Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay?
Abstract
:1. Introduction
2. Heavy Metals and the Kidney
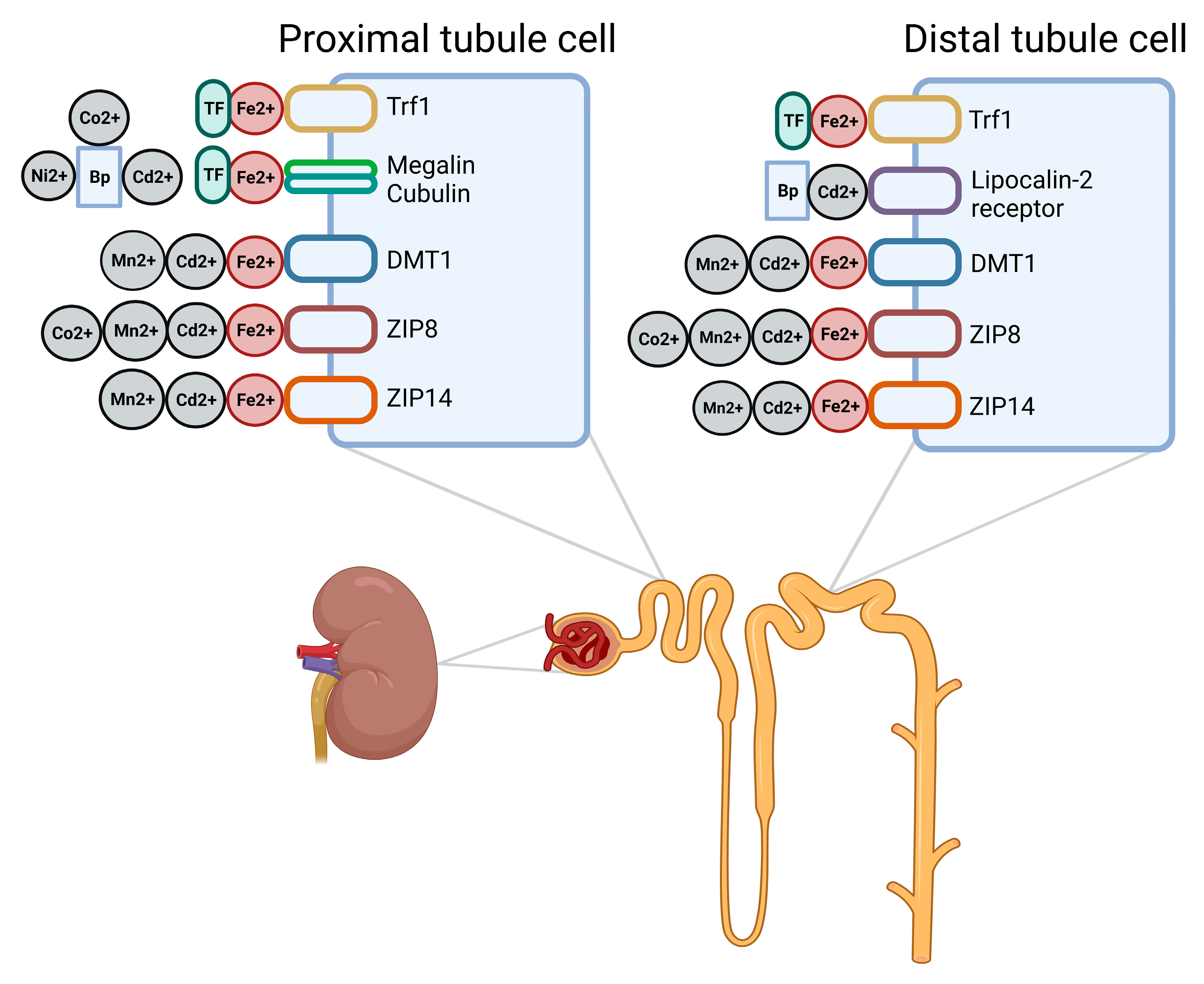
3. Handling of Heavy Metals by the Kidney
| Metal (Most Common Oxidation State) | Primary Locations of Accumulation | Receptors Suggested to Be Involved in Renal Accumulation |
|---|---|---|
| Cadmium (Cd2+) | Kidneys and liver [72] | Cd2+: DMT1 [52,73], ZIP8 1 [52,74,75], ZIP14 [52] Cd2+- protein complexes: megalin–cubulin, lipocalin 2/NGAL/24p3 receptor [68,71,76] |
| Lead (Pb2+) | Bones [77] | Pb2+: Ca2+ channels [62] |
| Nickel (Ni2+) | Respiratory tract [78,79] | Ni2+: Ca2+ channels [64] |
| Manganese (Mn2+, Mn4+, and Mn7+) | Brain [80] | Mn2+: DMT1 [52], ZIP8 [36,52,81], ZIP14 [52] |
| Cobalt (Co2+ and Co3+) | Kidneys and liver [82] | Co2+: ZIP8 [61] |
| Mercury (Hg+ and Hg2+) | Kidneys [27] | Hg2+: OAT1 (basolateral) [65,66] |
4. Hepatic Transporters and Heavy Metal Accumulation in the Kidney
5. Iron Deficiency in CKD
6. Absorption of Iron in the Gut
7. Iron Handling in the Kidney
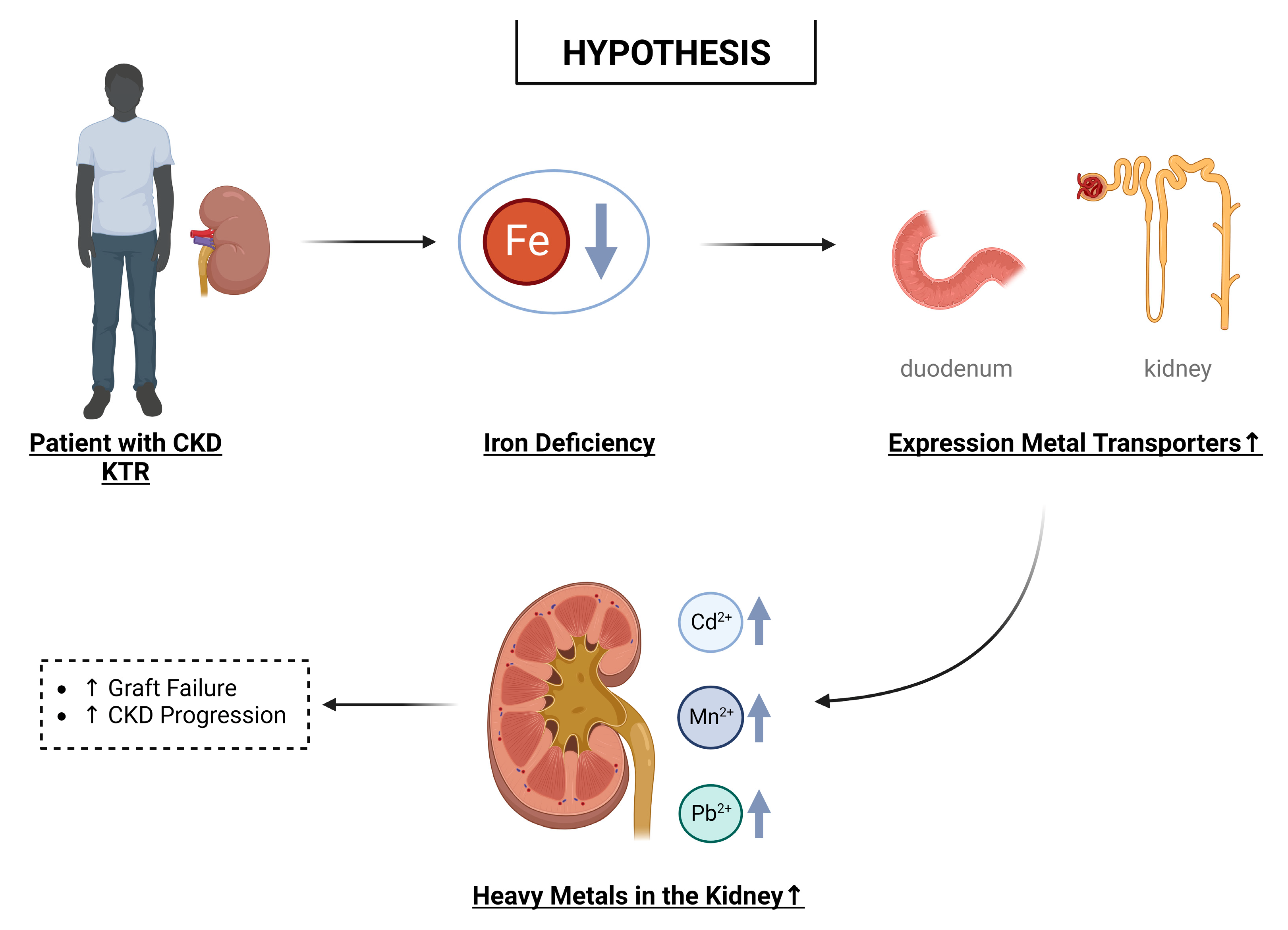
8. Hypothesis: Iron Deficiency and Nephrotoxic Effects of Heavy Metals
9. Possible Implications
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, p. 133. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Heavy Metal Emissions in Europe. Available online: https://www.eea.uropa.eu/ims/heavy-metal-emissions-in-europe (accessed on 10 January 2023).
- Yabe, J.; Ishizuka, M.; Umemura, T. Current levels of heavy metal pollution in Africa. J. Vet. Med. Sci. 2010, 72, 1257–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Chen, F.; Li, Y.; Ren, B. Trends and Sources of Heavy Metal Pollution in Global River and Lake Sediments from 1970 to 2018; Springer: Cham, Switzerland, 2020; pp. 1–35. [Google Scholar]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Chang, J.Y.; Hong, J.; Shin, S.; Park, J.S.; Oh, S. Low-Level Toxic Metal Exposure in Healthy Weaning-Age Infants: Association with Growth, Dietary Intake, and Iron Deficiency. Int. J. Environ. Res. Public Health 2017, 14, 388. [Google Scholar] [CrossRef] [Green Version]
- Jarup, L.; Hellstrom, L.; Alfven, T.; Carlsson, M.D.; Grubb, A.; Persson, B.; Pettersson, C.; Spang, G.; Schutz, A.; Elinder, C.G. Low level exposure to cadmium and early kidney damage: The OSCAR study. Occup. Environ. Med. 2000, 57, 668–672. [Google Scholar] [CrossRef] [Green Version]
- Nordberg, G.; Fowler, B.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Vogel, N.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Doyle, U.; Kampfe, A.; Hora, C.; Hildebrand, J.; Schafer, M.; Drexler, H.; et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany-Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2021, 237, 113822. [Google Scholar] [CrossRef]
- European Food Safety Authority. Cadmium in food-Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 7, 980. [Google Scholar] [CrossRef]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Krajcovicova-Kudladkova, M.; Ursinyova, M.; Masanova, V.; Bederova, A.; Valachovicova, M. Cadmium blood concentrations in relation to nutrition. Cent. Eur. J. Public Health 2006, 14, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Snoj Tratnik, J.; Kocman, D.; Horvat, M.; Andersson, A.M.; Juul, A.; Jacobsen, E.; Olafsdottir, K.; Klanova, J.; Andryskova, L.; Janasik, B.; et al. Cadmium exposure in adults across Europe: Results from the HBM4EU Aligned Studies survey 2014–2020. Int. J. Hyg. Environ. Health 2022, 246, 114050. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment Information System: Toxicity Summary for Cadmium. Available online: https://rais.ornl.gov/tox/profiles/cadmium.html (accessed on 24 February 2023).
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Lentini, P.; Zanoli, L.; Granata, A.; Signorelli, S.S.; Castellino, P.; Dell’Aquila, R. Kidney and heavy metals-The role of environmental exposure (Review). Mol. Med. Rep. 2017, 15, 3413–3419. [Google Scholar] [CrossRef] [Green Version]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cui, W.; Wang, M.; Liang, Y.; Zhu, G.; Jin, T.; Chen, X. The association between life-time dietary cadmium intake from rice and chronic kidney disease. Ecotoxicol. Environ. Saf. 2021, 211, 111933. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.C.; Hsu, H.T.; Mao, Y.C.; Wu, C.C.; Ho, C.T.; Liu, C.S.; Chung, C.J. Association and mediation analyses among multiple metals exposure, plasma folate, and community-based impaired estimated glomerular filtration rate in central Taiwan. Environ. Health 2022, 21, 44. [Google Scholar] [CrossRef]
- Nan, Y.; Yang, J.; Ma, L.; Jin, L.; Bai, Y. Associations of nickel exposure and kidney function in U.S. adults, NHANES 2017–2018. J. Trace Elem. Med. Biol. 2022, 74, 127065. [Google Scholar] [CrossRef]
- Tsai, H.J.; Hung, C.H.; Wang, C.W.; Tu, H.P.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Kuo, C.H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Lopez-Chaves, C.; Gomez-Aracena, J.; Galindo, P.; Aranda, P.; Llopis, J. Association of plasma manganese levels with chronic renal failure. J. Trace Elem. Med. Biol. 2015, 31, 78–84. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury Draft for Public Comment; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2022. [Google Scholar]
- Kim, N.H.; Hyun, Y.Y.; Lee, K.B.; Chang, Y.; Ryu, S.; Oh, K.H.; Ahn, C. Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci. 2015, 30, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lee, B.K. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the Korean general population: Analysis of 2008–2010 Korean National Health and Nutrition Examination Survey data. Environ. Res. 2012, 118, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sommar, J.N.; Svensson, M.K.; Bjor, B.M.; Elmstahl, S.I.; Hallmans, G.; Lundh, T.; Schon, S.M.; Skerfving, S.; Bergdahl, I.A. End-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ. Health 2013, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.H.; Ke, D.Y.; Wang, J.E.; Chan, C.C. Associations between renal functions and exposure of arsenic and polycyclic aromatic hydrocarbon in adults living near a petrochemical complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef]
- Butler-Dawson, J.; James, K.A.; Krisher, L.; Jaramillo, D.; Dally, M.; Neumann, N.; Pilloni, D.; Cruz, A.; Asensio, C.; Johnson, R.J.; et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 461–471. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Xiao, Y.; Li, Y.; Yu, Y.; Mo, T.; Jiang, H.; Li, X.; Yang, H.; Xu, C.; et al. Associations of plasma metal concentrations with the decline in kidney function: A longitudinal study of Chinese adults. Ecotoxicol. Environ. Saf. 2020, 189, 110006. [Google Scholar] [CrossRef]
- Vyskocil, A.; Senft, V.; Viau, C.; Cizkova, M.; Kohout, J. Biochemical renal changes in workers exposed to soluble nickel compounds. Hum. Exp. Toxicol. 1994, 13, 257–261. [Google Scholar] [CrossRef]
- Martin, P.; Fareh, M.; Poggi, M.C.; Boulukos, K.E.; Pognonec, P. Manganese is highly effective in protecting cells from cadmium intoxication. Biochem. Biophys. Res. Commun. 2006, 351, 294–299. [Google Scholar] [CrossRef]
- Fujishiro, H.; Himeno, S. New Insights into the Roles of ZIP8, a Cadmium and Manganese Transporter, and Its Relation to Human Diseases. Biol. Pharm. Bull. 2019, 42, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Hendryx, M. Metal mixtures and kidney function: An application of machine learning to NHANES data. Environ. Res. 2020, 191, 110126. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Gelein, R.M.; Cherian, M.G. Shifts in the dose-effect relationship for the nephropathy induced by cadmium-metallothionein in rats after a reduction in renal mass. J. Pharmacol. Exp. Ther. 1992, 262, 1256–1266. [Google Scholar] [PubMed]
- Danziger, J.; Dodge, L.E.; Hu, H.; Mukamal, K.J. Susceptibility to Environmental Heavy Metal Toxicity among Americans with Kidney Disease. Kidney360 2022, 3, 1191–1196. [Google Scholar] [CrossRef]
- Mishra, M.; Nichols, L.; Dave, A.A.; Pittman, E.H.; Cheek, J.P.; Caroland, A.J.V.; Lotwala, P.; Drummond, J.; Bridges, C.C. Molecular Mechanisms of Cellular Injury and Role of Toxic Heavy Metals in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 11105. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, L.; Elinder, C.G.; Dahlberg, B.; Lundberg, M.; Jarup, L.; Persson, B.; Axelson, O. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. [Google Scholar] [CrossRef]
- Palaneeswari, M.S.; Rajan, P.M.; Silambanan, S.; Jothimalar. Blood Arsenic and Cadmium Concentrations in End-Stage Renal Disease Patients who were on Maintenance Haemodialysis. J. Clin. Diagn. Res. 2013, 7, 809–813. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Eisenga, M.F.; Knobbe, T.J.; IJmker, J.; Lammerts, R.G.M.; de Borst, M.H.; Berger, S.P.; Nolte, I.M.; et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int. 2021, 99, 1213–1224. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Giubergia, F.; Groothof, D.; Ferreccio, C.; Nolte, I.M.; Navis, G.J.; Gomes-Neto, A.W.; Kremer, D.; Knobbe, T.J.; Eisenga, M.F.; et al. Plasma Lead Concentration and Risk of Late Kidney Allograft Failure: Findings From the TransplantLines Biobank and Cohort Studies. Am. J. Kidney Dis. 2022, 80, 87–97.e81. [Google Scholar] [CrossRef]
- Fransson, M.N.; Barregard, L.; Sallsten, G.; Akerstrom, M.; Johanson, G. Physiologically-Based Toxicokinetic Model for Cadmium Using Markov-Chain Monte Carlo Analysis of Concentrations in Blood, Urine, and Kidney Cortex from Living Kidney Donors. Toxicol. Sci. 2014, 141, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, M.; Gougoux, A.; Noël, J.; Parent, L. The Renal Fanconi Syndrome|The Online Metabolic and Molecular Bases of Inherited Disease|OMMBID|; McGraw Hill Medical: New York, NY, USA, 1989. [Google Scholar]
- Garrick, M.D.; Singleton, S.T.; Vargas, F.; Kuo, H.C.; Zhao, L.; Knopfel, M.; Davidson, T.; Costa, M.; Paradkar, P.; Roth, J.A.; et al. DMT1: Which metals does it transport? Biol. Res. 2006, 39, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Bannon, D.I.; Abounader, R.; Lees, P.S.; Bressler, J.P. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell Physiol. 2003, 284, C44–C50. [Google Scholar] [CrossRef] [Green Version]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, R.O.; Zoller, H.; Theuri, I.; Obrist, P.; Egg, G.; Strohmayer, W.; Vogel, W.; Weiss, G. Distribution of DMT 1 within the human glandular system. Histol. Histopathol. 2003, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, S.; van Swelm, R.; Bouman, K.; Cliteur, M.; van den Heuvel, M.C.; Pertijs, J.; Patel, D.; Bass, P.; van Goor, H.; Unwin, R.; et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 2018, 8, 9353. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Balusikova, K.; Dostalikova-Cimburova, M.; Tacheci, I.; Kovar, J. Expression profiles of iron transport molecules along the duodenum. J. Cell. Mol. Med. 2022, 26, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [Green Version]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Ohrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [Green Version]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebert, D.W.; Galvez-Peralta, M.; Hay, E.B.; Li, H.; Johansson, E.; Yin, C.; Wang, B.; He, L.; Soleimani, M. ZIP14 and ZIP8 zinc/bicarbonate symporters in Xenopus oocytes: Characterization of metal uptake and inhibition. Metallomics 2012, 4, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Verouti, S.N.; Pujol-Gimenez, J.; Bermudez-Lekerika, P.; Scherler, L.; Bhardwaj, R.; Thomas, A.; Lenglet, S.; Siegrist, M.; Hofstetter, W.; Fuster, D.G.; et al. The Allelic Variant A391T of Metal Ion Transporter ZIP8 (SLC39A8) Leads to Hypotension and Enhanced Insulin Resistance. Front. Physiol. 2022, 13, 912277. [Google Scholar] [CrossRef] [PubMed]
- Bogden, J.D.; Gertner, S.B.; Christakos, S.; Kemp, F.W.; Yang, Z.; Katz, S.R.; Chu, C. Dietary calcium modifies concentrations of lead and other metals and renal calbindin in rats. J. Nutr. 1992, 122, 1351–1360. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [Green Version]
- Refsvik, T.; Andreassen, T. Surface binding and uptake of nickel(II) in human epithelial kidney cells: Modulation by ionomycin, nicardipine and metals. Carcinogenesis 1995, 16, 1107–1112. [Google Scholar] [CrossRef]
- Koh, A.S.; Simmons-Willis, T.A.; Pritchard, J.B.; Grassl, S.M.; Ballatori, N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: Transport by the renal organic anion transporter-1. Mol. Pharmacol. 2002, 62, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.M.; Dnyanmote, A.V.; Bush, K.T.; Wu, W.; Nigam, S.K. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J. Biol. Chem. 2011, 286, 26391–26395. [Google Scholar] [CrossRef] [Green Version]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Metallothioneins and Related Chelators; Royal Society of Chemistry Cambridge: Cambridge, UK, 2009; pp. 1–514. [Google Scholar] [CrossRef]
- Fels, J.; Scharner, B.; Zarbock, R.; Zavala Guevara, I.P.; Lee, W.K.; Barbier, O.C.; Thevenod, F. Cadmium Complexed with beta2-Microglubulin, Albumin and Lipocalin-2 rather than Metallothionein Cause Megalin:Cubilin Dependent Toxicity of the Renal Proximal Tubule. Int. J. Mol. Sci. 2019, 20, 2379. [Google Scholar] [CrossRef] [Green Version]
- Bal, W.; Sokolowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thevenod, F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [Google Scholar]
- Olivi, L.; Sisk, J.; Bressler, J. Involvement of DMT1 in uptake of Cd in MDCK cells: Role of protein kinase C. Am. J. Physiol. Cell Physiol. 2001, 281, C793–C800. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Hamao, S.; Isawa, M.; Himeno, S. Segment-specific and direction-dependent transport of cadmium and manganese in immortalized S1, S2, and S3 cells derived from mouse kidney proximal tubules. J. Toxicol. Sci. 2019, 44, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.N.; Liu, Z.; Wang, B.; Miller, M.L.; Afton, S.E.; Soleimani, M.; Nebert, D.W. Oral cadmium in mice carrying 5 versus 2 copies of the Slc39a8 gene: Comparison of uptake, distribution, metal content, and toxicity. Int. J. Toxicol. 2014, 33, 14–20. [Google Scholar] [CrossRef]
- Thevenod, F.; Wolff, N.A. Iron transport in the kidney: Implications for physiology and cadmium nephrotoxicity. Metallomics 2016, 8, 17–42. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.-W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2007; Volume 582. [Google Scholar]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef]
- Toxicology Department. Nickel Toxicological Overview Key Points; Public Health England: London, UK, 2009. [Google Scholar]
- Williams, M.; Todd, G.D.; Roney, N.; Crawford, J.; Coles, C.; Garey, J.D.; Zaccaria, K.; Citra, M.; Williams, M.; Todd, G.D.; et al. Toxicological Profile for Manganese; 8004471544; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012; pp. 143–150. [Google Scholar]
- Lin, W.; Vann, D.R.; Doulias, P.T.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J.; et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investig. 2017, 127, 2407–2417. [Google Scholar] [CrossRef] [Green Version]
- Paustenbach, D.J.; Tvermoes, B.E.; Unice, K.M.; Finley, B.L.; Kerger, B.D. A review of the health hazards posed by cobalt. Crit. Rev. Toxicol. 2013, 43, 316–362. [Google Scholar] [CrossRef]
- Jorge-Nebert, L.F.; Galvez-Peralta, M.; Landero Figueroa, J.; Somarathna, M.; Hojyo, S.; Fukada, T.; Nebert, D.W. Comparing gene expression during cadmium uptake and distribution: Untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol. Sci. 2015, 143, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Malyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Awan, A.A.; Walther, C.P.; Richardson, P.A.; Shah, M.; Winkelmayer, W.C.; Navaneethan, S.D. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- 2018 Annual Data Report. Available online: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/prior-data-reports/2018 (accessed on 10 January 2023).
- Hamano, T.; Fujii, N.; Hayashi, T.; Yamamoto, H.; Iseki, K.; Tsubakihara, Y. Thresholds of iron markers for iron deficiency erythropoiesis-finding of the Japanese nationwide dialysis registry. Kidney Int. Suppl. 2015, 5, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Vinke, J.S.J.; Francke, M.I.; Eisenga, M.F.; Hesselink, D.A.; de Borst, M.H. Iron deficiency after kidney transplantation. Nephrol. Dial. Transplant. 2021, 36, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Schechter, A.; Rozen-Zvi, B. Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 2019, 142, 44–50. [Google Scholar] [CrossRef]
- Liang, C.C.; Wang, S.M.; Kuo, H.L.; Chang, C.T.; Liu, J.H.; Lin, H.H.; Wang, I.K.; Yang, Y.F.; Lu, Y.J.; Chou, C.Y.; et al. Upper gastrointestinal bleeding in patients with CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Lee, T.C.; Montez-Rath, M.E.; Paik, J.; Chertow, G.M.; Desai, M.; Winkelmayer, W.C. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J. Am. Soc. Nephrol. 2012, 23, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Ashby, D.R.; Gale, D.P.; Busbridge, M.; Murphy, K.G.; Duncan, N.D.; Cairns, T.D.; Taube, D.H.; Bloom, S.R.; Tam, F.W.; Chapman, R.S.; et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009, 75, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Zaritsky, J.; Young, B.; Wang, H.J.; Westerman, M.; Olbina, G.; Nemeth, E.; Ganz, T.; Rivera, S.; Nissenson, A.R.; Salusky, I.B. Hepcidin—A potential novel biomarker for iron status in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 649–651. [Google Scholar]
- Ems, T.; Lucia, K.S.; Huecker, M.R. Biochemistry, Iron Absorption. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448204 (accessed on 10 January 2023).
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria-a complex interrelationship. Gut Microbes 2021, 13, 1874855. [Google Scholar] [CrossRef]
- Norden, A.G.; Lapsley, M.; Lee, P.J.; Pusey, C.D.; Scheinman, S.J.; Tam, F.W.; Thakker, R.V.; Unwin, R.J.; Wrong, O. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001, 60, 1885–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Raaij, S.E.G.; Rennings, A.J.; Biemond, B.J.; Schols, S.E.M.; Wiegerinck, E.T.G.; Roelofs, H.M.J.; Hoorn, E.J.; Walsh, S.B.; Nijenhuis, T.; Swinkels, D.W.; et al. Iron handling by the human kidney: Glomerular filtration and tubular reabsorption both contribute to urinary iron excretion. Am. J. Physiol. Ren. Physiol. 2019, 316, F606–F614. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Spektor, L.; Cohen, A.L.; Lifshitz, L.; Magid Gold, I.; Zhang, D.L.; Truman-Rosentsvit, M.; Leichtmann-Bardoogo, Y.; Nyska, A.; Addadi, S.; et al. Orchestrated regulation of iron trafficking proteins in the kidney during iron overload facilitates systemic iron retention. PLoS ONE 2018, 13, e0204471. [Google Scholar] [CrossRef] [Green Version]
- Carrero, J.J.; Gonzalez-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Molne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources. Environ. Res. 2010, 110, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Akesson, A.; Nermell, B.; Vahter, M. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ. Health Perspect. 1994, 102, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Sand, S.; Nalsen, C.; Lundh, T.; Enghardt Barbieri, H.; Pearson, M.; Lindroos, A.K.; Jonsson, B.A.; Barregard, L.; Darnerud, P.O. Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food Chem. Toxicol. 2013, 57, 161–169. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, J.E.; Lee, D.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Nah, J.W.; Kim, S.K. Prevalence and Relationships of Iron Deficiency Anemia with Blood Cadmium and Vitamin D Levels in Korean Women. J. Korean Med. Sci. 2016, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Brantsaeter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Valberg, L.S.; Ludwig, J.; Olatunbosun, D. Alteration in cobalt absorption in patients with disorders of iron metabolism. Gastroenterology 1969, 56, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bradman, A.; Eskenazi, B.; Sutton, P.; Athanasoulis, M.; Goldman, L.R. Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ. Health Perspect. 2001, 109, 1079–1084. [Google Scholar] [CrossRef]
- Kwong, W.T.; Friello, P.; Semba, R.D. Interactions between iron deficiency and lead poisoning: Epidemiology and pathogenesis. Sci. Total Environ. 2004, 330, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Kazi, T.G.; Afridi, H.I.; Baig, J.A.; Khan, S.; Kolachi, N.F.; Wadhwa, S.K.; Shah, A.Q. Environmental exposure of lead and iron deficit anemia in children age ranged 1–5 years: A cross sectional study. Sci. Total Environ. 2010, 408, 5325–5330. [Google Scholar] [CrossRef]
- Danziger, J.; Mukamal, K.J. Levels of Lead in Residential Drinking Water and Iron Deficiency among Patients with End Stage Kidney Disease. Kidney360 2022, 3, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.J.; Gellein, K.; Syversen, T.; Aschner, M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol. Sci. 2007, 95, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Kim, K.Y.; Choi, B.S.; Youn, P.; Ryu, D.Y.; Klaassen, C.D.; Park, J.D. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch. Toxicol. 2007, 81, 327–334. [Google Scholar] [CrossRef]
- Reuber, S.; Kreuzer, M.; Kirchgessner, M. Interactions of cobalt and iron in absorption and retention. J. Trace Elem. Electrolytes Health Dis. 1994, 8, 151–158. [Google Scholar]
- Ryu, D.Y.; Lee, S.J.; Park, D.W.; Choi, B.S.; Klaassen, C.D.; Park, J.D. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol. Lett. 2004, 152, 19–25. [Google Scholar] [CrossRef]
- Schafer, S.G.; Schwegler, U.; Schumann, K. Retention of cadmium in cadmium-naive normal and iron-deficient rats as well as in cadmium-induced iron-deficient animals. Ecotoxicol. Environ. Saf. 1990, 20, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Allerson, C.R.; Polycarpou-Schwarz, M.; Rofts, A.; Rogers, J.T.; Kishi, F.; Hentze, M.W.; Rouault, T.A.; Andrews, N.C.; Hediger, M.A. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001, 509, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoller, H.; Koch, R.O.; Theurl, I.; Obrist, P.; Pietrangelo, A.; Montosi, G.; Haile, D.J.; Vogel, W.; Weiss, G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001, 120, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; Mackenzie, B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, C862–C871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Raaij, S.E.G.; Srai, S.K.S.; Swinkels, D.W.; van Swelm, R.P.L. Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells. Biometals 2019, 32, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Schneider, S.N.; Dragin, N.; Girijashanker, K.; Dalton, T.P.; He, L.; Miller, M.L.; Stringer, K.F.; Soleimani, M.; Richardson, D.D.; et al. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am. J. Physiol. Cell Physiol. 2007, 292, C1523–C1535. [Google Scholar] [CrossRef] [Green Version]
- Canonne-Hergaux, F.; Gros, P. Expression of the iron transporter DMT1 in kidney from normal and anemic mk mice. Kidney Int. 2002, 62, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Wareing, M.; Ferguson, C.J.; Delannoy, M.; Cox, A.G.; McMahon, R.F.; Green, R.; Riccardi, D.; Smith, C.P. Altered dietary iron intake is a strong modulator of renal DMT1 expression. Am. J. Physiol. Renal Physiol. 2003, 285, F1050–F1059. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Su, S.; Zhai, R.; Chen, K.; Jin, T.; Huang, B.; Zhou, Y.; Ge, X.; Wei, G.; Liao, R. Lack of reversal effect of EDTA treatment on cadmium induced renal dysfunction: A fourteen-year follow-up. Biometals 2004, 17, 435–441. [Google Scholar] [CrossRef]
- Group, K.D.I.G.O.K.A.W. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.E.; Hansen, J.L.; Peters, C.B.; Cheung, A.K.; Greene, T.; Sauer, B.C. An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic Veterans with predialysis chronic kidney disease. Kidney Int. 2019, 96, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Nolte, I.M.; van der Meer, P.; Bakker, S.J.L.; Gaillard, C. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018, 19, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.M.Y.; Tu, C.; Li, Y.; Perlman, R.L.; Pecoits-Filho, R.; Lopes, A.A.; Narita, I.; Reichel, H.; Port, F.K.; Sukul, N.; et al. Anemia and iron deficiency among chronic kidney disease Stages 3-5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: Often unmeasured, variably treated. Clin. Kidney J. 2020, 13, 613–624. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawee, P.; Kremer, D.; Nolte, I.M.; Leuvenink, H.G.D.; Touw, D.J.; De Borst, M.H.; Bakker, S.J.L.; Hanudel, M.R.; Eisenga, M.F. Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay? Int. J. Mol. Sci. 2023, 24, 5315. https://doi.org/10.3390/ijms24065315
Rawee P, Kremer D, Nolte IM, Leuvenink HGD, Touw DJ, De Borst MH, Bakker SJL, Hanudel MR, Eisenga MF. Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay? International Journal of Molecular Sciences. 2023; 24(6):5315. https://doi.org/10.3390/ijms24065315
Chicago/Turabian StyleRawee, Pien, Daan Kremer, Ilja M. Nolte, Henri G. D. Leuvenink, Daan J. Touw, Martin H. De Borst, Stephan J. L. Bakker, Mark R. Hanudel, and Michele F. Eisenga. 2023. "Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay?" International Journal of Molecular Sciences 24, no. 6: 5315. https://doi.org/10.3390/ijms24065315
APA StyleRawee, P., Kremer, D., Nolte, I. M., Leuvenink, H. G. D., Touw, D. J., De Borst, M. H., Bakker, S. J. L., Hanudel, M. R., & Eisenga, M. F. (2023). Iron Deficiency and Nephrotoxic Heavy Metals: A Dangerous Interplay? International Journal of Molecular Sciences, 24(6), 5315. https://doi.org/10.3390/ijms24065315







