3. Energy Production
2.6.4. Lipid and Fatty Acid Metabolism
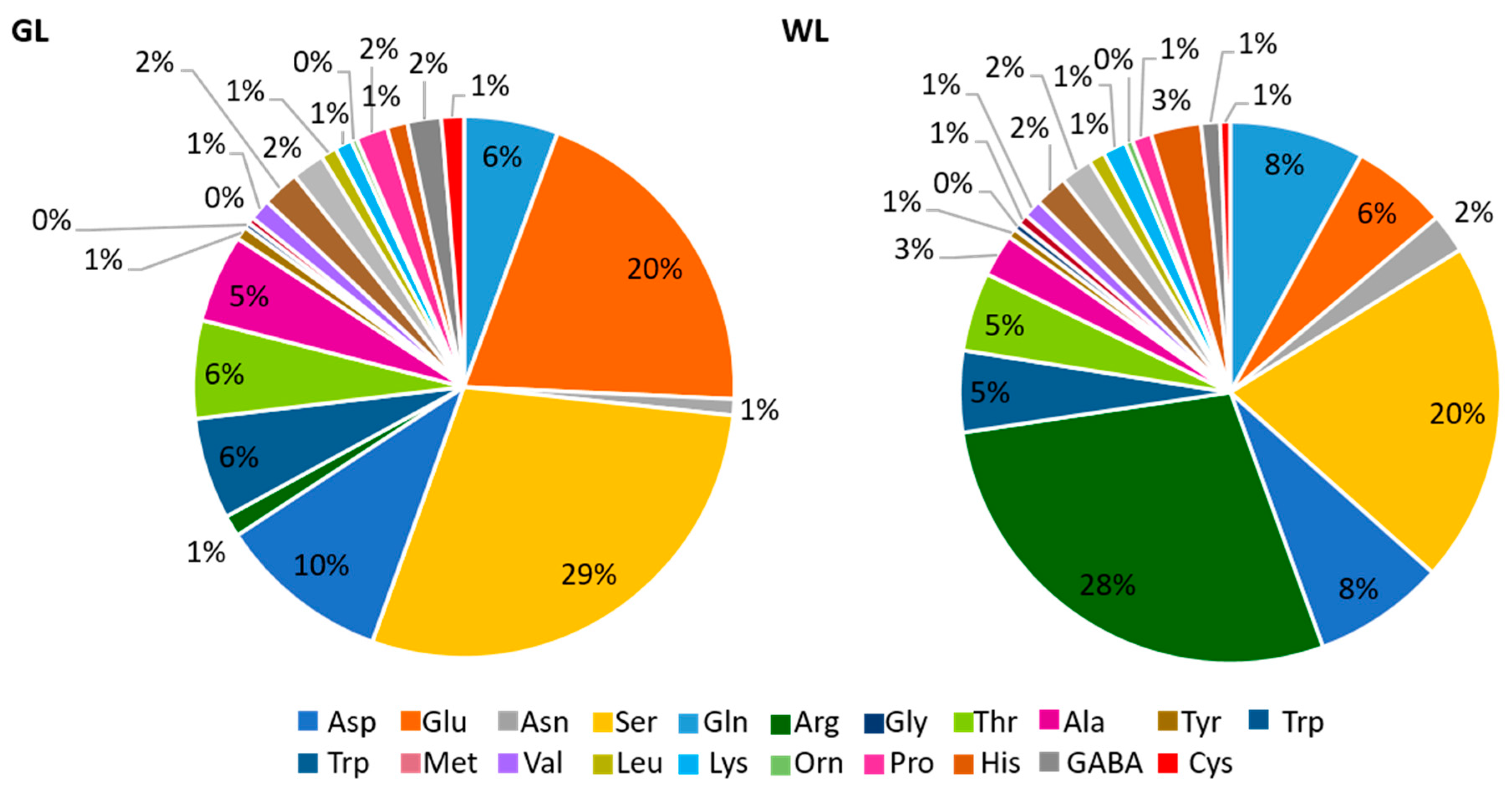
2.6.5. Nitrogen and Amino Acids Metabolism
2.6.6. Proteostasis
2.6.7. RNA Metabolism
2.6.8. DNA Metabolism
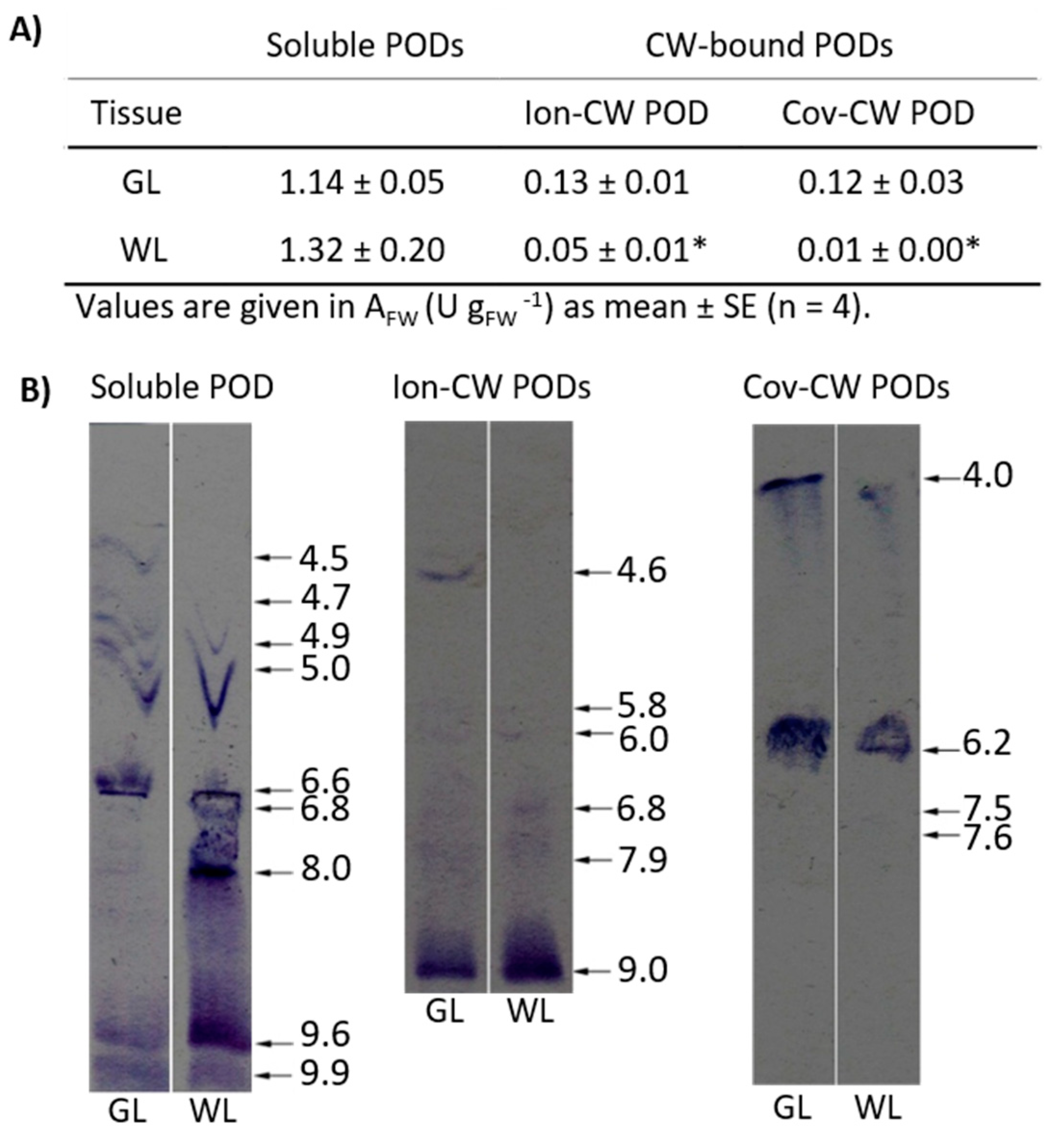
2.6.9. Antioxidant Metabolism
2.6.10. Polyphenols
2.6.11. Cell Wall
2.6.12. Cytoskeleton
2.6.13. Metal Homeostasis
3. Discussion
3.1. De Novo Transcriptome Assembly of P. zonale WL and GL
3.2. Differentially Expressed Genes in P. zonale WL
3.3. WL Acts as a Carbon Sink Leaf Tissue
3.4. WL Acts as an AA Storage Compartment
3.5. WL Has an Efficient Protective System against (a)Biotic Stress
3.6. Abnormal Metabolism in P. zonale WL Plastids
4. Materials and Methods
4.1. Plant Material and Growth
4.2. RNA Extraction, cDNA Library Construction, and Illumina High-Throughput Sequencing
4.3. Transcriptome De Novo Assembly
4.4. Gene Functional Annotation
4.5. Differential Expression Analysis and Functional Enrichment
4.6. Extraction and Analysis of Amino Acids
4.7. Polyphenol Analysis
4.8. Organic Acid Analysis
4.9. Peroxidase Extraction and Activity Measurements
4.10. Isoelectric Focusing of POD Isoforms
4.11. Metal Determination
4.12. qPCR
4.13. Statistics
5. Conclusions
- WL acts as a carbon sink and depends on photosynthetic and energy-generating processes in GL;
- WL can serve as a nitrogen storage for GL;
- Upregulated nitrogen and protein metabolism in WL might provide alternative respiratory substrates;
- WL exhibited upregulated H2O2 scavenging network, protein and DNA repair and pathogen defence system;
- Genes encoding motor proteins associated with cell division, DNA replication, modification, repair, and recombination were induced in WL.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidović, M.; Morina, F.; Milić, S.; Albert, A.; Zechmann, B.; Tosti, T.; Winkler, J.B.; Veljović-Jovanović, S. Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Physiol. Biochem. 2015, 93, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Tilney-Bassett, R.A.E. The control of plastid inheritance in Pelargonium IV. Heredity 1976, 37, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Tilney-Bassett, R.A.E.; Almouslem, A.B. Variation in plastid inheritance between pelargonium cultivars and their hybrids. Heredity 1989, 63, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Tilney-Bassett, R.A.E.; Almouslem, A.B.; Amoatey, H.M. Complementary genes control biparental plastid inheritance in Pelargonium. Theor. Appl. Genet. 1992, 85, 317–324. [Google Scholar] [CrossRef]
- Ming-Yin, L. Anatomical, Cytological and Histological Investigation of Somatic Variability of the Muted Clone of Pelargonium zonale ‘Kleiner Liebling’. Ph.D. Thesis, Humboldt University of Berlin, Berlin, Germany, 2005. [Google Scholar]
- Grieger, P. Untersuchungen zur Züchtung variegater Pelargonium x zonale Hybriden auf tetraploider Stufe. Ph.D. Thesis, Agricultural-Horticultural Faculty, Humboldt University of Berlin, Berlin, Germany, 2007. [Google Scholar]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Reski, R. Challenges to our current view on chloroplasts. Biol. Chem. 2020, 390, 731–738. [Google Scholar] [CrossRef]
- Sakamoto, W. Leaf-variegated mutations and their responsible genes in Arabidopsis thaliana. Genes Genet. Sys. 2003, 78, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.E.I.; Fu, A.; Aluru, M.; Park, S.; Xu, Y.; Liu, H.; Rodermel, S. Variegation mutants and mechanisms of chloroplast biogenesis. Plant Cell Environ. 2007, 30, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Ahchige, M.W. Canalization of Plant Metabolism and Yield. Ph.D. Thesis, Mathematisch-Naturwissenschaftlichen Fakultät Institut für Biologie und Biochemie der Universität Potsdam, Potsdam, Germany, 2022. [Google Scholar]
- Kato, Y.; Miura, E.; Ido, K.; Ifuku, K.; Sakamoto, W. The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol. 2009, 151, 1790–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Q.; Zhang, Y.; Pan, M.; Wang, R.; Sun, Y.; An, L.; Liu, X.; Yu, F.; Qi, Y. VAR2/AtFtsH2 and EVR2/BCM1/CBD1 synergistically regulate the accumulation of PSII reaction centre D1 protein during de-etiolation in Arabidopsis. Plant Cell Environ. 2022, 45, 2395–2409. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. FtsH protease in the thylakoid membrane: Physiological functions and the regulation of protease activity. Front. Plant Sci. 2018, 20, 9855. [Google Scholar] [CrossRef] [Green Version]
- Aluru, M.R.; Stessman, D.; Spalding, M.H.; Rodermel, S.R. Alterations in photosynthesis in Arabidopsis lacking IMMUTANS, a chloroplast terminal oxidase. Photosynth. Res. 2007, 91, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Aluru, M.R.; Zola, J.; Foudree, A.; Rodermel, S.R. Chloroplast photooxidation-induced transcriptome reprogramming in Arabidopsis immutans white leaf sectors. Plant Physiol. 2009, 150, 904–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, T.H.; Lin, S.H.; Huang, M.Y.; Huang, W.D.; Yang, C.M. Transcriptome profile of the variegated Ficus microcarpa c.v. Milky Stripe Fig Leaf. Int. J. Mol. Sci. 2019, 20, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, V.; Simková, K.; Carrie, C.; Delannoy, E.; Giraud, E.; Whelan, J.; Small, I.D.; Apel, K.; Badger, M.R.; Pogson, B.J. The cytoskeleton and the peroxisomal-targeted snowy cotyledon3 protein are required for chloroplast development in Arabidopsis. Plant Cell 2010, 22, 3423–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.C.; Wu, Y.J.; Sheue, C.R.; Liao, P.C.; Chen, Y.H.; Li, S.J.; Liu, J.W.; Chan, H.T.; Liu, W.L.; Ko, Y.Z.; et al. Molecular basis underlying leaf variegation of a moth orchid mutant (Phalaenopsis aphrodite subsp. formosana). Front. Plant Sci. 2017, 8, 1333. [Google Scholar] [CrossRef] [Green Version]
- Vidović, M.; Morina, F.; Milić, S.; Vuleta, A.; Zechmann, B.; Prokić, L.; Veljović-Jovanović, S. Characterisation of antioxidants in photosynthetic and non-photosynthetic leaf tissues of variegated Pelargonium zonale plants. Plant Biol. 2016, 18, 669–680. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Prokić, L.; Milić-Komić, S.; Živanović, B.; Veljović Jovanović, S. Antioxidative response in variegated Pelargonium zonale leaves and generation of extracellular H2O2 in (peri) vascular tissue induced by sunlight and paraquat. J. Plant Physiol. 2016, 206, 25–39. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Sierla, M.; Rahikainen, M.; Salojärvi, J.; Kangasjärvi, J.; Kangasjärvi, S. Apoplastic and chloroplastic redox signaling networks in plant stress responses. Antioxid. Redox Signal. 2013, 18, 2220–2239. [Google Scholar] [CrossRef] [PubMed]
- Toshoji, H.; Katsumata, T.; Takusagawa, M.; Yusa, Y.; Sakai, A. Effects of chloroplast dysfunction on mitochondria: White sectors in variegated leaves have higher mitochondrial DNA levels and lower dark respiration rates than green sectors. Protoplasma 2012, 249, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadie, C.; Lamothe, M.; Mauve, C.; Gilard, F.; Tcherkez, G. Leaf green-white variegation is advantageous under N deprivation in Pelargonium hortorum. Funct. Plant Biol. 2015, 42, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Guérard, F.; Gilard, F.; Lamothe, M.; Mauve, C.; Gout, E.; Bligny, R. Metabolomic characterization of the functional division of nitrogen metabolism in variegated leaves. Funct. Plant Biol. 2012, 39, 959–967. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, C.; Zhou, P.; Gao, X.; Wang, M.; Tian, S.; Lu, C.; Wang, C.; Shen, C. Transcriptomic analyses reveal variegation-induced metabolic changes leading to high L-theanine levels in albino sectors of variegated tea (Camellia sinensis). Plant Physiol. Biochem. 2021, 169, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Royal FloraHolland Annual Report 2021. Available online: www.floraholland.com (accessed on 4 January 2023).
- Janson, C. Ornamental Plants Market Study the Western European Market from a Nicaraguan Perspective; Chemonics International Inc.: Washington, DC, USA, 2000. [Google Scholar]
- Gehl, C.; Wamhoff, D.; Schaarschmidt, F.; Serek, M. Improved leaf and flower longevity by expressing the etr1-1 allele in Pelargonium zonale under control of FBP1 and SAG12 promoters. Plant Growth Regul. 2018, 86, 351–363. [Google Scholar] [CrossRef]
- Vidović, M.; Ćuković, K. Isolation of high-quality RNA from recalcitrant leaves of variegated and resurrection plants. 3 Biotech 2020, 10, 286–294. [Google Scholar] [CrossRef]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Craig, R.; United States Patents and Trademark Office. Patent Genius: Alexandria. 1988. Available online: https://patentimages.storage.googleapis.com/dd/63/cb/958f43dbe7896b/USPP6218.pdf (accessed on 8 January 2023).
- Williams, C.A.; Newman, M.; Gibby, M. The application of leaf phenolic evidence for systematic studies within the genus Pelargonium (Geraniaceae). Biochem. Syst. Ecol. 2000, 28, 119–132. [Google Scholar] [CrossRef]
- Weng, M.L.; Ruhlman, T.A.; Gibby, M.; Jansen, R.K. Phylogeny, rate variation, and genome size evolution of Pelargonium (Geraniaceae). Mol. Phylogenet. Evol. 2012, 64, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Plaschil, S.; Abel, S.; Klocke, E. The variability of nuclear DNA content of different Pelargonium species estimated by flow cytometry. PLoS ONE 2022, 17, e0267496. [Google Scholar] [CrossRef]
- Bakker, F.T.; Culham, A.; Hettiarachi, P.; Touloumenidou, T.; Gibby, M. Phylogeny of Pelargonium (Geraniaceae) based on DNA sequences from three genomes. Taxon 2004, 53, 17–28. [Google Scholar] [CrossRef]
- Zhang, J.; Ruhlman, T.A.; Mower, J.P.; Jansen, R.K. Comparative analyses of two Geraniaceae transcriptomes using next-generation sequencing. BMC Plant Biol. 2013, 13, 228. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.H.; Hung, C.Y.; Qiu, J.; Chen, J.; Kittur, F.S.; Oldham, C.E.; Henny, R.J.; Burkey, K.O.; Fan, L.; Xie, J. Accumulation of high OPDA level correlates with reduced ROS and elevated GSH benefiting white cell survival in variegated leaves. Sci. Rep. 2017, 7, 44158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, E.; Kato, Y.; Sakamoto, W. Comparative transcriptome analysis of green/white variegated sectors in Arabidopsis yellow variegated2: Responses to oxidative and other stresses in white sectors. J. Exp. Bot. 2010, 61, 2433–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, W.; Hu, D.; Shen, P.; Zhang, G.; Zhu, Y. Comparative analysis of the metabolome and transcriptome between green and albino zones of variegated leaves from Hydrangea macrophylla ‘Maculata’ infected by hydrangea ringspot virus. Plant Physiol. Biochem. 2020, 157, 195–210. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Liu, M.; Ruan, J. Integrated analyses of the transcriptome and metabolome of the leaves of albino tea cultivars reveal coordinated regulation of the carbon and nitrogen metabolism. Sci. Hortic. 2018, 231, 272–281. [Google Scholar] [CrossRef]
- Ren, J.; Zou, J.; Zou, X.; Song, G.; Gong, Z.; Liu, Z.; Ji, R.; Feng, H. Fine Mapping of BoVl conferring the variegated leaf in ornamental kale (Brassica oleracea var. acephala). Int. J. Mol. Sci. 2022, 23, 14853. [Google Scholar] [CrossRef]
- Veljović-Jovanović, S.; Vidović, M.; Morina, F.; Prokić, L.; Todorović, D.M. Comparison of photoacoustic signals in photosynthetic and nonphotosynthetic leaf tissues of variegated Pelargonium zonale. Int. J. Thermophys. 2016, 37, 91–101. [Google Scholar] [CrossRef]
- Bashir, K.; Rasheed, S.; Kobayashi, T.; Seki, M.; Nishizawa, N.K. Regulating subcellular metal homeostasis: The key to crop improvement. Front. Plant Sci. 2016, 7, 1192. [Google Scholar] [CrossRef] [Green Version]
- Rasmusson, A.G.; Geisler, D.A.; Møller, I.M. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 2008, 8, 47–60. [Google Scholar] [CrossRef]
- Vidal, G.; Ribas-Carbo, M.; Garmier, M.; Dubertret, G.; Rasmusson, A.G.; Mathieu, C.; Foyer, C.H.; De Paepe, R. Lack of respiratory chain complex I impairs alternative oxidase engagement and modulates redox signaling during elicitor-induced cell death in Tobacco. Plant Cell 2007, 19, 640–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellido, A.M.; Distéfano, A.M.; Setzes, N.; Cascallares, M.M.; Oklestkova, J.; Novak, O.; Ramirez, J.A.; Zabaleta, E.J.; Fiol, D.F.; Pagnussat, G.C. A mitochondrial ADXR–ADX–P450 electron transport chain is essential for maternal gametophytic control of embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2000482119. [Google Scholar] [CrossRef] [PubMed]
- Deeken, R.; Engelmann, J.C.; Efetova, M.; Czirjak, T.; Müller, T.; Kaiser, W.M.; Tietz, O.; Krischke, M.; Mueller, M.J.; Palme, K.; et al. An integrated view of gene expression and solute profiles of Arabidopsis tumors: A genome-wide approach. Plant Cell 2006, 18, 3617–3634. [Google Scholar] [CrossRef] [Green Version]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Veyres, N.; Danon, A.; Aono, M.; Galliot, S.; Karibasappa, Y.B.; Diet, A.; Grandmottet, F.; Tamaoki, M.; Lesur, D.; Pilard, S.; et al. The Arabidopsis sweetie mutant is affected in carbohydrate metabolism and defective in the control of growth, development and senescence. Plant J. 2008, 55, 665–686. [Google Scholar] [CrossRef]
- Hernández, I.; Van Breusegem, F. Opinion on the possible role of flavonoids as energy escape valves: Novel tools for nature’s Swiss army knife? Plant Sci. 2010, 179, 297–301. [Google Scholar] [CrossRef]
- Martins, S.C.; Araújo, W.L.; Tohge, T.; Fernie, A.R.; DaMatta, F.M. In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS ONE 2014, 9, e94862. [Google Scholar] [CrossRef] [Green Version]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Chopin, F.; Orsel, M.; Dorbe, M.; Chardon, F.; Truong, H.; Miller, A.J.; Krapp, A.; Daniel-Vedele, F. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 2007, 19, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.; Chan, H.; Coruzzi, G.M.; Lam, H. Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol. 2004, 134, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.B.; Langer, J.D.; Jovanovic, M. Proteome turnover in the spotlight: Approaches, applications, and perspectives. Mol. Cell. Proteom. 2021, 20, 100016. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Satou, M.H.; Enoki, A.; Oikawa, D.; Ohta, K.; Saito, T.; Hachiya, H.; Sakakibara, M.; Kusano, A.; Fukushima, K.; Saito, M.; et al. Integrated analysis of transcriptome and metabolome of Arabidopsis albino or pale green mutants with disrupted nuclear-encoded chloroplast proteins. Plant Mol. Biol. 2014, 85, 411–428. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Papanga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kim, J.Y. Callose synthesis in higher plants. Plant Signal. Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L.; Baker, R.F.; Stubert, A.; Braun, D.M. Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiol. 2012, 160, 1540–1550. [Google Scholar] [CrossRef] [Green Version]
- Hess, W.; Golz, R.; Borner, T. Analysis of randomly selected cDNA reveals the expression of stress- and defence-related genes in the barley mutant albostrians. Plant Sci. 1998, 133, 191–201. [Google Scholar] [CrossRef]
- Liu, X.; Lan, J.; Huang, Y.; Cao, P.; Zhou, C.; Ren, Y.; He, N.; Liu, S.; Tian, Y.; Nguyen, T.; et al. WSL5, a pentatricopeptide repeat protein, is essential for chloroplast biogenesis in rice under cold stress. J. Exp. Bot. 2018, 69, 3949–3961. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Xu, B.; Li, Y.; Ma, Y.; Wang, G. Phenotype and transcriptome analysis reveals chloroplast development and pigment biosynthesis together influenced the leaf color formation in mutants of Anthurium andraeanum ‘Sonate’. Front. Plant Sci. 2015, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- TerBush, A.D.; MacCready, J.S.; Chen, C.; Ducat, D.C.; Osteryoung, K.W. Conserved dynamics of chloroplast cytoskeletal ftsz proteins across photosynthetic lineages. Plant Physiol. 2018, 176, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I.; Yang, W.C. The functions of kinesin and kinesin-related proteins in eukaryotes. Cell Adhes. Migr. 2020, 14, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hensel, G.; Mascher, M.; Melzer, M.; Budhagatapalli, N.; Rutten, T.; Himmelbach, A.; Beier, S.; Korzun, V.; Kumlehn, J.; et al. Leaf variegation and impaired chloroplast development caused by a truncated CCT domain gene in albostrians barley. Plant Cell 2019, 31, 1430–1445. [Google Scholar] [CrossRef] [Green Version]
- Robles, P.; Micol, J.L.; Quesada, V. Mutations in the plant-conserved MTERF9 alter chloroplast gene expression, development and tolerance to abiotic stress in Arabidopsis thaliana. Physiol. Plant. 2015, 154, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410–424. [Google Scholar]
- Bateman, A.; Birney, E.; Cerruti, L.; Durbin, R.; Etwiller, L.; Eddy, S.R.; Griffiths-Jones, S.; Howe, K.L.; Marshall, M.; Sonnhammer, E.L. The Pfam protein families database. Nucleic Acids Res. 2002, 30, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, S.C.; Shen, Y.C.; Yang, D.J. Comparison of various preparation methods for determination of organic acids in fruit vinegars with a simple ion-exclusion liquid chromatography. Food Anal. Methods 2021, 4, 531–539. [Google Scholar] [CrossRef]
- Kukavica, B.; Veljović-Jovanović, S.; Menckhoff, L.; Luthje, S. Cell wall-bound cationic and anionic class III isoperoxidases of pea root: Biochemical characterization and function in root growth. J. Exp. Bot. 2012, 63, 4631–4645. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of pro-tein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

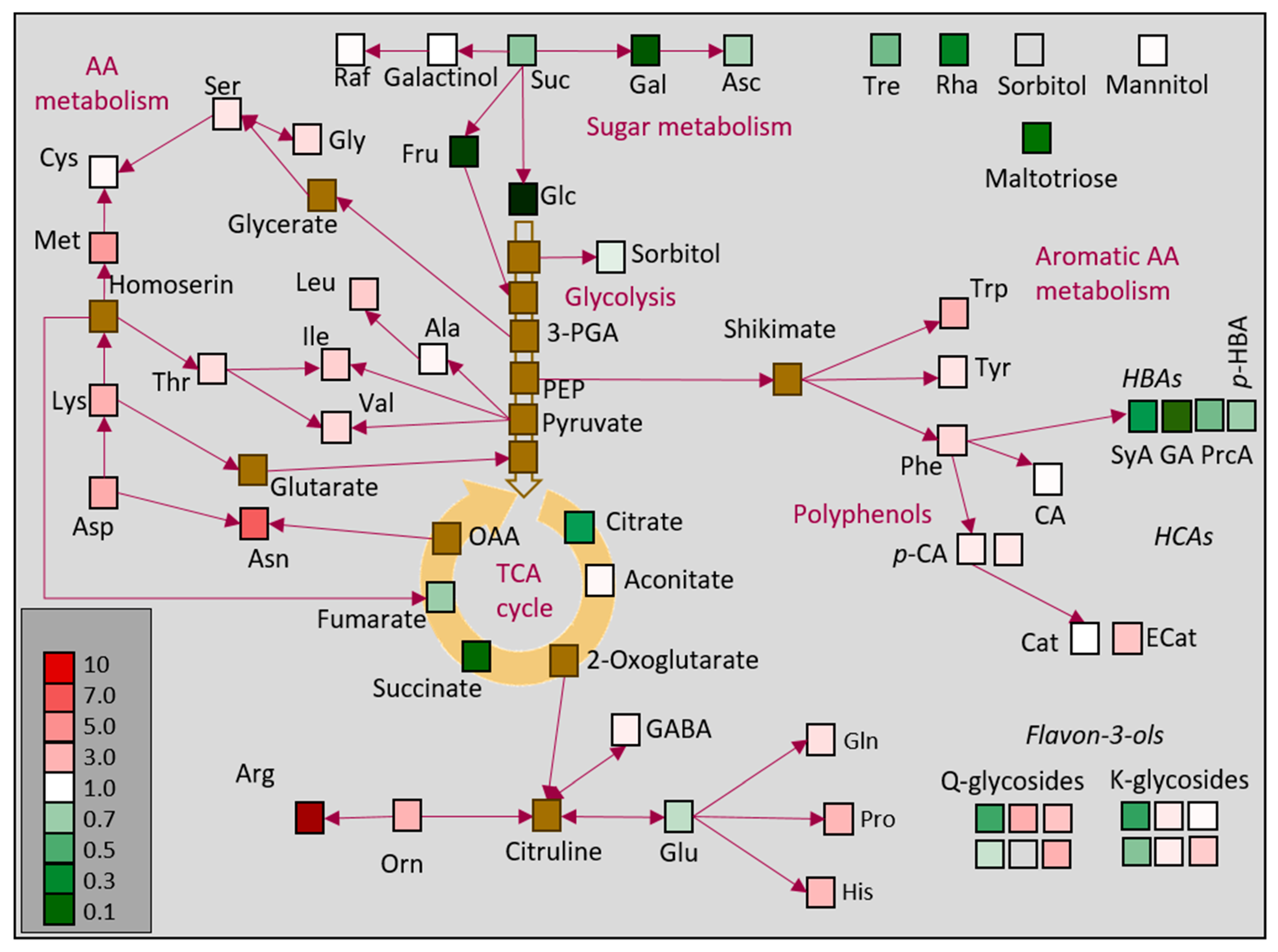
| Metabolite | GL | WL | Metabolite | GL | WL |
|---|---|---|---|---|---|
| Organic acids (µmol g–1 FW) | Amino acids (nmol g–1 FW) | ||||
| Oxalic acid | 1039 ± 9 | 1911 ± 38 ** | Aspartic acid | 358 ± 28 | 1433 ± 75 ** |
| Citric acid | 510 ± 30 | 221 ± 3 * | Glutamic acid | 1277 ± 104 | 1029 ± 65 |
| Tartaric acid | 439 ± 20 | 612 ± 4 * | Asparagine | 62 ± 9 | 420 ± 87 * |
| Aconitic acid | 229 ± 51 | 292 ± 6 | Serine | 1832 ± 266 | 3637 ± 260 * |
| Formic acid | 72 ± 14 | 379 ± 16 *** | Glutamine | 654 ± 87 | 1402 ± 158 * |
| Malic acid | 1075 ± 239 | 620 ± 14 | Arginine | 81 ± 4 | 5022 ± 770 ** |
| Fumaric acid | 3.7 ± 0.1 | 2.6 ± 0.4 | Glycine | 386 ± 54 | 868 ± 97 * |
| Succinic acid | 90 ± 9 | 11 ± 1 * | Threonine | 372 ± 23 | 840 ± 74 * |
| Ascorbate | 2.0 ± 0.1 | 1.5 ± 0.1 ** | Alanine | 335 ± 45 | 453 ± 35 |
| Hydroxybenzoic acids (µmol g–1 FW) | Tyrosine | 50 ± 7 | 100 ± 13 * | ||
| p-HBA | 49.1 ± 4.3 | 34.9 ± 3.2 * | Tryptophan | 21 ± 1 | 78 ± 7 * |
| Gallic acid | 4.5 ± 0.3 | 1.5 ± 0.1 *** | Methionine | 24 ± 3 | 110 ± 8 * |
| SyA | 1.5 ± 0.2 | 0.6 ± 0.1 *** | Valine | 81 ± 10 | 194 ± 10 * |
| PrcA | 2.4 ± 0.2 | 1.4 ± 0.1 *** | Phenylalanine | 142 ± 5 | 346 ± 49 * |
| Hydroxycinnamic acids (µmol g–1 FW) | Isoleucine | 124 ± 11 | 338 ± 53 * | ||
| p-CA | 1.2 ± 0.2 | 2.0 ± 0.3 ** | Leucine | 60 ± 11 | 172 ± 12 * |
| Caffeic acid | 0.3 ± 0.1 | 0.3 ± 0.1 | Lysine | 65 ± 9 | 247 ± 32 * |
| p-CA derivative | 1.2 ± 0.2 | 2.3 ± 0.3 ** | Ornithine | 22 ± 2 | 82 ± 9 * |
| Flavan-3-ols (µmol g–1 FW) | Proline | 121 ± 18 | 206 ± 29 * | ||
| Catechin | 1.9 ± 0.2 | 1.9 ± 0.2 | Histidine | 78 ± 11 | 536 ± 79 ** |
| Epicatechin | 0.4 ± 0.1 | 1.1 ± 0.1 *** | GABA | 129 ± 17 | 202 ± 24 * |
| Flavon-3-ols (µmol g–1 FW) | Cysteine | 85 ± 8 | 107 ± 12 | ||
| Q-O-Rha-galactose | 0.05 ± 0.01 | 0.05 ± 0.01 | a Hexose (mmol g–1 FW) | ||
| Q-O-Rha-glucose | 0.12 ± 0.01 | 0.06 ± 0.01 *** | Glucose | 7.73 ± 0.77 | 0.08 ± 0.01 ** |
| Q-3-O-galactose | 0.57 ± 0.07 | 2.25 ± 0.33 ** | Fructose | 5.85 ± 0.63 | 0.17 ± 0.03 ** |
| Q-3-O-glucose | 0.59 ± 0.06 | 1.85 ± 0.22 *** | Galactose | 0.81 ± 0.15 | 0.04 ± 0.01 ** |
| Q-O-arabinose | 0.06 ± 0.01 | 0.05 ± 0.01 | a Disaccharides (µmol g–1 FW) | ||
| Q-O-xylose | 0.30 ± 0.03 | 1.14 ± 0.10 *** | Sucrose | 1.04 ± 0.15 | 0.24 ± 0.04 ** |
| K-O-glucose-Rha-Rha | 0.55 ± 0.07 | 0.25 ± 0.03 ** | Trehalose | 0.041 ± 0.005 | 0.024 ± 0.005 |
| K-3-O-rutinose | 0.30 ± 0.05 | 0.19 ± 0.02 | a Sugar alcohols (µmol g–1 FW) | ||
| K-3-O-galactose | 0.78 ± 0.13 | 1.41 ± 0.06 *** | Sorbitol | 0.09 ± 0.01 | 0.08 ± 0.01 |
| K-3-O-glucose | 0.33 ± 0.04 | 0.57 ± 0.05 ** | Galactinol | 0.07 ± 0.01 | 0.07 ± 0.01 |
| K-O-arabinose | 0.19 ± 0.02 | 0.29 ± 0.05 | Mannitol | 0.06 ± 0.01 | 0.07 ± 0.01 |
| K-O-xylose | 0.02 ± 0.01 | 0.06 ± 0.01 ** | a Trisaccharides (µmol g–1 FW) | ||
| a Pentose (µmol g–1 FW) | Maltotriose | 0.023 ± 0.003 | 0.004 ± 0.001 * | ||
| Rhamnose | 0.44 ± 0.05 | 0.12 ± 0.01 * | Raffinose | 0.06 ± 0.01 | 0.07 ± 0.01 |
| Element | GL | WL |
|---|---|---|
| Fe, µg g–1DW | 64.5 ± 6.2 | 102.5 ± 9.9 * |
| Cu, µg g–1DW | 7.0 ± 0.6 | 11.1 ± 0.1 * |
| Ca, mg g–1DW | 23.4 ± 2.7 | 10.8 ± 1.3 * |
| Mn, µg g–1DW | 113.0 ± 4.3 | 243.0 ± 9.3 ** |
| Zn, µg g–1DW | 28.8 ± 2.1 | 50.4 ± 3.7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milić, D.; Pantelić, A.; Banović Đeri, B.; Samardžić, J.; Vidović, M. Contrasting Metabolisms in Green and White Leaf Sectors of Variegated Pelargonium zonale—An Integrative Transcriptomic and Metabolomic Study. Int. J. Mol. Sci. 2023, 24, 5288. https://doi.org/10.3390/ijms24065288
Milić D, Pantelić A, Banović Đeri B, Samardžić J, Vidović M. Contrasting Metabolisms in Green and White Leaf Sectors of Variegated Pelargonium zonale—An Integrative Transcriptomic and Metabolomic Study. International Journal of Molecular Sciences. 2023; 24(6):5288. https://doi.org/10.3390/ijms24065288
Chicago/Turabian StyleMilić, Dejana, Ana Pantelić, Bojana Banović Đeri, Jelena Samardžić, and Marija Vidović. 2023. "Contrasting Metabolisms in Green and White Leaf Sectors of Variegated Pelargonium zonale—An Integrative Transcriptomic and Metabolomic Study" International Journal of Molecular Sciences 24, no. 6: 5288. https://doi.org/10.3390/ijms24065288








