Potential Role of Fenestrated Septa in Axonal Transport of Golgi Cisternae and Gap Junction Formation/Function
Abstract
1. Introduction
2. Structure of Fenestrated Septa
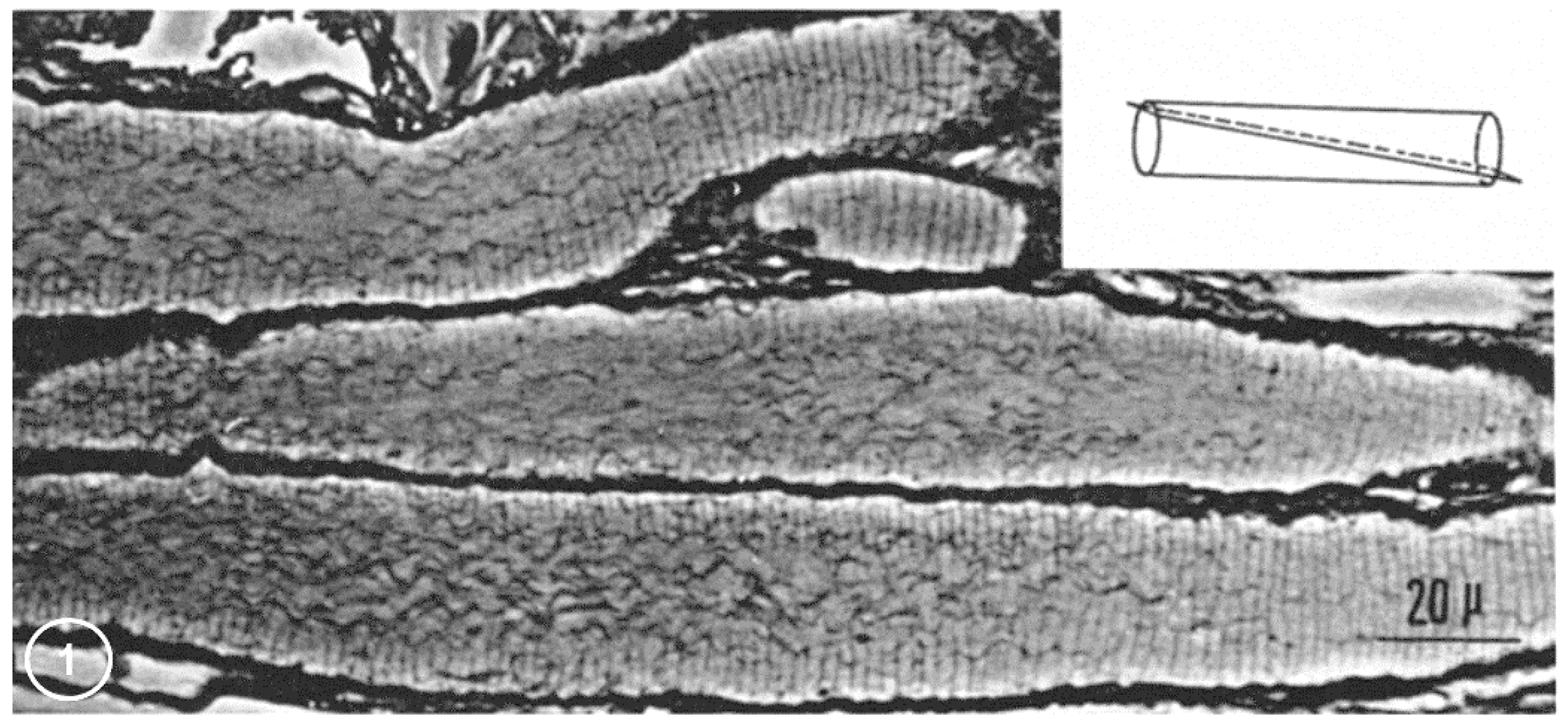
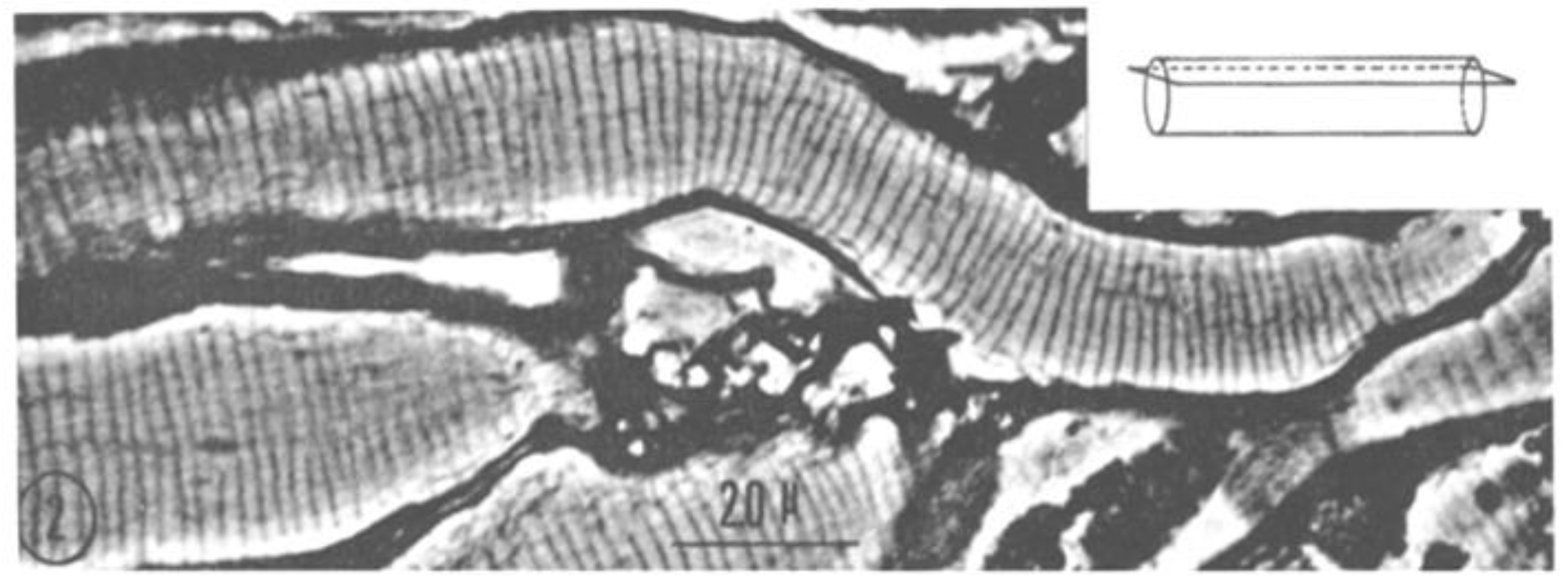
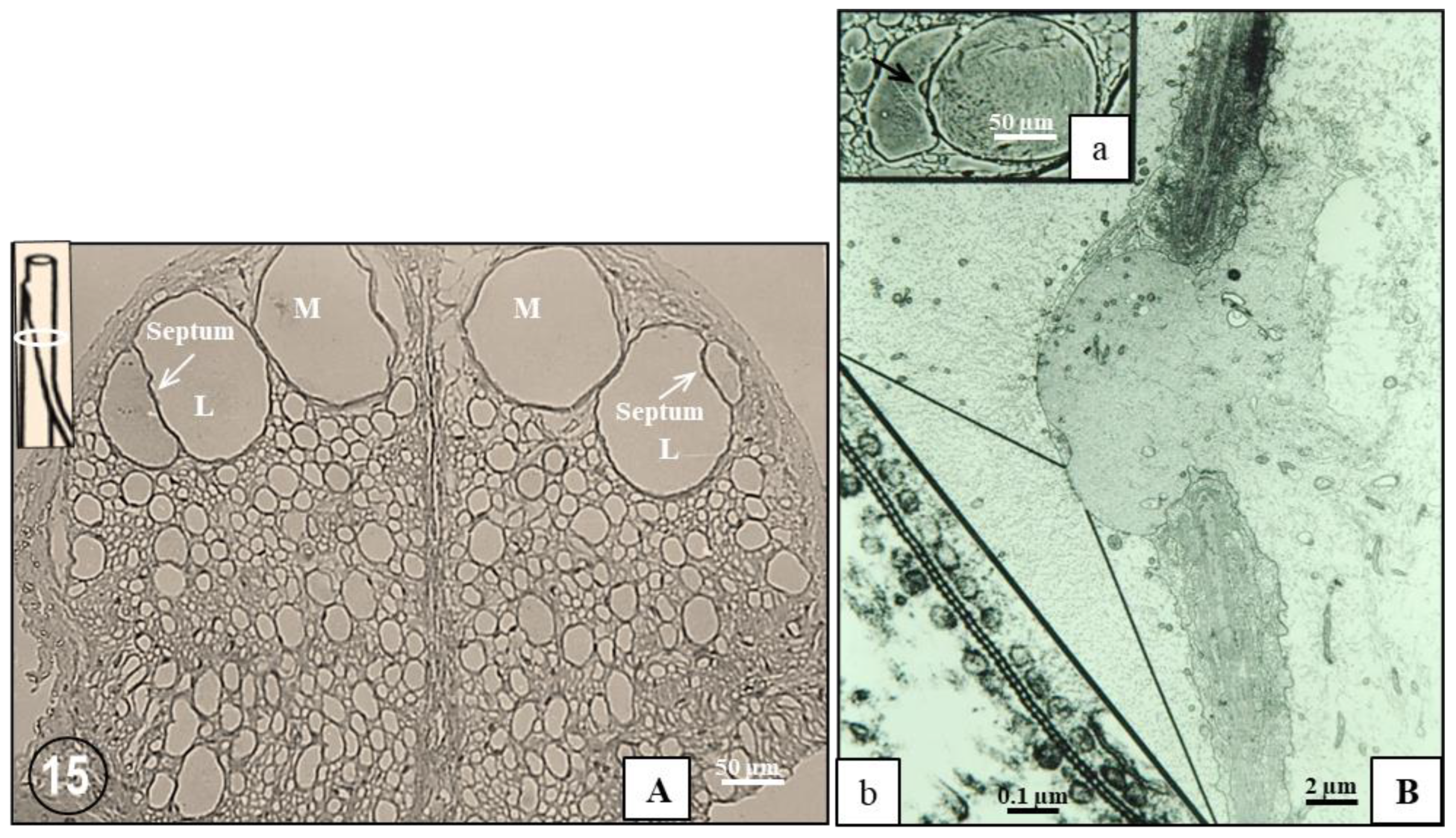
2.1. Phase-Contrast Microscopy of Fenestrated Septa
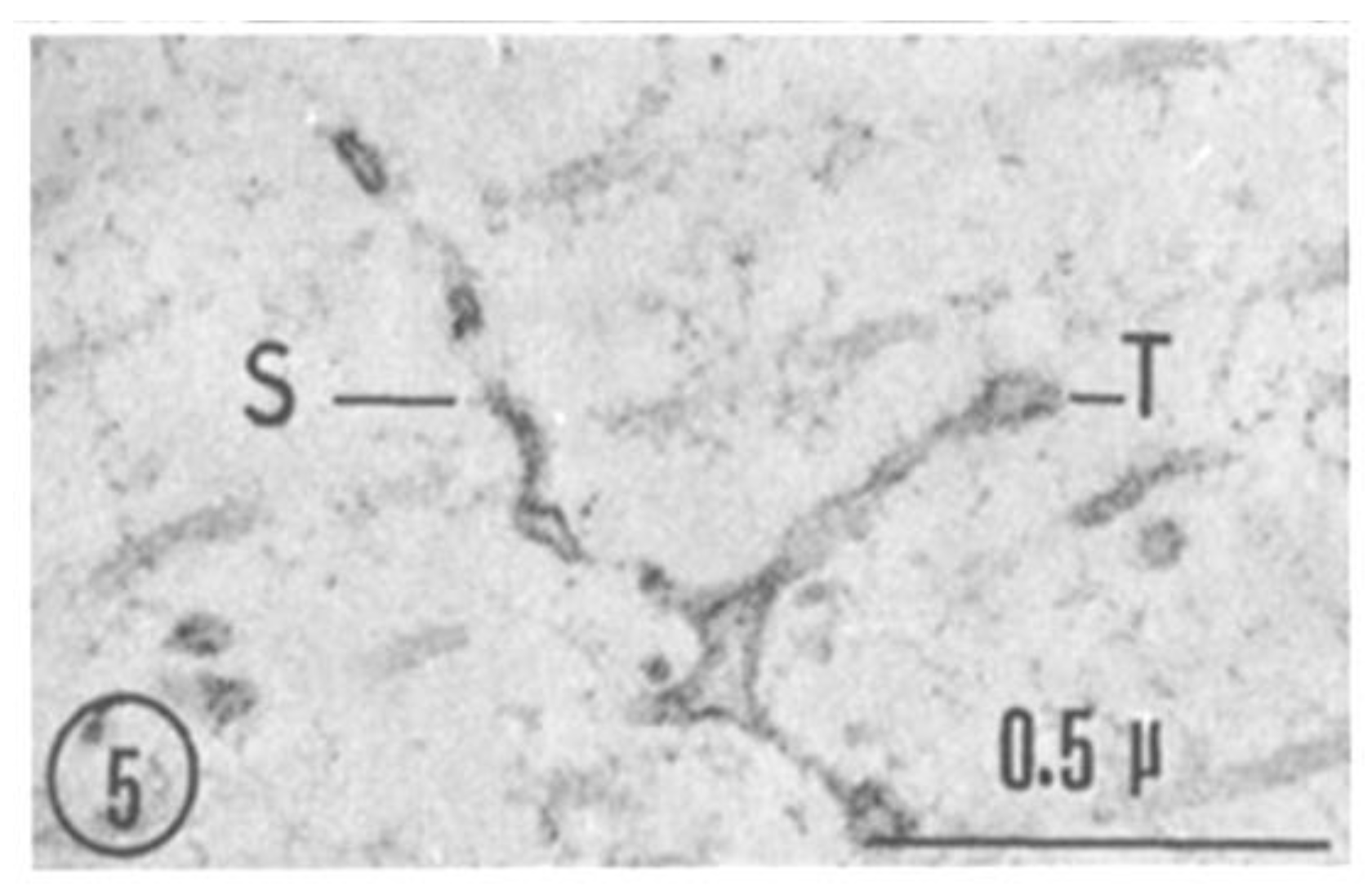
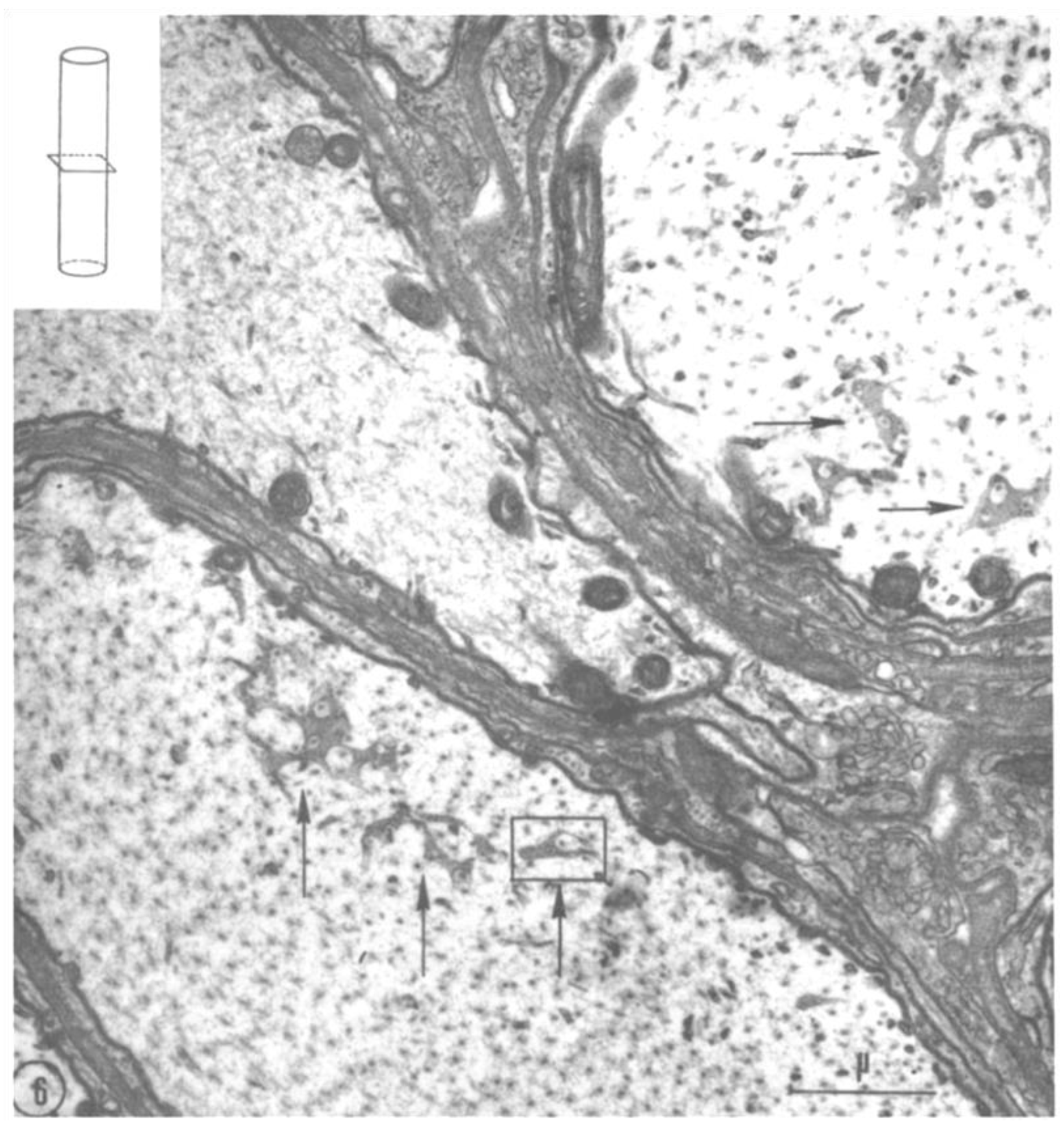
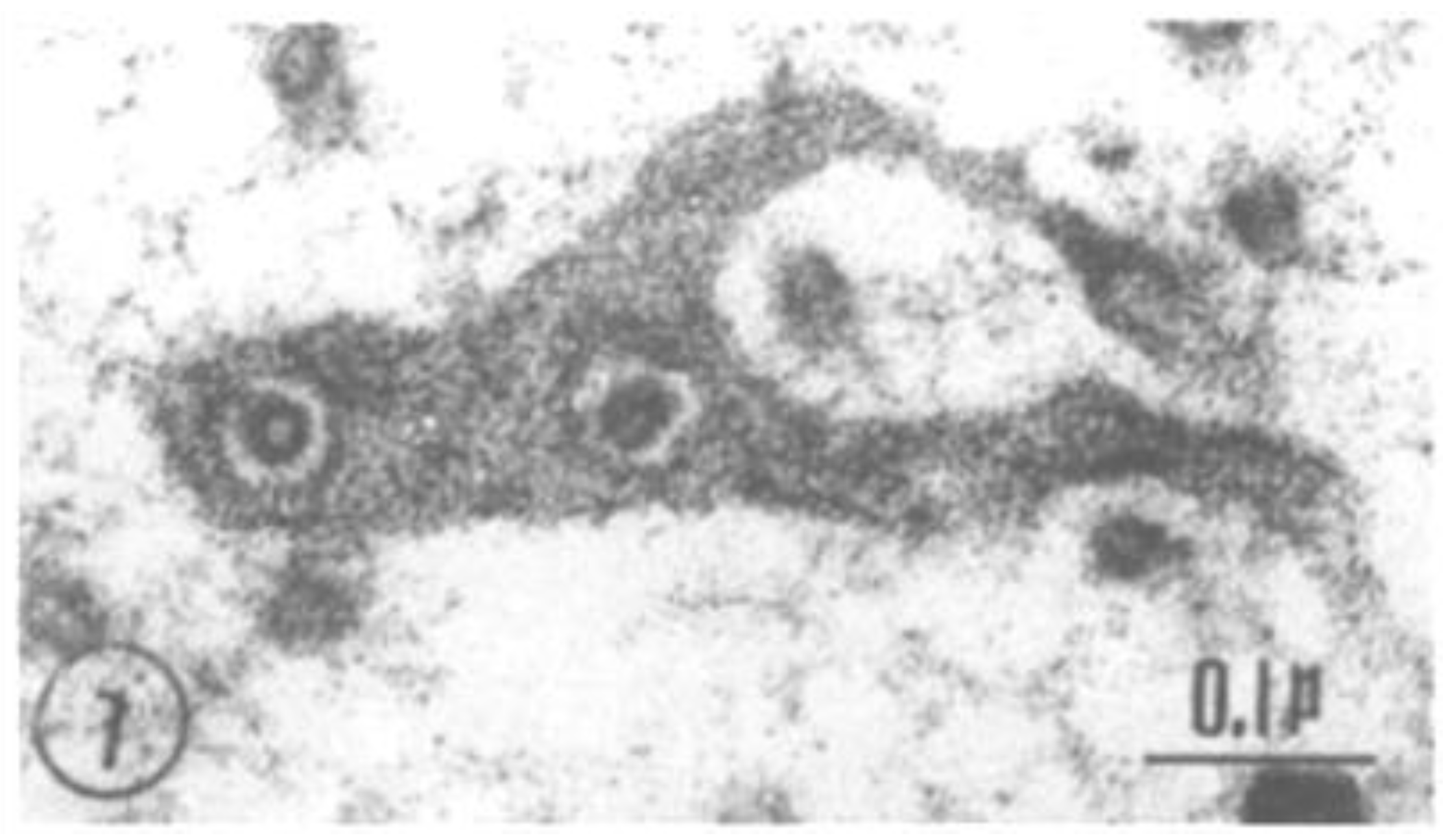
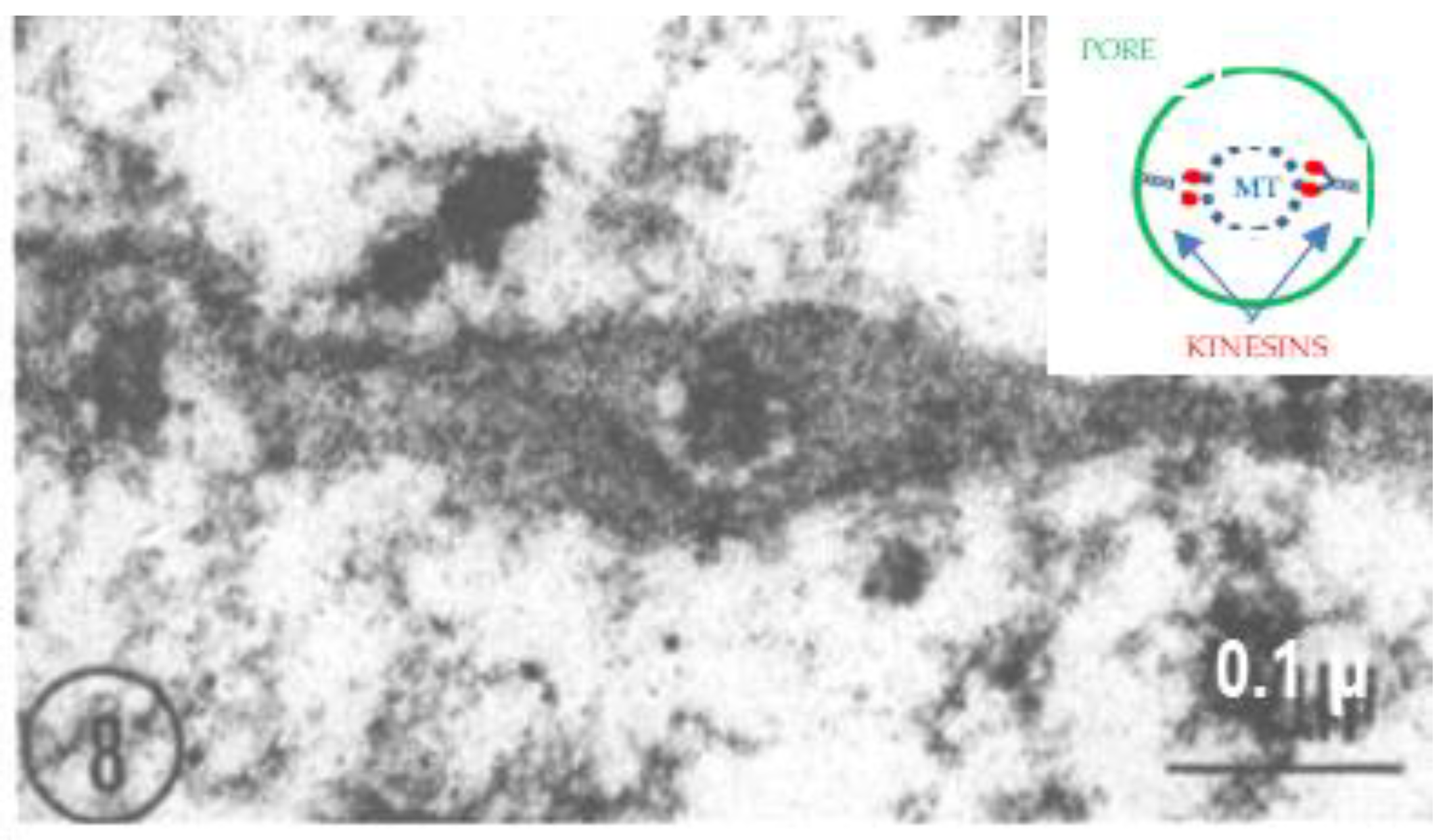
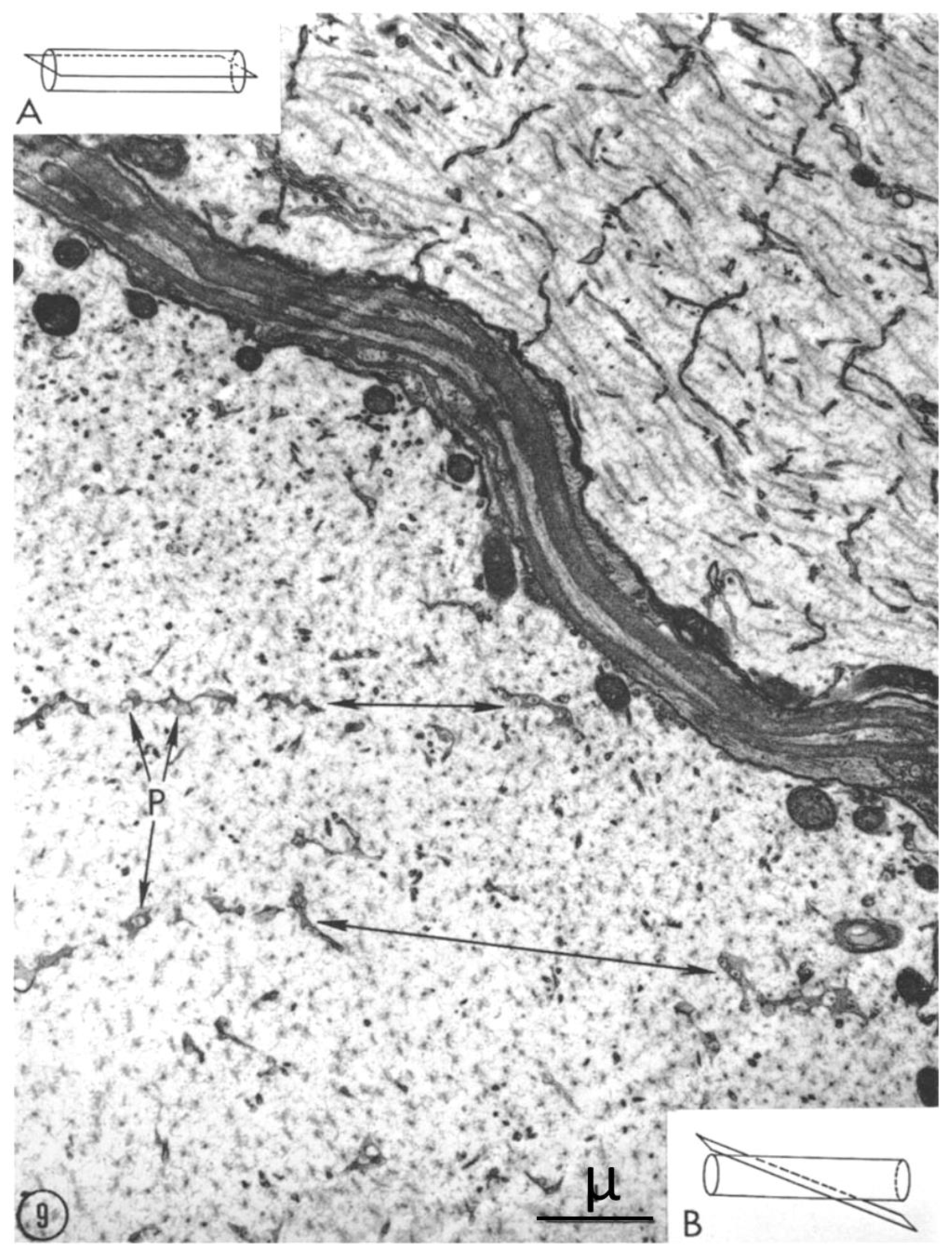
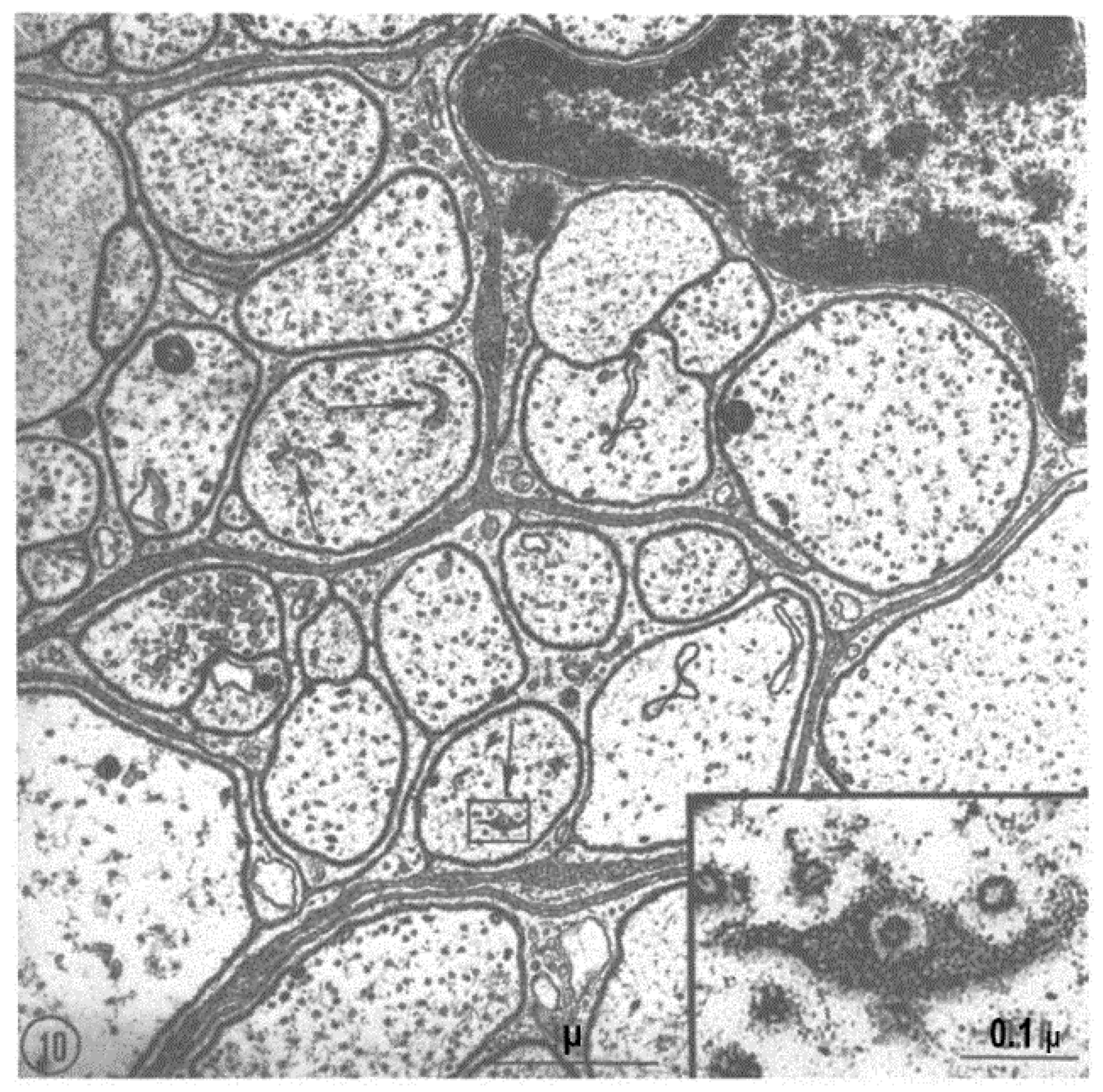
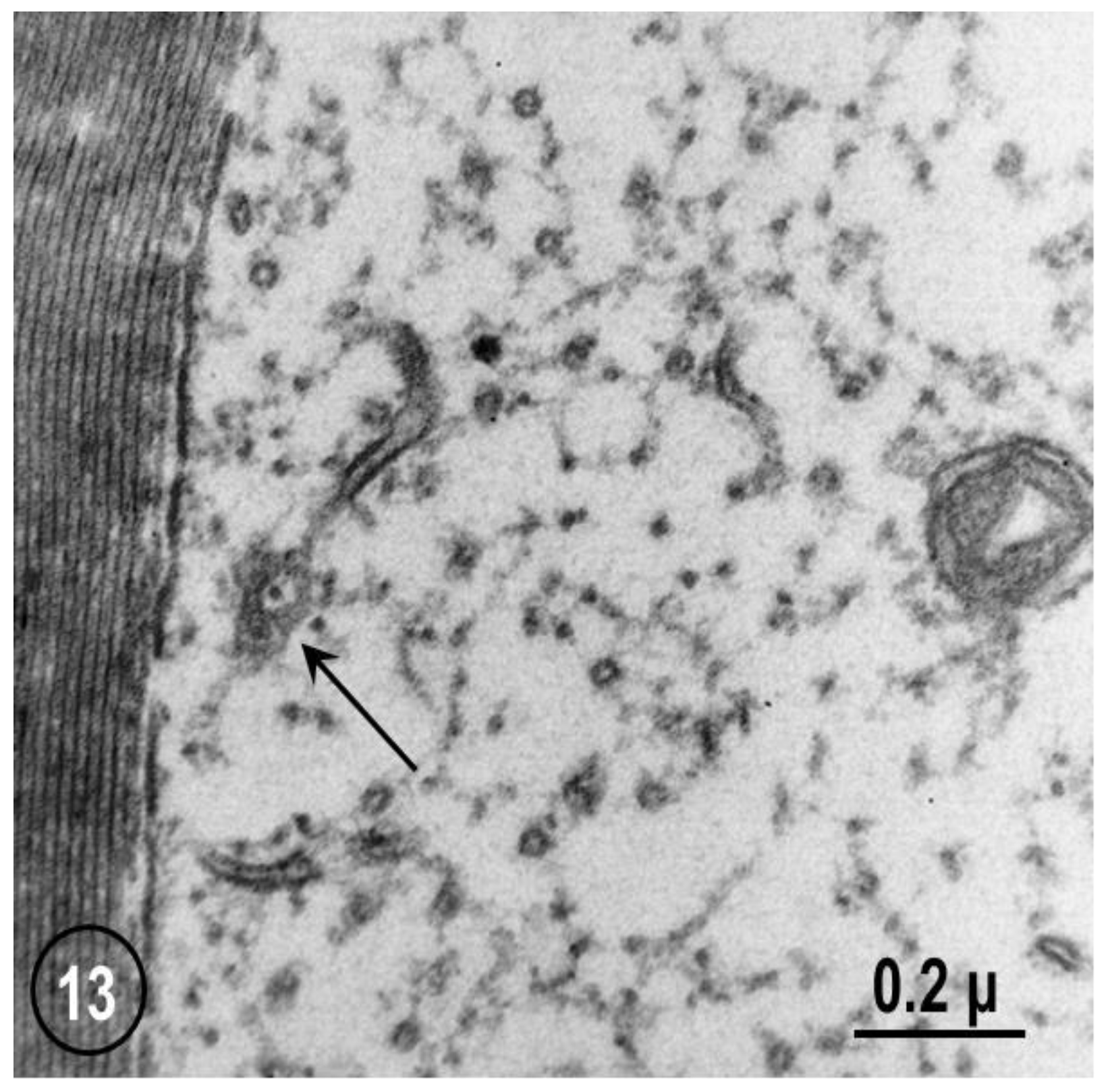
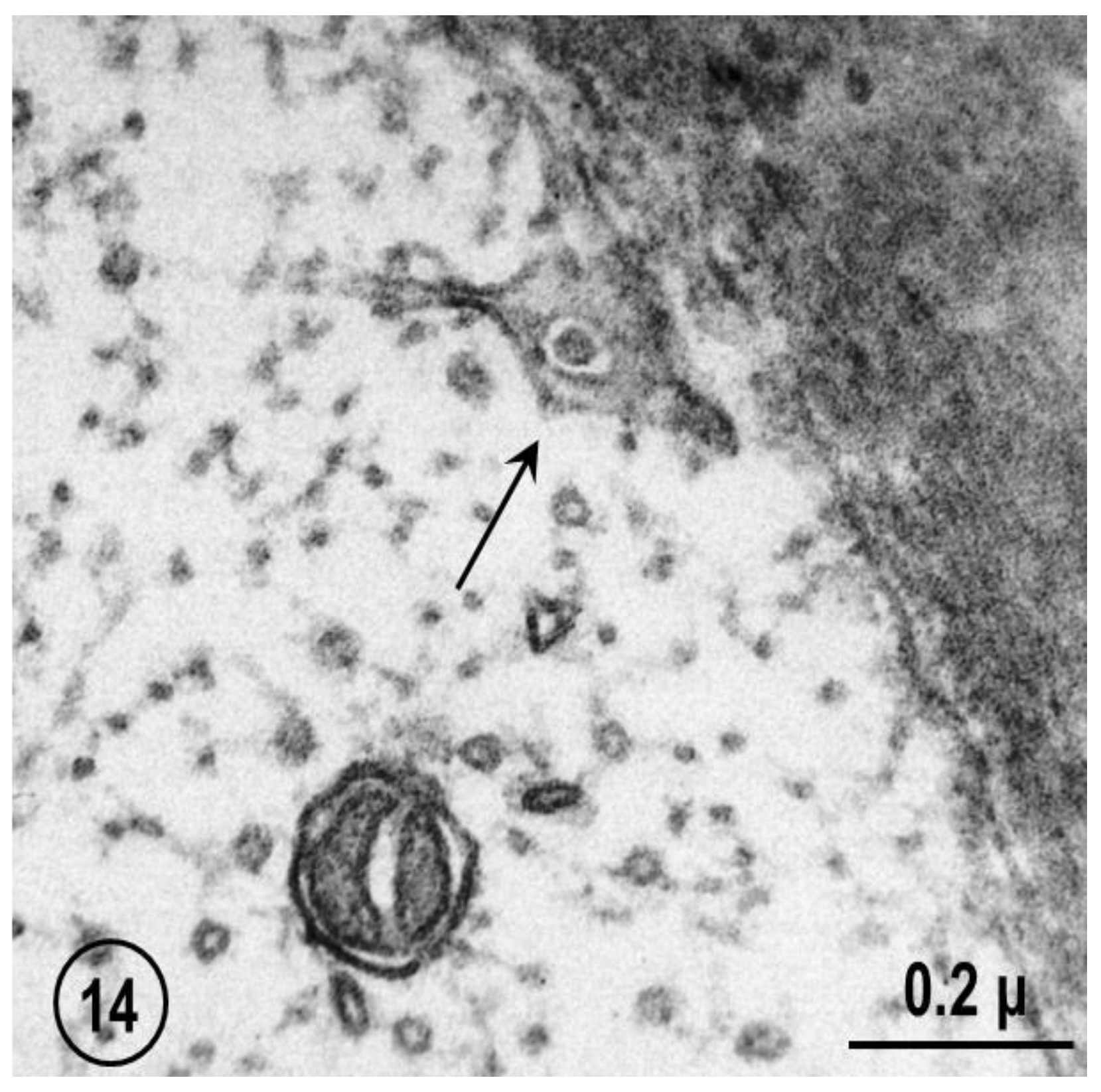
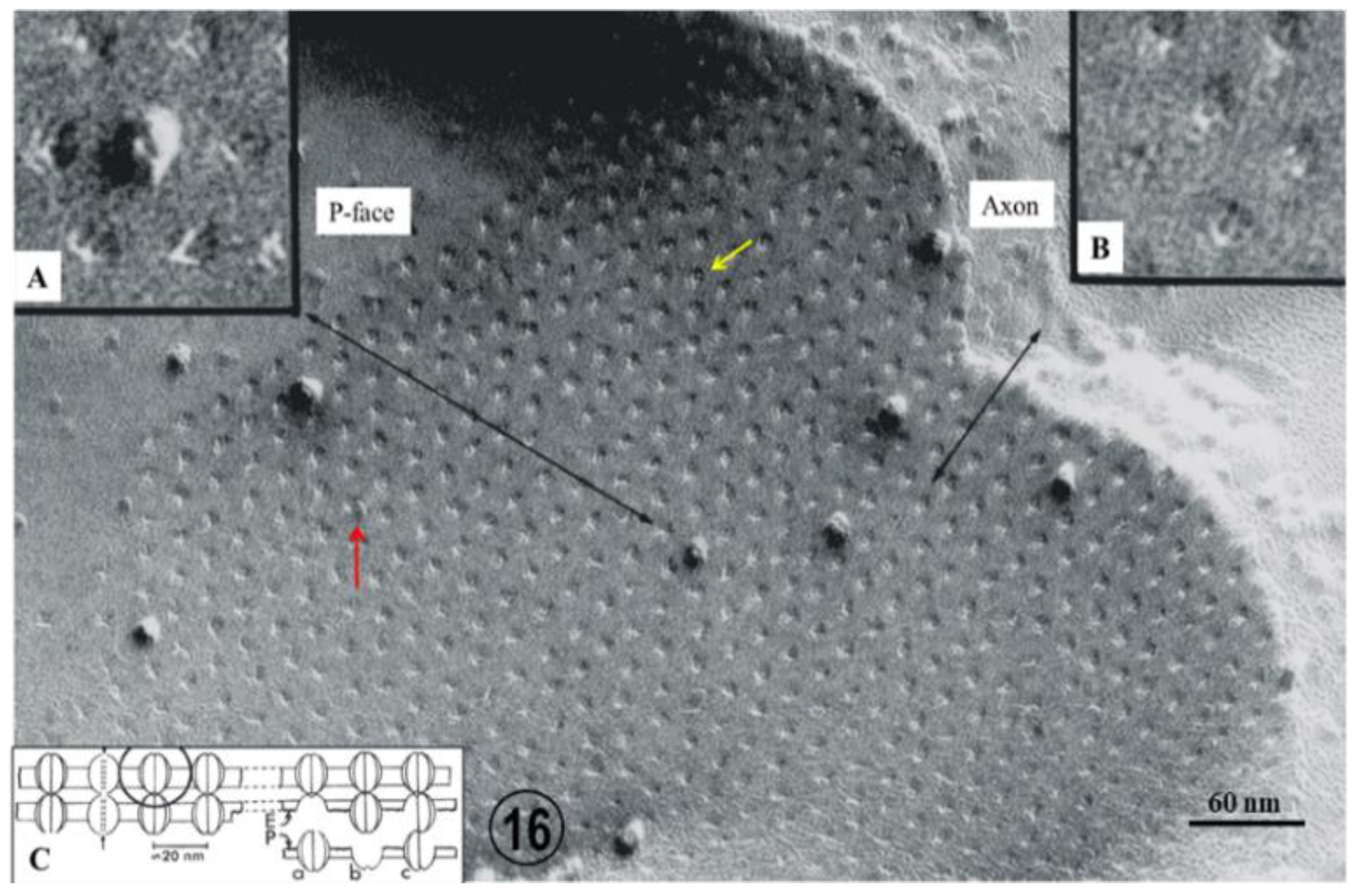
2.2. Electron Microscopy of Fenestrated Septa
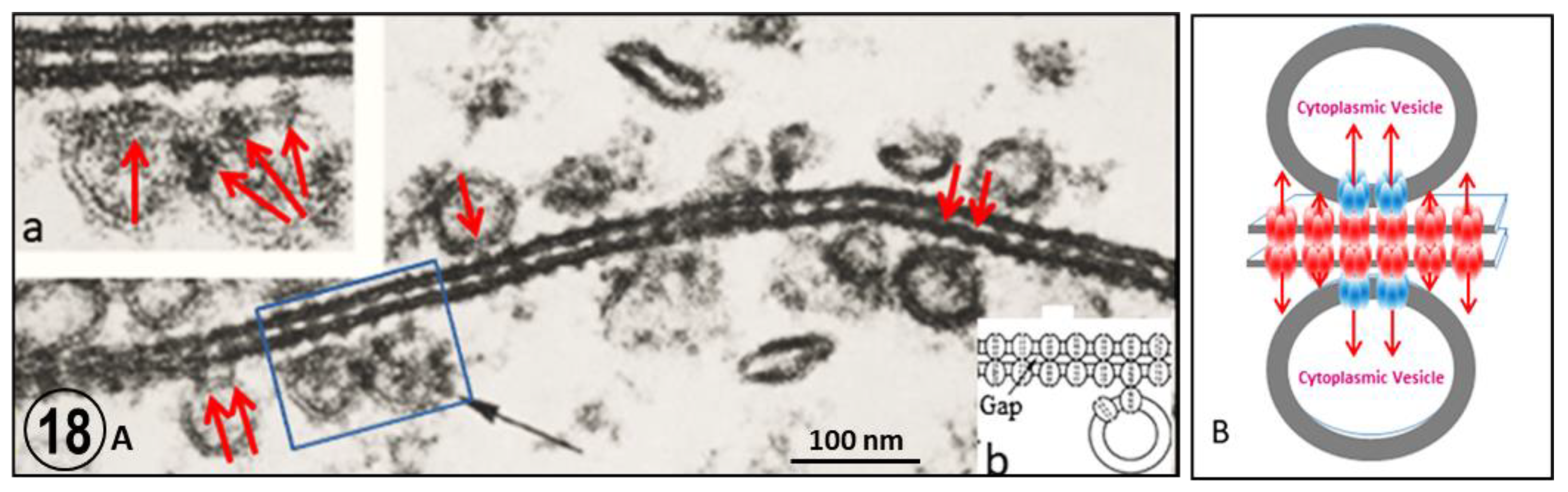
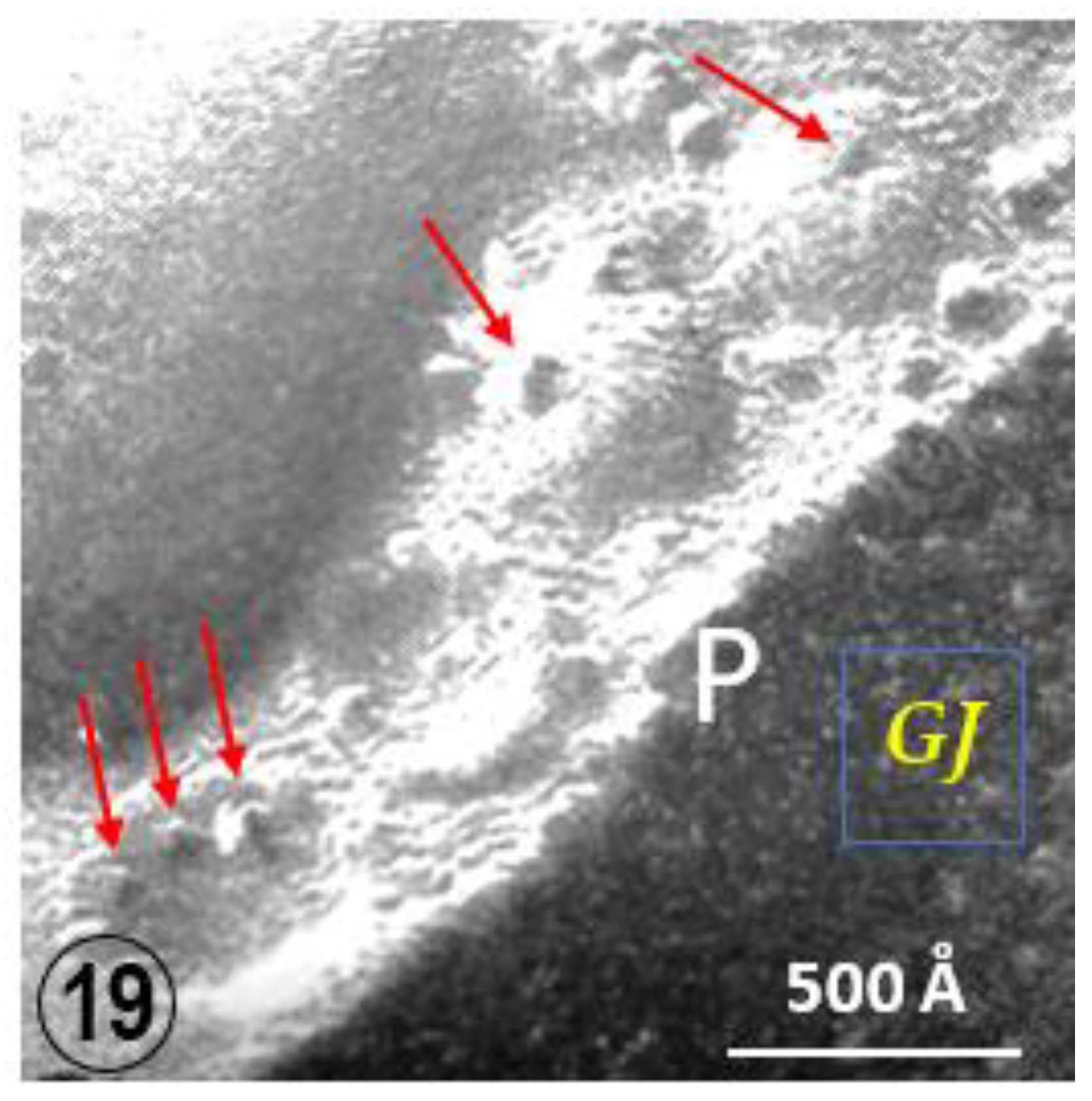
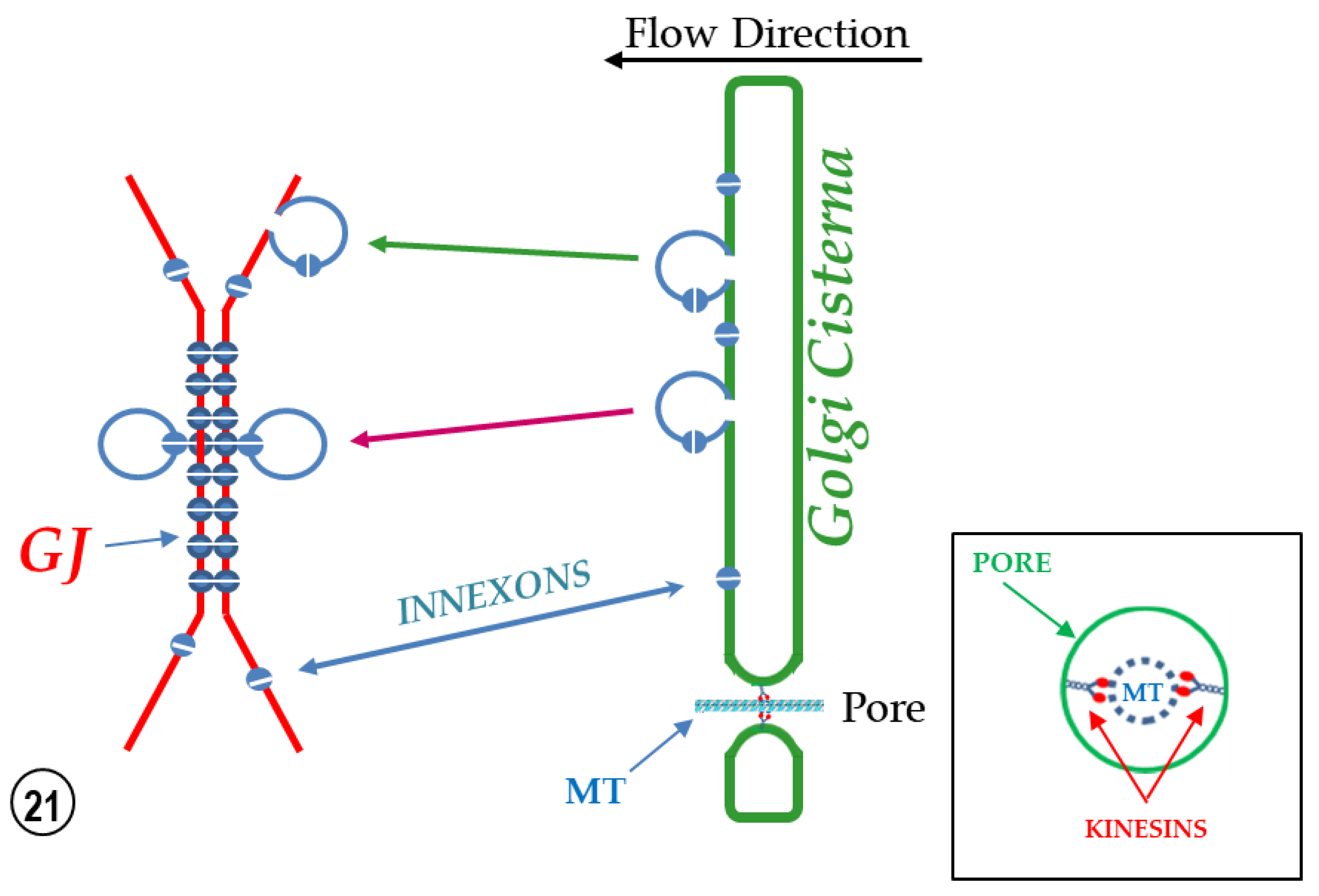
2.3. Potential Role of Fenestrated Septa in Gap Junction Formation/Function
3. Conclusions
Funding
Conflicts of Interest
References
- Ochs, S. Axoplasmic Transport and Its Relation to Other Nerve Functions; John Whiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, A. Axonal transport of membranous and nonmembranous cargoes: A unified perspective. J. Cell Biol. 2003, 160, 817–821. [Google Scholar] [CrossRef]
- Vallee, R.B.; Bloom, G.S. Mechanisms of fast and slow axonal transport. Annu. Rev. Neurosci. 1991, 14, 59–92. [Google Scholar] [CrossRef] [PubMed]
- Gennerich, A.; Vale, R.D. Walking the walk: How kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol 2009, 21, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. A system of parallel septa in crayfish nerve fibers. J. Cell Biol. 1970, 44, 125–133. [Google Scholar] [CrossRef]
- Wobst, H.; Schmitz, B.; Schachner, M.; Diestel, S.; Leshchyns′ka, I.; Sytnyk, V. Kinesin-1 promotes post-Golgi trafficking of NCAM140 and NCAM180 to the cell surface. J. Cell Sci. 2015, 128, 2816–2829. [Google Scholar] [CrossRef]
- Angerani, S.; Lindberg, E.; Klena, N.; Bleck, C.K.E.; Aumeier, C.; Winssinger, N. Kinesin-1 activity recorded in living cells with a precipitating dye. Nat. Commun. 2021, 12, 1463. [Google Scholar] [CrossRef]
- Jaarsma, D.; Hoogenraad, C.C. Cytoplasmic dynein and its regulatory proteins in Golgi pathology in nervous system disorders. Front. Neurosci. 2015, 9, 397. [Google Scholar] [CrossRef]
- Gonzalez, C.; Cornejo, V.H.; Couve, A. Golgi bypass for local delivery of axonal proteins, fact or fiction? Curr. Opin. Cell Biol. 2018, 53, 9–14. [Google Scholar] [CrossRef]
- Merianda, T.T.; Lin, A.C.; Lam, J.S.; Vuppalanchi, D.; Willis, D.E.; Karin, N.; Holt, C.E.; Twiss, J.L. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell Neurosci. 2009, 40, 128–142. [Google Scholar] [CrossRef]
- Peracchia, C. Gap Junction Stucture and Chemical Regulation. In Direct Calmodulin Role in Cell-to-Cell Channel Gating; Academic Press, an Imprint of Elsevier: London, UK, 2019. [Google Scholar]
- Trujillo-Cenoz, O. The fine structure of a special type of nerve fiber found in the ganglia of Armadilidium vulgare (Crustacea-Isopoda). J. Biophys. Biochem. Cytol. 1960, 7, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Cenoz, O. Some aspects of the structural organization of the arthropod ganglia. Z. Zellforsch. Mikrosk. Anat. 1962, 56, 649–682. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.J.D.; Brzin, M.; Dettbarn, W.D. Fine structure and organization of nerve fibers and giant axons in Homarus americanus. J. Ultrastruct. Res. 1968, 24, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Boschek, C.B. On the fine structure of the peripheral retina and Lamina ganglionaris of the fly, Musca domestica. Z. Zellforsch. Mikrosk. Anat. 1971, 118, 369–409. [Google Scholar] [CrossRef]
- Toh, Y.; Kuwabara, M. Synaptic organization of the fleshfly ocellus. J. Neurocytol. 1975, 4, 271–287. [Google Scholar] [CrossRef]
- Aizu, S. Fine structure of cardiac ganglion trunk in prawn, Penaeus japonicus bates. Tissue Cell 1975, 7, 433–452. [Google Scholar] [CrossRef]
- Brodie, D.A.; Halcrow, K. The ultrastructure of the sinus gland of Gammarus oceanicus (crustacea: Amphipoda). Cell Tissue Res. 1977, 182, 557–564. [Google Scholar] [CrossRef]
- Lieberman, A.R. Microtubule-associated smooth endoplasmic reticulum in the frog’s brain. Z. Zellforsch. Mikrosk. Anat. 1971, 116, 564–577. [Google Scholar] [CrossRef]
- Santalova, I.M.; Moshkov, D.A. Smooth endoplasmic reticulum in fish Mauthner cells at different functional states. Neuroscience 1999, 89, 593–602. [Google Scholar] [CrossRef]
- Grainger, F.; James, D.W. Association of glial cells with the terminal parts of neurite bundles extending from chick spinal cord In Vitro. Z. Zellforsch. Mikrosk. Anat. 1970, 108, 93–104. [Google Scholar] [CrossRef]
- Maynard, E.A. Electron microscopy of stomatogastric ganglion in the lobster Homarus americanus. Tissue Cell 1971, 3, 137–160. [Google Scholar] [CrossRef]
- Smith, D.S.; Jarlfors, U.; Cameron, B.F. Morphological evidence for the participation of microtubules in axonal transport. Ann. N. Y. Acad. Sci. 1975, 253, 472–506. [Google Scholar] [CrossRef] [PubMed]
- Follenius, E. Special organization of the endoplasmic reticulum in some hypothalamic nerve processes of Gasterosteus aculeatus L. Z. Zellforsch. Mikrosk. Anat. 1970, 106, 61–68. [Google Scholar] [CrossRef]
- Hinds, J.W. Early neuron differentiation in the mouse olfactory bulb. II. Electron microscopy. J. Comp. Neurol. 1972, 146, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Hindelang-Gertner, C.; Stoeckel, M.E.; Porte, A.; Stutinsky, F. Colchicine effects on neurosecretory neurons and other hypothalamic and hypophysial cells, with special reference to changes in the cytoplasmic membranes. Cell Tissue Res. 1976, 170, 17–41. [Google Scholar] [CrossRef]
- Sato-Yoshitake, R.; Yorifuji, H.; Inagaki, M.; Hirokawa, N. The phosphorylation of kinesin regulates its binding to synaptic vesicles. J. Biol. Chem. 1992, 267, 23930–23936. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Sato-Yoshitake, R.; Kobayashi, N.; Pfister, K.K.; Bloom, G.S.; Brady, S.T. Kinesin associates with anterogradely transported membranous organelles in vivo. J. Cell Biol. 1991, 114, 295–302. [Google Scholar] [CrossRef]
- Okada, Y.; Sato-Yoshitake, R.; Hirokawa, N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. J. Neurosci. 1995, 15, 3053–3064. [Google Scholar] [CrossRef]
- Vale, R.D.; Reese, T.S.; Sheetz, M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985, 42, 39–50. [Google Scholar] [CrossRef]
- Vale, R.D.; Schnapp, B.J.; Mitchison, T.; Steuer, E.; Reese, T.S.; Sheetz, M.P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell 1985, 43, 623–632. [Google Scholar] [CrossRef]
- Peracchia, C. Low resistance junctions in crayfish. II. Structural details and further evidence for intercellular channels by freeze-fracture and negative staining. J. Cell Biol. 1973, 57, 54–65. [Google Scholar] [CrossRef]
- Markham, R.; Frey, S.; Hills, G.J. Methods for the enhancement of image detail and accentuation of structure in electron microscopy. Virology 1963, 20, 88–102. [Google Scholar] [CrossRef]
- Peracchia, C. Low resistance junctions in crayfish. I. Two arrays of globules in junctional membranes. J. Cell Biol. 1973, 57, 66–76. [Google Scholar] [CrossRef]
- Peracchia, C. Connexin/Innexin Channels in Cytoplasmic Organelles. Are There Intracellular Gap Junctions? A Hypothesis! Int. J. Mol. Sci. 2020, 21, 2163. [Google Scholar] [CrossRef]
- Hanna, R.B.; Keeter, J.S.; Pappas, G.D. The fine structure of a rectifying electrotonic synapse. J. Cell Biol. 1978, 79, 764–773. [Google Scholar] [CrossRef]
- Musil, L.S.; Goodenough, D.A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 1993, 74, 1065–1077. [Google Scholar] [CrossRef]
- Martin, P.E.; Evans, W.H. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 2004, 62, 378–387. [Google Scholar] [CrossRef]
- Koval, M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006, 16, 159–166. [Google Scholar] [CrossRef]
- Evans, W.H.; Ahmad, S.; Diez, J.; George, C.H.; Kendall, J.M.; Martin, P.E. Trafficking pathways leading to the formation of gap junctions. Novartis. Found. Symp. 1999, 219, 44–54, discussion 54–59. [Google Scholar] [PubMed]
- Falk, M.M. Cell-free synthesis for analyzing the membrane integration, oligomerization, and assembly characteristics of gap junction connexins. Methods 2000, 20, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchia, C. Potential Role of Fenestrated Septa in Axonal Transport of Golgi Cisternae and Gap Junction Formation/Function. Int. J. Mol. Sci. 2023, 24, 5385. https://doi.org/10.3390/ijms24065385
Peracchia C. Potential Role of Fenestrated Septa in Axonal Transport of Golgi Cisternae and Gap Junction Formation/Function. International Journal of Molecular Sciences. 2023; 24(6):5385. https://doi.org/10.3390/ijms24065385
Chicago/Turabian StylePeracchia, Camillo. 2023. "Potential Role of Fenestrated Septa in Axonal Transport of Golgi Cisternae and Gap Junction Formation/Function" International Journal of Molecular Sciences 24, no. 6: 5385. https://doi.org/10.3390/ijms24065385
APA StylePeracchia, C. (2023). Potential Role of Fenestrated Septa in Axonal Transport of Golgi Cisternae and Gap Junction Formation/Function. International Journal of Molecular Sciences, 24(6), 5385. https://doi.org/10.3390/ijms24065385





