Convenient and Sensitive Measurement of Lactosylceramide Synthase Activity Using Deuterated Glucosylceramide and Mass Spectrometry
Abstract
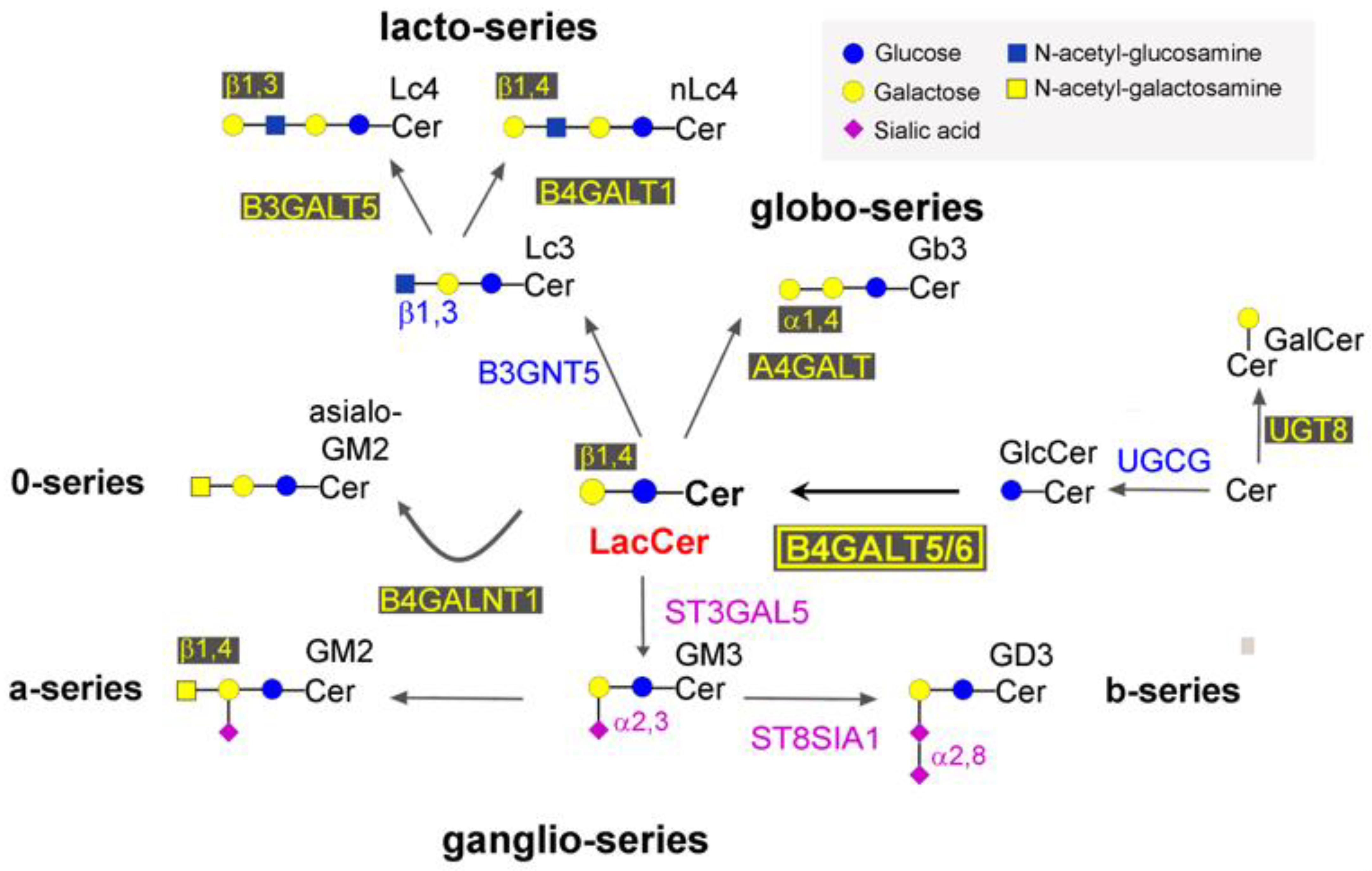
:1. Introduction
2. Results
2.1. Detection of LacCer Synthase Activity by Radiolabeled UDP-Gal and Chromatography
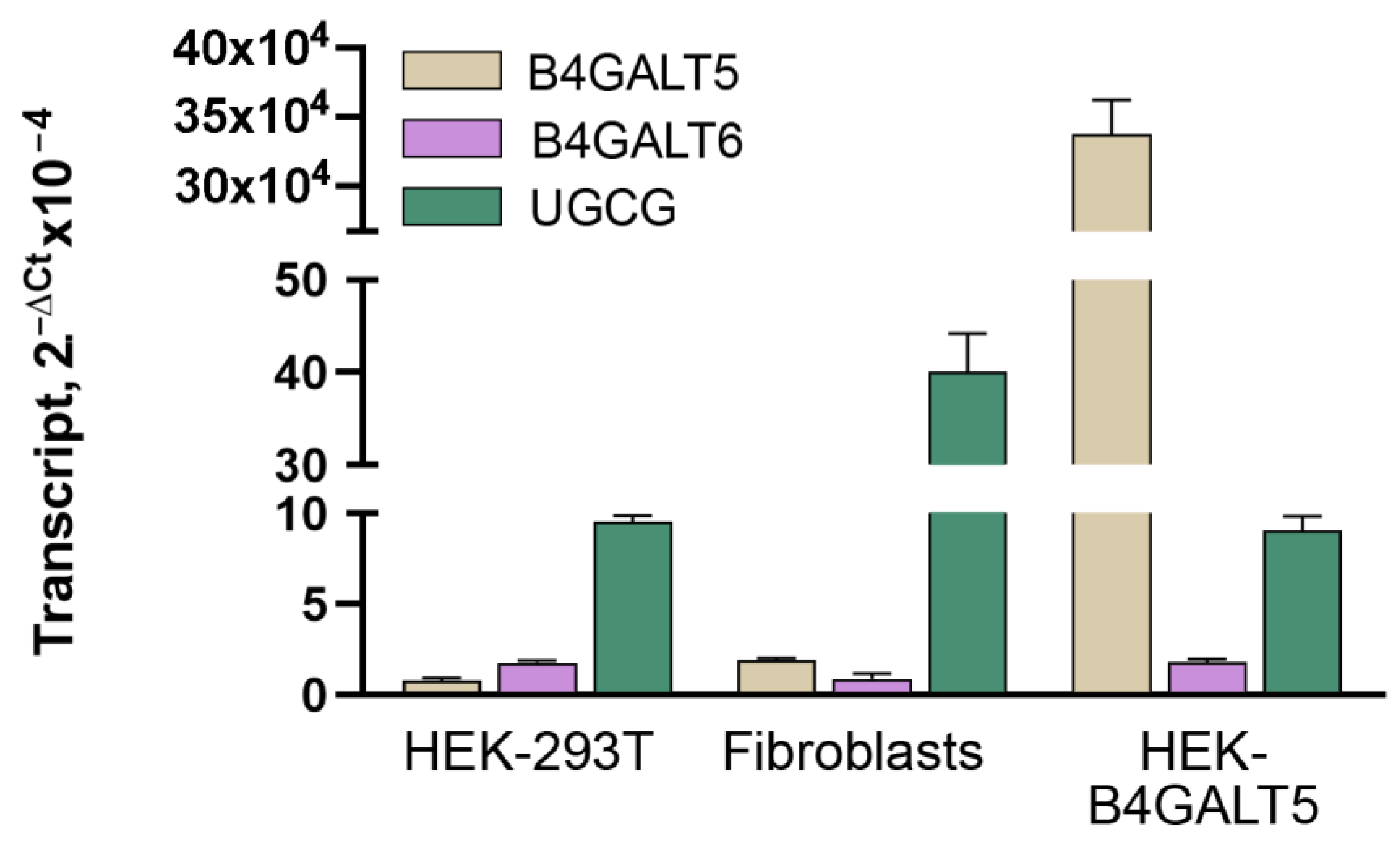
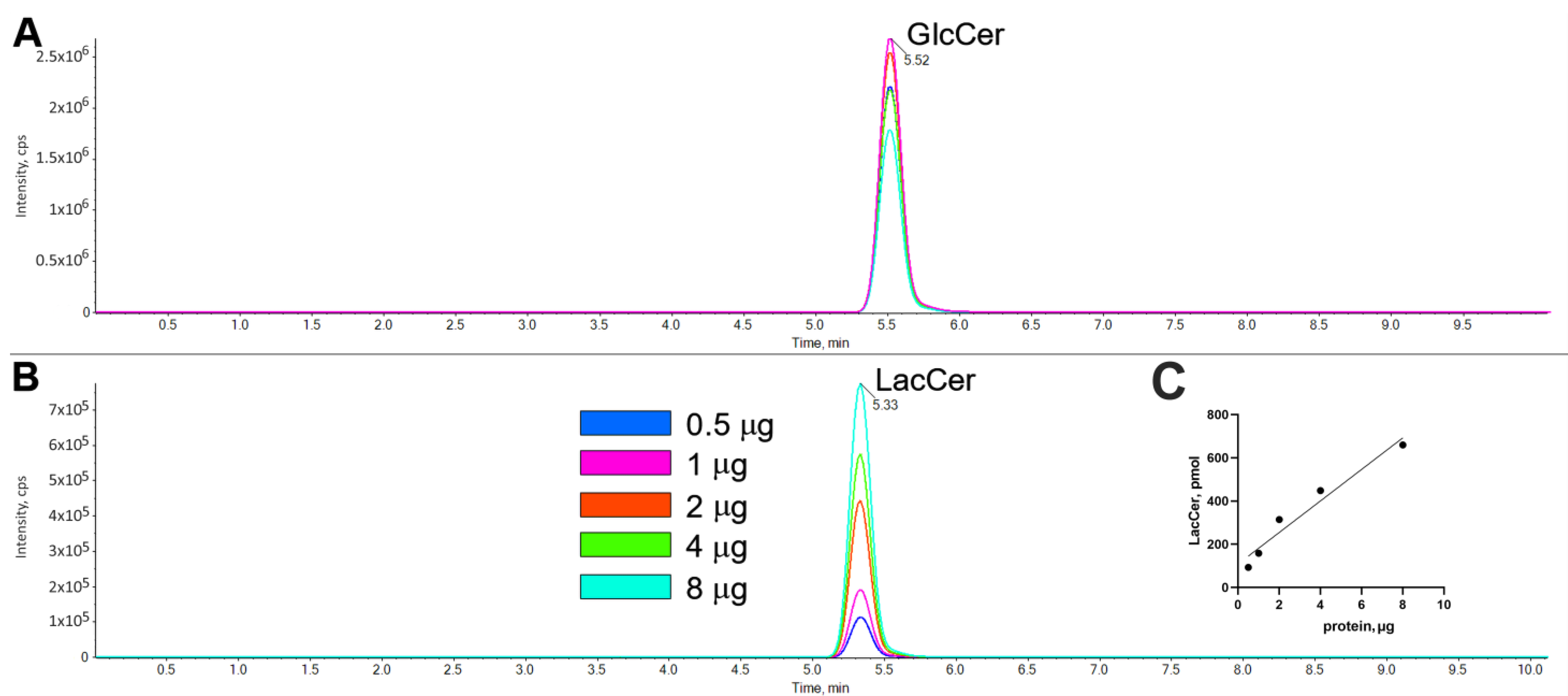
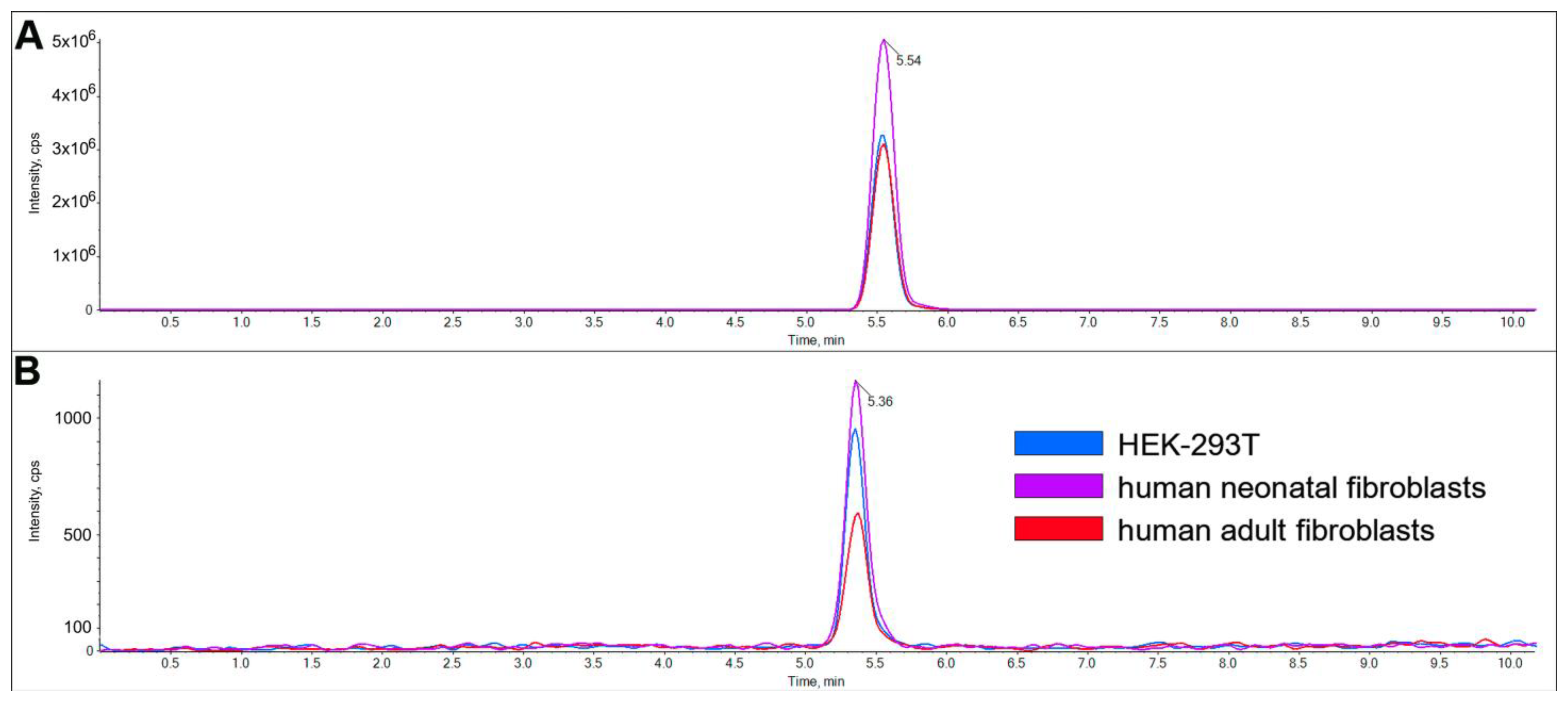
2.2. Characterization of LacCer Synthase Activity Detected by GlcCerd7 and LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. DNA Constructs
4.2. Cell Culture, Transfection, and Processing
4.3. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction
4.4. LacCer Synthase Activity Assay
4.5. Reaction Product Characterization and Quantification by LC–MS/MS
4.6. Preparation of Golgi Apparatus Fraction from Fibroblasts
4.7. Equations and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| B4GALT | β1,4 galactosyltransferases (according to HUGO nomenclature) |
| CE | collision energy |
| DP | declustering potential |
| GlcCer | glucosylceramide |
| GlcCerd7 | deuterated glucosylceramide |
| LacCer | lactosylceramide |
| LacCerd7 | deuterated lactosylceramide |
| LC-MS/MS | liquid chromatography coupled with tandem mass spectrometry |
| KO | knock-out |
| NBD—ceramide | 6-((N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)amino)hexanoyl)Sphingosine |
| RSA | relative specific activity |
| UGCG | UDP-glucose: ceramide glucosyltransferase |
References
- Nomura, T.; Takizawa, M.; Aoki, J.; Arai, H.; Inoue, K.; Wakisaka, E.; Yoshizuka, N.; Imokawa, G.; Dohmae, N.; Takio, K.; et al. Purification, CDNA Cloning, and Expression of UDP-Gal: Glucosylceramide Beta-1,4-Galactosyltransferase from Rat Brain. J. Biol. Chem. 1998, 273, 13570–13577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, T.; Sato, T.; Natsuka, S.; Kobayashi, Y.; Zhou, D.; Shinkai, T.; Hayakawa, S.; Furukawa, K. Involvement of Murine β-1,4-Galactosyltransferase V in Lactosylceramide Biosynthesis. Glycoconj. J. 2010, 27, 685–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuda, N.; Numata, S.; Li, X.; Nomura, T.; Takizawa, M.; Kondo, Y.; Yamashita, Y.; Hashimoto, N.; Kiyono, T.; Urano, T.; et al. Β4GalT6 Is Involved in the Synthesis of Lactosylceramide with Less Intensity than Β4GalT5. Glycobiology 2013, 23, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, T.; Tanaka, M.; Yokoyama, M.; Sato, T.; Shinkai, T.; Furukawa, K. Early Lethality of Beta-1,4-Galactosyltransferase V-Mutant Mice by Growth Retardation. Biochem. Biophys. Res. Commun. 2009, 379, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Satake, H.; Nishie, T.; Okino, N.; Hatta, T.; Otani, H.; Naruse, C.; Suzuki, H.; Sugihara, K.; Kamimura, E.; et al. Lactosylceramide Synthases Encoded by B4galt5 and 6 Genes Are Pivotal for Neuronal Generation and Myelin Formation in Mice. PLoS Genet. 2018, 14, e1007545. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; van Eijkeren, R.J.; Gabriel, T.L.; de Haar, C.; Gijzel, S.M.W.; Hamers, N.; Ferraz, M.J.; Aerts, J.M.F.G.; Schipper, H.S.; van Eijk, M.; et al. Adipocytes Harbor a Glucosylceramide Biosynthesis Pathway Involved in INKT Cell Activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1157–1167. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Mayo, L.; Trauger, S.A.; Blain, M.; Nadeau, M.; Patel, B.; Alvarez, J.I.; Mascanfroni, I.D.; Yeste, A.; Kivisäkk, P.; Kallas, K.; et al. Regulation of Astrocyte Activation by Glycolipids Drives Chronic CNS Inflammation. Nat. Med. 2014, 20, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-C.; Gutiérrez-Vázquez, C.; Rothhammer, V.; Mayo, L.; Wheeler, M.A.; Tjon, E.C.; Zandee, S.E.J.; Blain, M.; de Lima, K.A.; Takenaka, M.C.; et al. Metabolic Control of Astrocyte Pathogenic Activity via CPLA2-MAVS. Cell 2019, 179, 1483–1498.e22. [Google Scholar] [CrossRef]
- Chatterjee, S.B.; Hou, J.; Bandaru, V.V.R.; Pezhouh, M.K.; Syed Rifat Mannan, A.A.; Sharma, R. Lactosylceramide Synthase β-1,4-GalT-V: A Novel Target for the Diagnosis and Therapy of Human Colorectal Cancer. Biochem. Biophys. Res. Commun. 2019, 508, 380–386. [Google Scholar] [CrossRef]
- Tang, W.; Li, M.; Qi, X.; Li, J. Β1,4-Galactosyltransferase V Modulates Breast Cancer Stem Cells through Wnt/β-Catenin Signaling Pathway. Cancer Res. Treat. 2020, 52, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, Z.; Wu, Q.; Liu, H.; Sun, Z.; Wu, Y.; Luo, J. B4GALT5 High Expression Associated with Poor Prognosis of Hepatocellular Carcinoma. BMC Cancer 2022, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, B.; Chen, X.; Geng, X.; Zhang, Z. Circ_0009910 Sponges MiR-491-5p to Promote Acute Myeloid Leukemia Progression through Modulating B4GALT5 Expression and PI3K/AKT Signaling Pathway. Int. J. Lab. Hematol. 2022, 44, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Riley, C.L.; Yu, J.; Keffler, J.A.; Clarke, C.J.; Van Laer, A.O.; Baicu, C.F.; Zile, M.R.; Gudz, T.I. Lactosylceramide Contributes to Mitochondrial Dysfunction in Diabetes. J. Lipid Res. 2016, 57, 546–562. [Google Scholar] [CrossRef] [Green Version]
- Filimoniuk, A.; Blachnio-Zabielska, A.; Imierska, M.; Lebensztejn, D.M.; Daniluk, U. Sphingolipid Analysis Indicate Lactosylceramide as a Potential Biomarker of Inflammatory Bowel Disease in Children. Biomolecules 2020, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Hocine, T.; Blaise, S.; Hachet, C.; Guillot, A.; Sartelet, H.; Maurice, P.; Bennasroune, A.; Martiny, L.; Duca, L.; Romier-Crouzet, B.; et al. Lactosylceramide Induced by Elastin-Derived Peptides Decreases Adipocyte Differentiation. J. Physiol. Biochem. 2020, 76, 457–467. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Nakayama, H.; Hanafusa, K. Lactosylceramide-Enriched Microdomains Mediate Human Neutrophil Immunological Functions via Carbohydrate-Carbohydrate Interaction. Glycoconj. J. 2022, 39, 239–246. [Google Scholar] [CrossRef]
- Hayashi, Y.; Horibata, Y.; Sakaguchi, K.; Okino, N.; Ito, M. A Sensitive and Reproducible Assay to Measure the Activity of Glucosylceramide Synthase and Lactosylceramide Synthase Using HPLC and Fluorescent Substrates. Anal. Biochem. 2005, 345, 181–186. [Google Scholar] [CrossRef]
- Dei Cas, M.; Casati, S.; Roda, G.; Sardi, P.; Paroni, R.; Fonzo, A.; Trinchera, M. A Sensitive Method for Determining UDP-Glucose: Ceramide Glucosyltransferase (UGCG) Activity in Biological Samples Using Deuterated Glucosylceramide as Acceptor Substrate. Glycobiology 2022, 33, 88–94. [Google Scholar] [CrossRef]
- Zulueta, A.; Caretti, A.; Signorelli, P.; Dall’Olio, F.; Trinchera, M. Transcriptional Control of the B3GALT5 Gene by a Retroviral Promoter and Methylation of Distant Regulatory Elements. FASEB J. 2014, 28, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Indellicato, R.; Parini, R.; Domenighini, R.; Malagolini, N.; Iascone, M.; Gasperini, S.; Masera, N.; Dall’Olio, F.; Trinchera, M. Total Loss of GM3 Synthase Activity by a Normally Processed Enzyme in a Novel Variant and in All ST3GAL5 Variants Reported to Cause a Distinct Congenital Disorder of Glycosylation. Glycobiology 2019, 29, 229–241. [Google Scholar] [CrossRef]
- Aronica, A.; Avagliano, L.; Caretti, A.; Tosi, D.; Bulfamante, G.P.; Trinchera, M. Unexpected Distribution of CA19.9 and Other Type 1 Chain Lewis Antigens in Normal and Cancer Tissues of Colon and Pancreas: Importance of the Detection Method and Role of Glycosyltransferase Regulation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3210–3220. [Google Scholar] [CrossRef]
- Trinchera, M.; Fiorilli, A.; Ghidoni, R. Localization in the Golgi Apparatus of Rat Liver UDP-Gal:Glucosylceramide Beta 1----4galactosyltransferase. Biochemistry 1991, 30, 2719–2724. [Google Scholar] [CrossRef]
- Salvini, R.; Bardoni, A.; Valli, M.; Trinchera, M. Beta 1,3-Galactosyltransferase Beta 3Gal-T5 Acts on the GlcNAcbeta 1-->3Galbeta 1-->4GlcNAcbeta 1-->R Sugar Chains of Carcinoembryonic Antigen and Other N-Linked Glycoproteins and Is down-Regulated in Colon Adenocarcinomas. J. Biol. Chem. 2001, 276, 3564–3573. [Google Scholar] [CrossRef] [Green Version]
- Indellicato, R.; Domenighini, R.; Malagolini, N.; Cereda, A.; Mamoli, D.; Pezzani, L.; Iascone, M.; Dall’olio, F.; Trinchera, M. A Novel Nonsense and Inactivating Variant of ST3GAL3 in Two Infant Siblings Suffering Severe Epilepsy and Expressing Circulating CA19.9. Glycobiology 2020, 30, 95–104. [Google Scholar] [CrossRef]




| Total Protein | Ovalbumin Galactosyltransferase | Asialofetuin Sialyltransferase | LacCer Synthase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| μg | (%) | Specific Activity | (RSA) | Recovery % | Specific Activity | (RSA) | Recovery % | Specific Activity | |
| Total homogenate | 5670 | 100 | 16.7 | 1 | 100 | 10.5 | 1 | 100 | ND |
| Golgi fraction | 93.6 | 1.65 | 337 | 20.2 | 33.3 | 300 | 28.5 | 47.1 | 7.5 |
| LOD | LOQ | CV% LacCer | CV% GlcCer | |
|---|---|---|---|---|
| pmol/mg × min | pmol/mg × min | n = 6 | n = 6 | |
| LC-MS/MS | 0.05 | 0.2 | 3.34 | 5.07 |
| Radiochemical method | 100 | 330 | 10.7 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei Cas, M.; Montavoci, L.; Casati, S.; Malagolini, N.; Dall’Olio, F.; Trinchera, M. Convenient and Sensitive Measurement of Lactosylceramide Synthase Activity Using Deuterated Glucosylceramide and Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 5291. https://doi.org/10.3390/ijms24065291
Dei Cas M, Montavoci L, Casati S, Malagolini N, Dall’Olio F, Trinchera M. Convenient and Sensitive Measurement of Lactosylceramide Synthase Activity Using Deuterated Glucosylceramide and Mass Spectrometry. International Journal of Molecular Sciences. 2023; 24(6):5291. https://doi.org/10.3390/ijms24065291
Chicago/Turabian StyleDei Cas, Michele, Linda Montavoci, Sara Casati, Nadia Malagolini, Fabio Dall’Olio, and Marco Trinchera. 2023. "Convenient and Sensitive Measurement of Lactosylceramide Synthase Activity Using Deuterated Glucosylceramide and Mass Spectrometry" International Journal of Molecular Sciences 24, no. 6: 5291. https://doi.org/10.3390/ijms24065291
APA StyleDei Cas, M., Montavoci, L., Casati, S., Malagolini, N., Dall’Olio, F., & Trinchera, M. (2023). Convenient and Sensitive Measurement of Lactosylceramide Synthase Activity Using Deuterated Glucosylceramide and Mass Spectrometry. International Journal of Molecular Sciences, 24(6), 5291. https://doi.org/10.3390/ijms24065291









