Effects of Osmotic Stress on the mRNA Expression of prl, prlr, gr, gh, and ghr in the Pituitary and Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii
Abstract
1. Introduction
2. Results
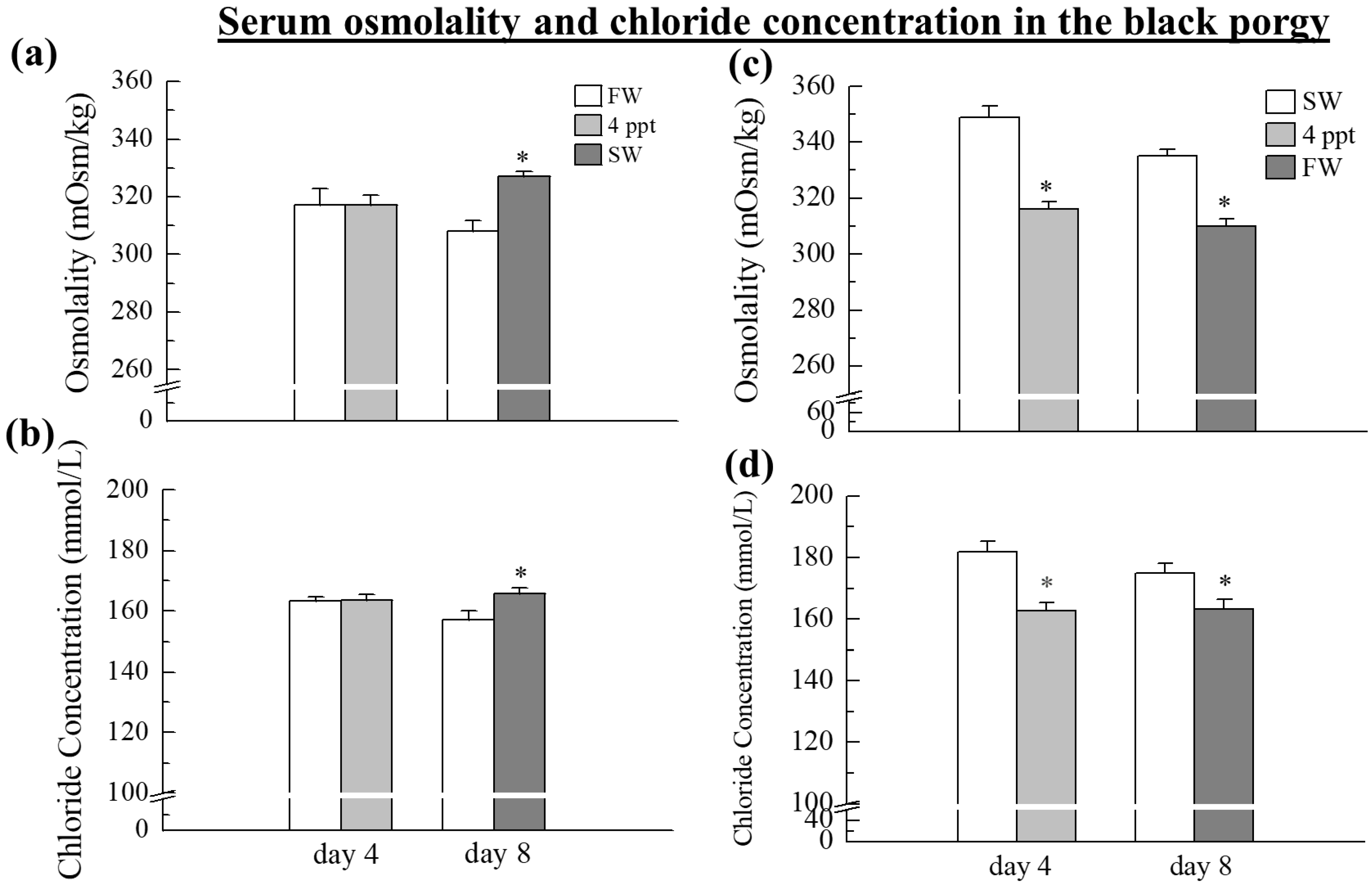
2.1. Serum Osmolality and Chloride Concentrations
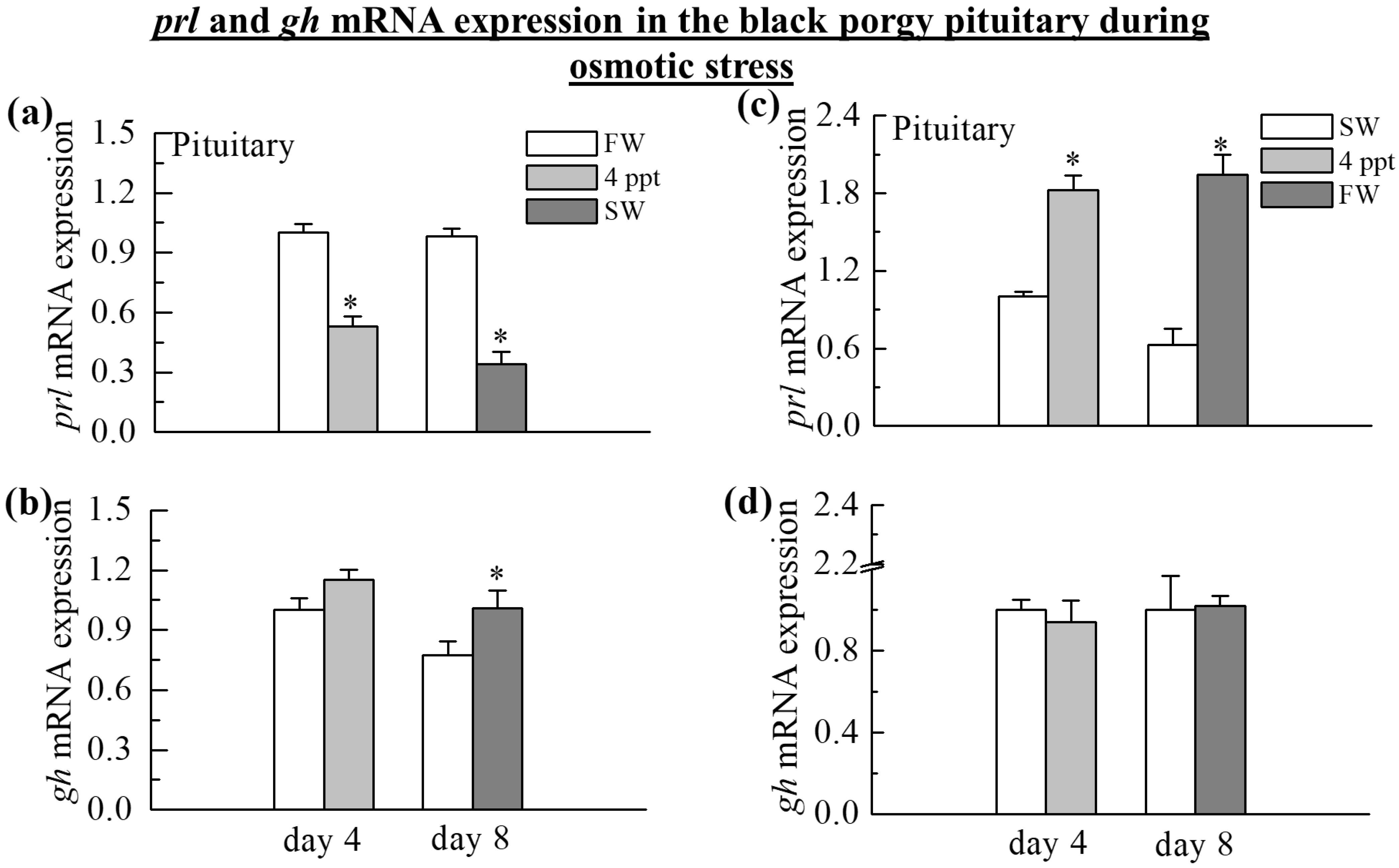
2.2. The Expression of prl and gh Transcripts in the Pituitary
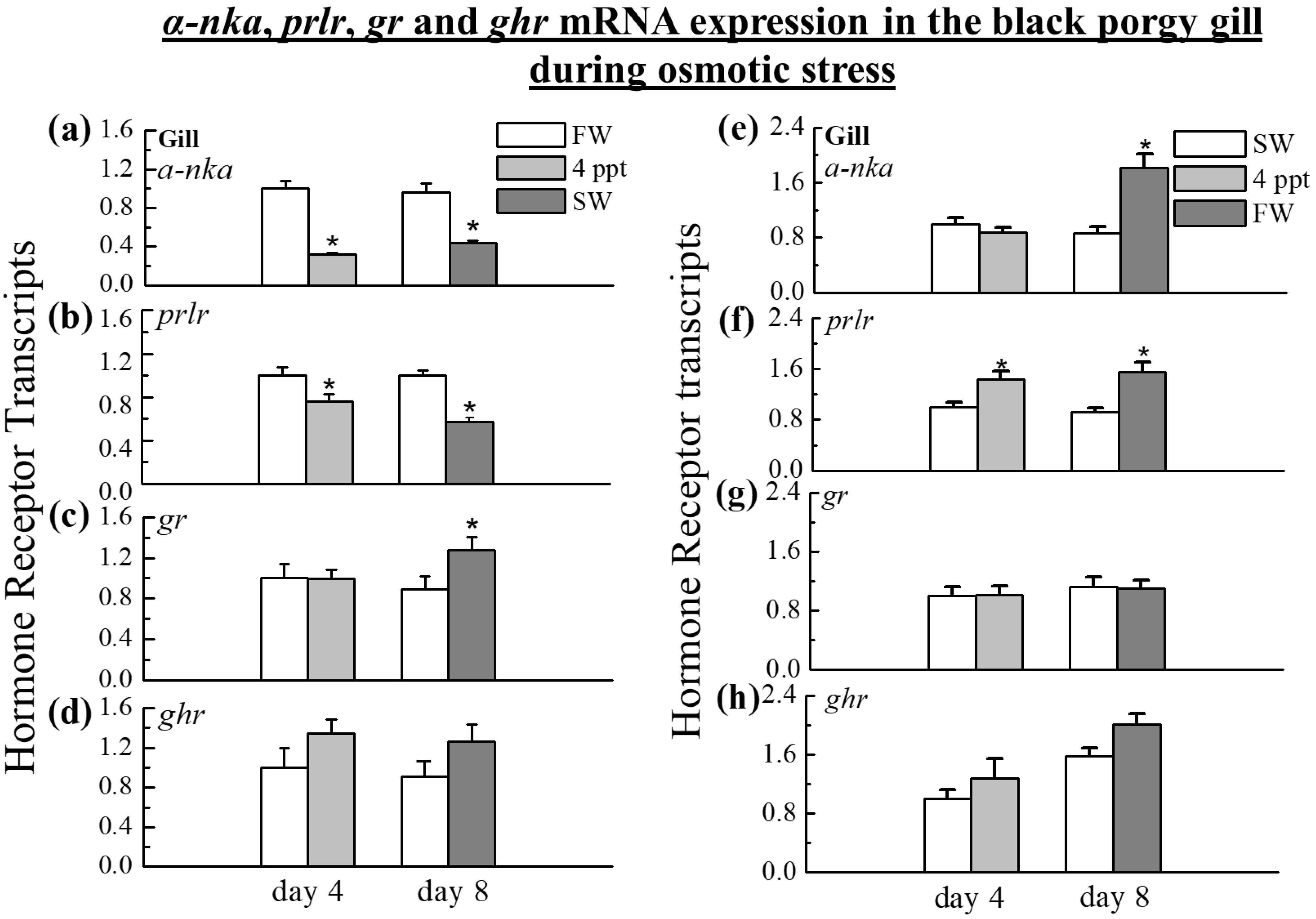
2.3. The Expression of α-nka, prlr, gr, and ghr Transcripts in the Gill
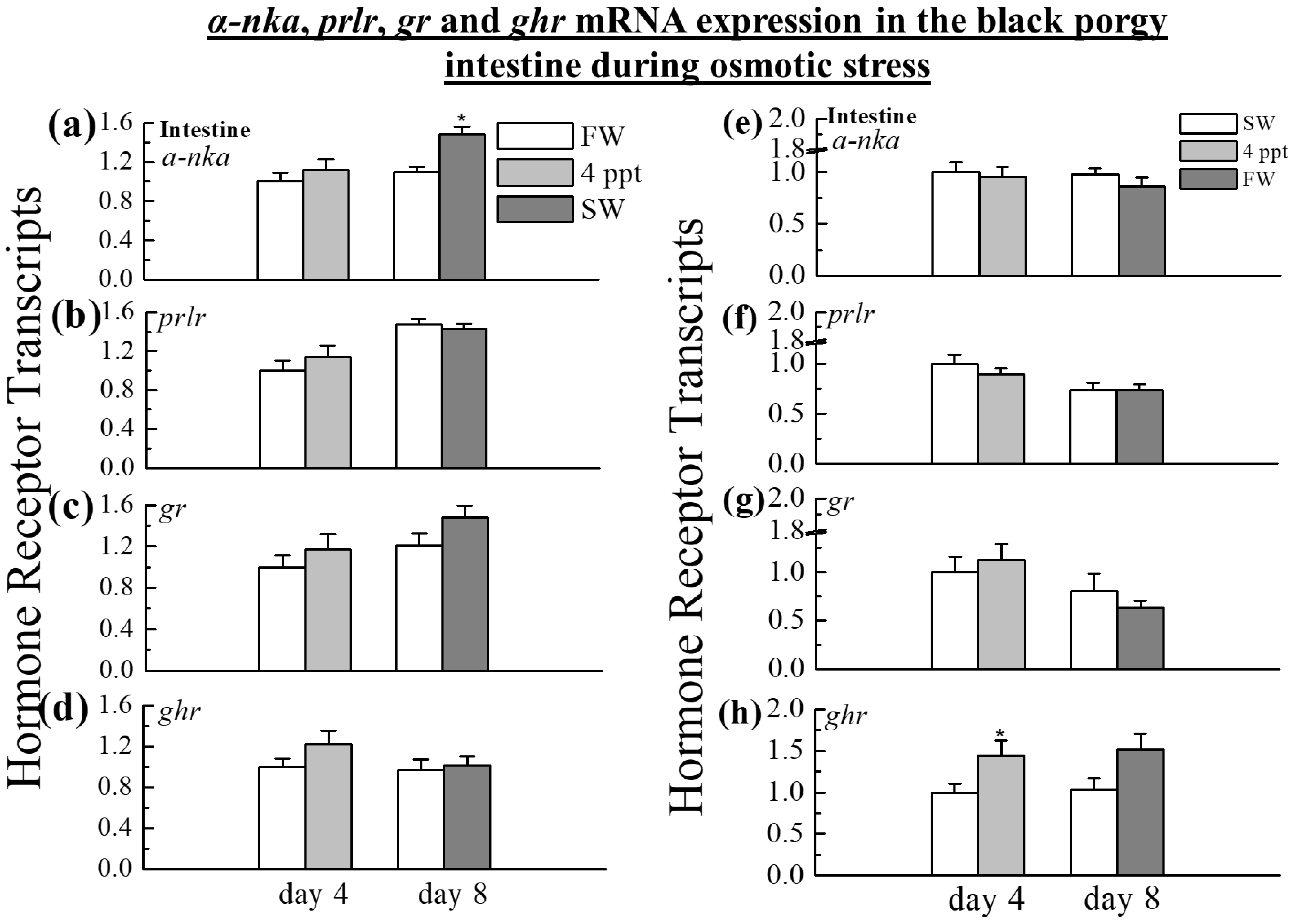
2.4. The Expression of α-nka, prlr, gr, and ghr in the Intestine
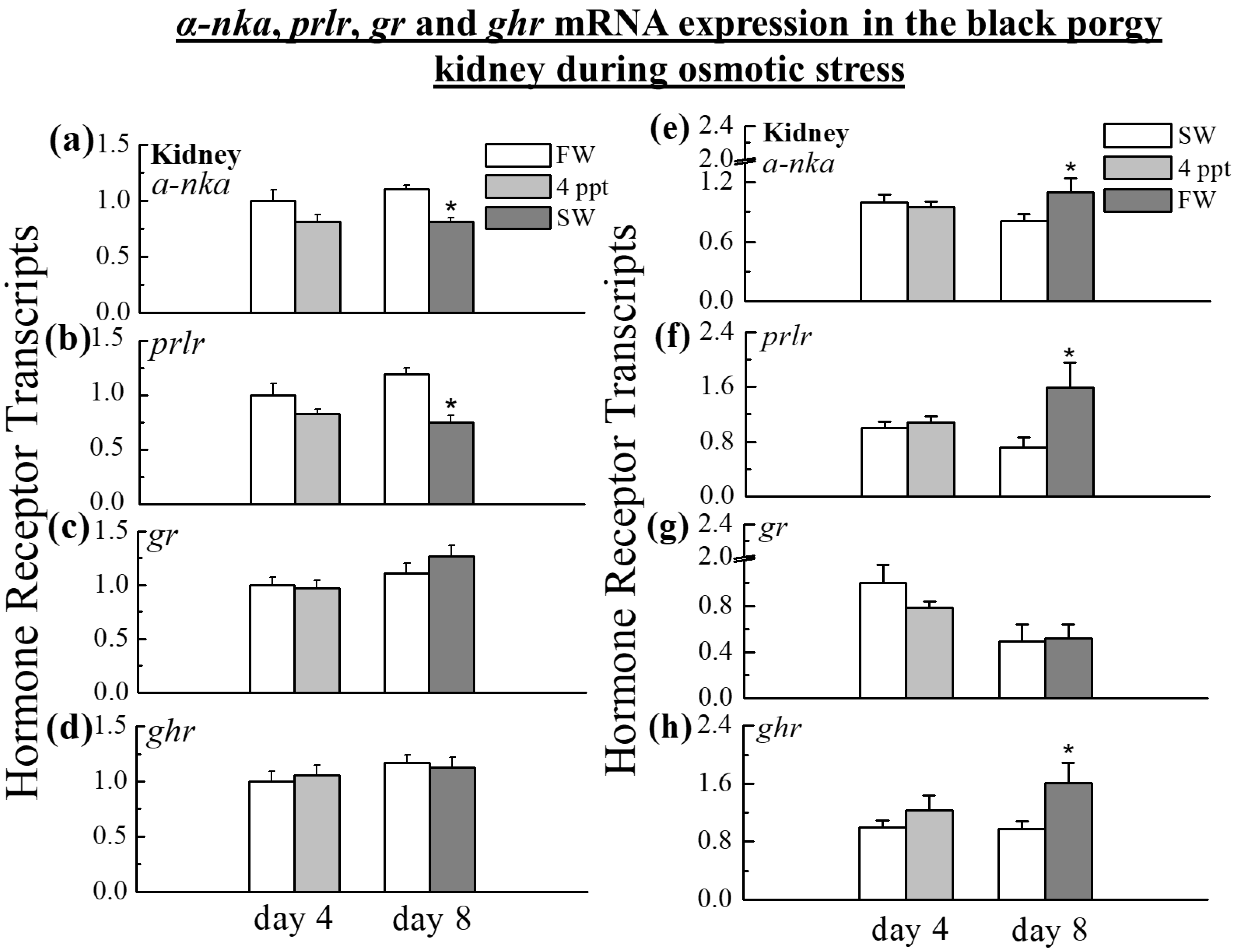
2.5. The Expression of α-nka, prlr, gr, and ghr in the Kidney
3. Discussion
4. Materials and Methods
4.1. Experimental Fish
4.2. Experiment of Osmotic Transfer
4.3. RNA Extraction, the First-Strand cDNA Synthesis, and Cloning
4.4. Quantification of α-nka, prl, prlr, gr, and ghr by Q-PCR Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aruna, A.; Nagarajan, G.; Chang, C.F. Involvement of corticotrophin releasing hormone and corticosteroid receptor in the brain-pituitary-gill of tilapia (Oreochromis mossambicus) during the course of seawater acclimation. J. Neuroendocrinol. 2012, 24, 818–830. [Google Scholar] [CrossRef]
- Rose, A.J.; Vegiopoulos, A.; Herzig, S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: Insights from genetic manipulations. J. Steroid Biochem. Mol. Biol. 2010, 122, 10–20. [Google Scholar] [CrossRef]
- Garabedian, M.J.; Harris, C.A.; Jeanneteau, F. Glucocorticoid receptor action in metabolic and neuronal function. F1000Research 2017, 6, 1208. [Google Scholar] [CrossRef]
- Lapp, H.E.; Bartlett, A.A.; Hunter, R. Stress and glucocorticoid receptor regulation of mitochondrial gene expression. J. Mol. Endocrinol. 2019, 62, R121–R128. [Google Scholar] [CrossRef]
- Greenwood, A.K.; Butler, P.C.; White, R.B.; DeMarco, U.; Pearce, D.; Fernald, R.D. Multiple corticosteroid receptors in a teleost fish: Distinct sequences, expression patterns, and transcriptional activities. Endocrinology 2003, 144, 4226–4236. [Google Scholar] [CrossRef]
- Bury, N.R.; Sturm, A.; Le Rouzic, P.; Lethimonier, C.; Ducouret, B.; Guiguen, Y.; Robinson Rechavi, M.; Laudet, V.; Rafestin-Oblin, M.E.; Prunet, P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003, 31, 141–156. [Google Scholar] [CrossRef]
- Lethimonier, C.; Tujague, M.; Kern, L.; Ducouret, B. Peptide insertion in the DNAbinding domain of fish glucocorticoid receptor is encoded by an additional exon and confers particular functional properties. Mol. Cell. Endocrinol. 2002, 194, 107–116. [Google Scholar] [CrossRef]
- Takeo, J.; Hata, J.; Segawa, C.; Toyohara, H.; Yamashita, S. Fish glucocorticoid receptor with splicing variants in the DNA binding domain. FEBS Lett. 1996, 389, 244–248. [Google Scholar] [CrossRef]
- Aruna, A.; Nagarajan, G.; Chang, C.F. Differential expression pattern and localization of glucocorticoid and mineralocorticoid receptors transcripts in osmoregulatory organs of freshwater and seawater acclimated tilapia. Gen. Comp. Endocrinol. 2012, 179, 465–476. [Google Scholar] [CrossRef]
- Aruna, A.; Nagarajan, G.; Chang, C.F. The acute salinity changes activate the dual pathways of endocrine responses in the brain and pituitary of tilapia. Gen. Comp. Endocrinol. 2015, 211, 154–164. [Google Scholar] [CrossRef]
- Alderman, S.L.; McGuire, A.; Bernier, N.J.; Vijayan, M.M. Central and peripheral glucocorticoid receptors are involved in the plasma cortisol response to an acute stressor in rainbow trout. Gen. Comp. Endocrinol. 2012, 176, 79–85. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. B 2016, 199, 136–145. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Gao, X.; Du, C.; Hou, C.; Wu, X.; Shen, W.; Zhu, J. Effects of acute low temperature stress on the hormones and gene expression of glucocorticoid receptor of large yellow croaker Larimichthys crocea. J. Therm. Biol. 2021, 99, 103018. [Google Scholar] [CrossRef]
- Marshall, W.S.; Cozzi, R.R.; Pelis, R.M.; McCormick, S.D. Cortisol receptor blockade and seawater adaptation in the euryhaline teleost Fundulus heteroclitus. J. Exp. Zool. 2005, 303, 132–142. [Google Scholar] [CrossRef]
- Weng, C.F.; Chiang, C.C.; Gong, H.; Chen, M.C.; Lin, C.J.; Huang, W.T.; Cheng, C.Y.; Hwang, P.P.; Wu, J.L. Acute Changes in Gill Na-K-ATPase and Creatine Kinase in Response to Salinity Changes in the Euryhaline Teleost, Tilapia (Oreochromis mossambicus). Physiol. Biochem. Zool. 2002, 75, 29–36. [Google Scholar] [CrossRef]
- Aruna, A.; Lin, C.J.; Nagarajan, G.; Chang, C.F. Neurohypophysial Hormones Associated with Osmotic Challenges in the Brain and Pituitary of the Euryhaline Black Porgy, Acanthopagrus schlegelii. Cells 2021, 10, 3086. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, Q.; Wang, T.; Lu, Q.; Tang, L.; Wang, Y.; Lu, W. Dynamic responses of prolactin, growth hormone and their receptors to hyposmotic acclimation in the olive flounder Paralichthys olivaceus. Gen. Comp. Endocrinol. 2017, 254, 8–13. [Google Scholar] [CrossRef]
- Richards, J.G.; Semple, J.W.; Bystriansky, J.S.; Schulte, P.M. Na+ ⁄ K+ -ATPase isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J. Exp. Biol. 2003, 206, 4475–4486. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y. Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am. J Physiol. 2004, 287, 1054–1063. [Google Scholar] [CrossRef]
- Tomy, S.; Chang, Y.M.; Chen, Y.H.; Cao, J.C.; Wang, T.P.; Chang, C.F. Salinity effects on the expression of osmoregulatory genes in the euryhaline black porgy Acanthopagrus schlegeli. Gen. Comp. Endocrinol. 2009, 161, 123–132. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in fish. Am. Zool. 2001, 41, 781–794. [Google Scholar]
- Breves, J.P.; Popp, E.E.; Rothenberg, E.F.; Rosenstein, C.W.; Maffett, K.M.; Guertin, R.R. Osmoregulatory actions of prolactin in the gastrointestinal tract of fishes. Gen. Comp. Endocrinol. 2020, 298, 113589. [Google Scholar] [CrossRef]
- Sakamoto, T.; McCormick, S.D. Prolactin and growth hormone in fish osmoregulation. Gen. Comp. Endocrinol. 2006, 147, 24–30. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Edery, M.; Young, G.; Bern, H.A.; Steiny, S. Prolactin receptors in tilapia (Sarotherodon mossambicus) tissues: Binding studies using I-125 labeled ovine prolactin. Gen. Comp. Endocrinol. 1984, 56, 19–23. [Google Scholar] [CrossRef]
- Auperin, B.; Rentier-Delrue, F.; Martial, J.A.; Prunet, P. Regulation of gill prolactin receptors in tilapia (Oreochromis niloticus) after a change in salinity or hypophysectomy. J. Endocrinol. 1995, 45, 213–220. [Google Scholar] [CrossRef]
- Sandra, O.; Sohm, F.; de Luze, A.; Prunet, P.; Edery, M.; Kelly, P.A. Expression cloning of a cDNA encoding a fish prolactin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 6037–6041. [Google Scholar] [CrossRef]
- Weng, C.F.; Lee, T.H.; Hwang, P.P. Immune localization of prolactin receptor in themitochondria-rich cells of the euryhaline teleost (Oreochromis mossambicus) gill. FEBS Lett. 1997, 405, 91–94. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Vet. Immunol. Immunopathol. 2000, 77, 63–176. [Google Scholar] [CrossRef]
- San Martín, R.; Hurtado, W.; Quezada, C.; Reyes, A.E.; Vera, M.I.; Krauskopf, M. Gene structure and seasonal expression of carp fish prolactin short receptor isoforms. J. Cell. Biochem. 2007, 100, 970–980. [Google Scholar] [CrossRef]
- Manzon, L.A. The role of prolactin in fish osmoregulation: A review. Gen. Comp. Endocrinol. 2002, 125, 291–310. [Google Scholar] [CrossRef]
- Seale, A.P.; Riley, L.G.; Leedom, T.A.; Kajimura, S.; Dores, R.M.; Hirano, T.; Grau, E.G. Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen. Comp. Endocrinol. 2002, 128, 91–101. [Google Scholar] [CrossRef]
- Seale, A.P.; Stagg, J.J.; Yamaguchi, Y.; Breves, J.P.; Soma, S.; Watanabe, S.; Kaneko, T.; Cnaani, A.; Harpaz, S.; Lerner, D.T. Effects of salinity and prolactin on gene transcript levels of ion transporters, ion pumps and prolactin receptors in Mozambique tilapia intestine. Gen. Comp. Endocrinol. 2014, 206, 146–154. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Breves, J.P.; Haws, M.C.; Lerner, D.T.; Grau, E.G.; Seale, A.P. Acute salinity tolerance and the control of two prolactins and their receptors in the Nile tilapia (Oreochromis niloticus) and Mozambique tilapia (O. mossambicus): A comparative study. Gen. Comp. Endocrinol. 2018, 257, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.P.; Fiess, J.C.; Hirano, T.; Cooke, I.M.; Grau, E.G. Disparate release of prolactin and growth hormone from the tilapia pituitary in response to osmotic stimulation. Gen. Comp. Endocrinol. 2006, 145, 222–231. [Google Scholar] [CrossRef]
- Hirano, T. The spectrum of prolactin action in teleosts. In Comparative Endocrinology: Developments and Directions; Ralph, C.R., Liss, A.R., Eds.; Wiley: New York, NY, USA, 1986; pp. 53–74. [Google Scholar]
- Sandra, O.; Rouzic, P.L.; Rentier-Delrue, F.; Prunet, P. Transfer of Tilapia (Oreochromis niloticus) to a Hyperosmotic Environment Is Associated with Sustained Expression of Prolactin Receptor in Intestine, Gill, and Kidney. Gen. Comp. Endocrinol. 2001, 123, 295–307. [Google Scholar] [CrossRef]
- Bollinger, R.J.; Ellis, L.V.; Bossus, M.C.; Tipsmark, C.K. Prolactin controls Na+,Cl− cotransporter via Stat5 pathway in the teleost gill. Mol. Cell. Endocrinol. 2018, 477, 163–171. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, A.; Zhang, J.; Yang, S.; Cui, W.; Xia, D.; Qu, J. Cloning and molecular characterization of PRL and PRLR from turbot (Scophthalmus maximus) and their expressions in response to short-term and long-term low salt stress. Fish Physiol. Biochem. 2020, 46, 501–517. [Google Scholar] [CrossRef]
- Prunet, P.; Sandra, O.; Le Rouzic, P.; Marchand, O.; Laudet, V. Molecular characterization of the prolactin receptor in two fish species, tilapia Oreochromis niloticus and rainbow trout, Oncorhynchus mykiss: A comparative approach. Can. J. Physiol. Pharmacol. 2000, 78, 1086–1096. [Google Scholar] [CrossRef]
- Tse, D.L.Y.; Chow, B.K.C.; Chan, C.B.; Lee, L.T.O.; Cheng, C.H.K. Molecular cloning and expression studies of a prolactin receptor in goldfish (Carassius auratus). Life Sci. 2000, 66, 593–605. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Nakao, N.; Ohkubo, T.; Tanaka, M.; Nakashima, K. Structure and tissue distribution of prolactin receptor mRNA in Japanese flounder (Paralichtys olivaceus): Conserved and preferential expression in osmoregulatory organs. Gen. Comp. Endocrinol. 2001, 123, 170–179. [Google Scholar] [CrossRef]
- Santos, C.R.A.; Ingleton, P.M.; Cavaco, J.E.B.; Kelly, P.A.; Edery, M.; Power, D.M. Cloning, characterization, and tissue distribution of prolactin receptor in the sea bream (Sparus aurata). Gen. Comp. Endocrinol. 2001, 121, 32–47. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, B.; Fung, C.K.; Zhang, Y.; Ho, W.K.; Chan, C.B.; Lin, H.; Wang, D.; Cheng, C.H.K. The presence of two distinct prolactin receptors in seabream with different tissue distribution patterns, signal transduction pathways and regulation of gene expression by steroid hormones. J. Endocrinol. 2007, 194, 373–392. [Google Scholar] [CrossRef]
- Lee, K.M.; Kaneko, T.; Aida, K. Prolactin and prolactin receptor expression in a marine teleost, pufferfish Takifugu rubripes. Gen. Comp. Endocrinol. 2006, 146, 318–328. [Google Scholar] [CrossRef]
- Pierce, A.L.; Fox, B.K.; Davis, L.K.; Visitacion, N.; Kitashashi, T.; Hirano, T.; Grau, E.G. Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: Tissue specific expression and differential regulation by salinity and fasting. Gen. Comp. Endocrinol. 2007, 154, 31–40. [Google Scholar] [CrossRef]
- Fiol, D.F.; Sanmarti, E.; Sacchi, R.; Kültz, D. A novel tilapia prolactin receptor is functionally distinct from its paralog. J. Exp. Biol. 2009, 212, 2007–2015. [Google Scholar] [CrossRef]
- Breves, J.P.; Serizier, S.B.; Goffin, V.; McCormick, S.D.; Karlstrom, R.O. Prolactin regulates transcription of the ion uptake Na+/Cl− cotransporter (ncc) gene in zebrafish gill. Mol. Cell. Endocrinol. 2013, 369, 98–106. [Google Scholar] [CrossRef]
- Nagarajan, G.; Aruna, A.; Yousef, A.A.; Roshman, T.M.; Chang, C.F. Localization of the Neuropeptide Arginine Vasotocin and Its Receptor in the Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii: Gills, Kidneys, and Intestines. Int. J. Mol. Sci. 2022, 23, 13421. [Google Scholar] [CrossRef]
- Mancera, J.M.; Pérez-Figares, J.M.; Fernández-Llebrez, P. Osmoregulatory responses to abrupt salinity changes in the euryhaline gilthead sea bream (Sparus aurata L.). Comp. Biochem. Physiol. A 1993, 106, 245–250. [Google Scholar] [CrossRef]
- Min, B.H.; Choi, C.Y.; Chang, Y.J. Comparison of physiological conditions on black porgy, Acanthopagrus schlegeli acclimated and reared in freshwater and seawater. J. Aquacult. 2005, 18, 37–44. [Google Scholar]
- Chang, Y.J.; Min, B.H.; Choi, C.Y. Black porgy (Acanthopagrus schlegeli) prolactin cDNA sequence: mRNA expression and blood physiological responses during freshwater acclimation. Comp. Biochem. Physiol. 2007, 147, 122–128. [Google Scholar] [CrossRef]
- Breves, J.P.; Fox, B.K.; Pierce, A.L.; Thirano, T.; Grau, E.G. Gene expression of growth hormone family and glucocorticoid receptors, osmosensors, and ion transporters in the gill during seawater acclimation of mozambique tilapia, Oreochromis mossambicus. J. Exp. Zool. 2010, 313A, 432–441. [Google Scholar] [CrossRef]
- Ayson, F.G.; Kaneko, T.; Hasegawa, S.; Hirano, T. Differential expression of two prolactin and growth hormone genes during early development of tilapia (Oreochromis mossambicus) in fresh water and seawater: Implications for possible involvement in osmoregulation during early life stages. Gen. Comp. Endocrinol. 1994, 95, 143–152. [Google Scholar] [CrossRef]
- Tine, M.; de Lorgeril, J.; Panfili, J.; Diop, K.; Bonhomme, F.; Durand, J.D. Growth hormone and prolactin-1 gene transcription in natural populations of the black chinned tilapia Sarotherodon melanotheron acclimatised to different salinities. Comp. Biochem. Physiol. B 2007, 147, 541–549. [Google Scholar] [CrossRef]
- Varsamos, S.; Xuereb, B.; Commes, T.; Flik, G.; Spanings-Pierrot, C. Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J. Endocrinol. 2006, 191, 473–480. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hirano, T. Expression of insulin-like growth factor I gene in osmoregulatory organs during seawater adaptation of the salmonid fish: Possible mode of osmoregulatory action of growth hormone. Proc. Natl. Acad. Sci. USA 1993, 90, 1912–1916. [Google Scholar] [CrossRef]
- Link, K.; Shved1, N.; Serrano, N.; Akgül, G.; Caelers, A.; Faass, O.; Mouttet, F.; Raabe, O.; D’Cotta, H.; Baroiller, J.F.; et al. Effects of seawater and freshwater challenges on the Gh/Igf system in the saline-tolerant black chin tilapia (Sarotherodon melanotheron). Front. Endocrinol. 2022, 13, 976488. [Google Scholar] [CrossRef]
- Fuentes, J.; Brinca, L.; Guerreiro, P.M.; Power, D.M. PRL and GH synthesis and release from the sea bream (Sparus auratus L.) pituitary gland in vitro in response to osmotic challenge. Gen. Comp. Endocrinol. 2010, 168, 95–102. [Google Scholar] [CrossRef]
- Breves, J.P.; McCormick, S.D.; Karlstrom, R.O. Prolactin and teleost ionocytes: New insights into cellular and molecular targets of prolactin in vertebrate epithelia. Gen. Comp. Endocrinol. 2014, 203, 21–28. [Google Scholar] [CrossRef]
- Seale, A.P.; Watanabe, S.; Grau, E.G. Osmoreception: Perspectives on signal transduction and environmental modulation. Gen. Comp. Endocrinol. 2012, 176, 354–360. [Google Scholar] [CrossRef]
- Moorman, B.; Yamaguchi, Y.; Lerner, D.T.; Gordon, E.G.; Seale, A.P. Rearing Mozambique tilapia in tidally-changing salinities: Effects on growth and the growth hormone/Insulin like growth factor 1 axis. Com. Biochem. Physiol. 2016, 198, 8–14. [Google Scholar] [CrossRef]
- Seale, A.P.; Pavlosky, K.K.; Celino-Brady, F.T.; Yamaguchi, Y.; Breves, J.P.; Lerner, D.T. Systemic versus tissue-level prolactin signaling in a teleost during a tidal cycle. J. Comp. Physiol. 2019, 189, 581–594. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; Mancera, J.M.; Martınez-Rodrıguez, G. Acclimation to different environmental salinities induces molecular endocrine changes in the GH/IGF-I axis of juvenile gilthead sea bream (Sparus aurata L.). J. Comp. Physiol. B 2015, 185, 87–101. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; González, A.A.; Suárez, R.A.; Galal-Khallaf, A.; MartosSitcha, J.A.; Ibrahim, H.M. Molecular performance of prl and Gh/Igf1 axis in the Mediterranean meagre, Argyrosomus regius, acclimated to different rearing salinities. Fish Physiol. Biochem. 2017, 43, 203–216. [Google Scholar] [CrossRef]
- Yan, J.J.; Lee, Y.C.; Tsou, Y.L.; Tseng, Y.C.; Hwang, P.P. Insulin-like growth factor 1 triggers salt secretion machinery in fish under acute salinity stress. J. Endocrinol. 2020, 246, 277–288. [Google Scholar] [CrossRef]
- Bortoletti, M.; Maccatrozzo, L.; Peruzzi, S.; Espen, J.; Strand, T.; Jobling, M. Dietary effects on biomarkers of growth, stress, and welfare of diploid and triploid Atlantic salmon (Salmo salar) during parr-smolt transformation. Aquaculture Rep. 2022, 24, 101123. [Google Scholar] [CrossRef]
- Choi, C.Y.; An, K.W. Cloning and expression of Na+/K+ -ATPase and osmotic stress transcription factor 1 mRNA in black porgy, Acanthopagrus schlegeli during osmotic stress. Comp. Biochem. Physiol. B 2008, 149, 91–100. [Google Scholar] [CrossRef]
- Seidelin, M.; Madsen, S.S.; Blenstrup, H.; Tipsmark, C.K. Time-course changes in the expression of Na+, K+-ATPase in gills and pyloric caeca of brown trout (Salmo trutta) during acclimation to seawater. Physiol. Biochem. Zool. 2000, 73, 446–453. [Google Scholar] [CrossRef]
- Marshall, W.S.; Grosell, M. Ion transport, osmoregulation, and acid-base balance. In The Physiology of Fishes, 3rd ed.; Evans, D.H., Clairborne, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 177–230. [Google Scholar]
- McCormick, S.D. The hormonal control of osmoregulation in teleost fish. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1466–1473. [Google Scholar]
- Yada, T.; Hyodo, S.; Schreck, C.B. Effects of seawater acclimation on mRNA levels of corticosteroid receptor genes in osmoregulatory and immune systems in trout. Gen. Comp. Endocrinol. 2008, 156, 622–627. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.; Kiilerich, P.; Bjornsson, B.T.; Madsen, S.S.; McCormick, S.D.; Stefansson, S.O. Endocrine systems in juvenile anadromous and landlocked Atlantic salmon (Salmo salar): Seasonal development and seawater acclimation. Gen. Comp. Endocrinol. 2008, 155, 762–772. [Google Scholar] [CrossRef]
- Karnaky, K.J. Regulating epithelia from the apical side: New insights. Focus on “Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells”. Am. J. Physiol. 1998, 275, C1417–C1418. [Google Scholar] [CrossRef]
- Loretz, C.A. Electrophysiology of ion transport in the teleost intestinal cells. In Cellular and Molecular Approaches to Fish Ionic Regulation, Fish Physiology; Wood, C.M., Shuttleworth, T.J., Eds.; Academic Press: New York, NY, USA, 1995; Volume 14, pp. 25–26. [Google Scholar]
- Collie, N.L.; Hirano, T. Mechanisms of hormone actions on intestinal transport. In Vertebrate Endocrinology: Fundamentals and Biomedical Implications, Regulation of Water and Electrolytes; Pang, P.K.T., Schreibman, M.P., Eds.; Academic Press, Inc.: New York, NY, USA, 1987; Volume 2, pp. 239–270. [Google Scholar]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakamoto, T.; Hyodo, S.; Shepherd, B.S.; Kanedo, T.; Grau, E. Expression of glucocorticoid receptor in the intestine of a euryhaline teleost, the Mozambique tilapia (Oreochrmis mossambicus): Effect of seawater exposure and cortisol treatment. Life Sci. 2006, 78, 2329–2335. [Google Scholar] [CrossRef]
- Colombe, L.; Fostier, A.; Bury, N.; Pakdel, F.; Guiguen, Y. A mineralocorticoid-like receptor in the rainbow trout, Oncorhynchus mykiss: Cloning and characterization of its steroid binding domain. Steroids 2000, 65, 319–328. [Google Scholar] [CrossRef]
- Stolte, E.H.; Verburg, B.M.L.; Kemenade, V.; Savelkoul, H.F.J.; Flik, G. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J. Endocrinol. 2006, 190, 17–28. [Google Scholar] [CrossRef]
- Aruna, A.; Lan, D.S.; Nagarajan, G.; Wang, T.P.; Cao, J.C.; Chen, Y.H.; Chang, C. Differential expression of hypothalamic gill-crh system with osmotic stress in the euryh aline black porgy, Acanthopagrus Schlegelii. Front. Physiol. 2021, 12, 768122. [Google Scholar] [CrossRef]
| Gene | Orientation | Nucleotide Sequences | Usage |
|---|---|---|---|
| prl | F | 5′-AGTCGGACCTGTTGTCATTGG-3′ | Q-PCR |
| R | 5′-AGGCTGTTGGCAGAGTTGGA-3′ | Q-PCR | |
| prlr | F | 5′-CAACGCGCTGGGAAGAAC-3′ | Q-PCR |
| R | 5′-AGGATTGGGCTTGACGATGTAC-3′ | Q-PCR | |
| gh | F | 5′-CCTGCAGGATTTCTGTAACTCTGAT-3′ | Q-PCR |
| R | 5′-TGGGCTGCGCTGTGTCT-3′ | Q-PCR | |
| ghr | F | 5′-CCGTCAGCTTTCCTGATGATG-3 | Q-PCR |
| R | 5′-TGTCCTGGTCGTGGAGATCTG-3′ | Q-PCR | |
| gr | F | 5′-AACACAGATGGCCAGCACAAC-3′ | Q-PCR |
| R | 5′-CTTCCTTCGGATCTTATCGATGAT-3′ | Q-PCR | |
| α-nka | F | 5′-ACCGTGGCCCACATGTG-3′ | Q-PCR |
| R | 5′-GGTCCCGCTCTGGTTCTCA-3′ | Q-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagarajan, G.; Aruna, A.; Chang, Y.-M.; Alkhamis, Y.A.; Mathew, R.T.; Chang, C.-F. Effects of Osmotic Stress on the mRNA Expression of prl, prlr, gr, gh, and ghr in the Pituitary and Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii. Int. J. Mol. Sci. 2023, 24, 5318. https://doi.org/10.3390/ijms24065318
Nagarajan G, Aruna A, Chang Y-M, Alkhamis YA, Mathew RT, Chang C-F. Effects of Osmotic Stress on the mRNA Expression of prl, prlr, gr, gh, and ghr in the Pituitary and Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii. International Journal of Molecular Sciences. 2023; 24(6):5318. https://doi.org/10.3390/ijms24065318
Chicago/Turabian StyleNagarajan, Ganesan, Adimoolam Aruna, Yu-Ming Chang, Yousef Ahmed Alkhamis, Roshmon Thomas Mathew, and Ching-Fong Chang. 2023. "Effects of Osmotic Stress on the mRNA Expression of prl, prlr, gr, gh, and ghr in the Pituitary and Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii" International Journal of Molecular Sciences 24, no. 6: 5318. https://doi.org/10.3390/ijms24065318
APA StyleNagarajan, G., Aruna, A., Chang, Y.-M., Alkhamis, Y. A., Mathew, R. T., & Chang, C.-F. (2023). Effects of Osmotic Stress on the mRNA Expression of prl, prlr, gr, gh, and ghr in the Pituitary and Osmoregulatory Organs of Black Porgy, Acanthopagrus schlegelii. International Journal of Molecular Sciences, 24(6), 5318. https://doi.org/10.3390/ijms24065318







