Arteriosclerosis Derived from Cutaneous Inflammation Is Ameliorated by the Deletion of IL-17A and IL-17F
Abstract
:1. Introduction
2. Results
2.1. Histological Analysis
2.2. Observation of the Abdominal Aorta and Peri-Aortic Adipose Tissue Using Electron Microscopy
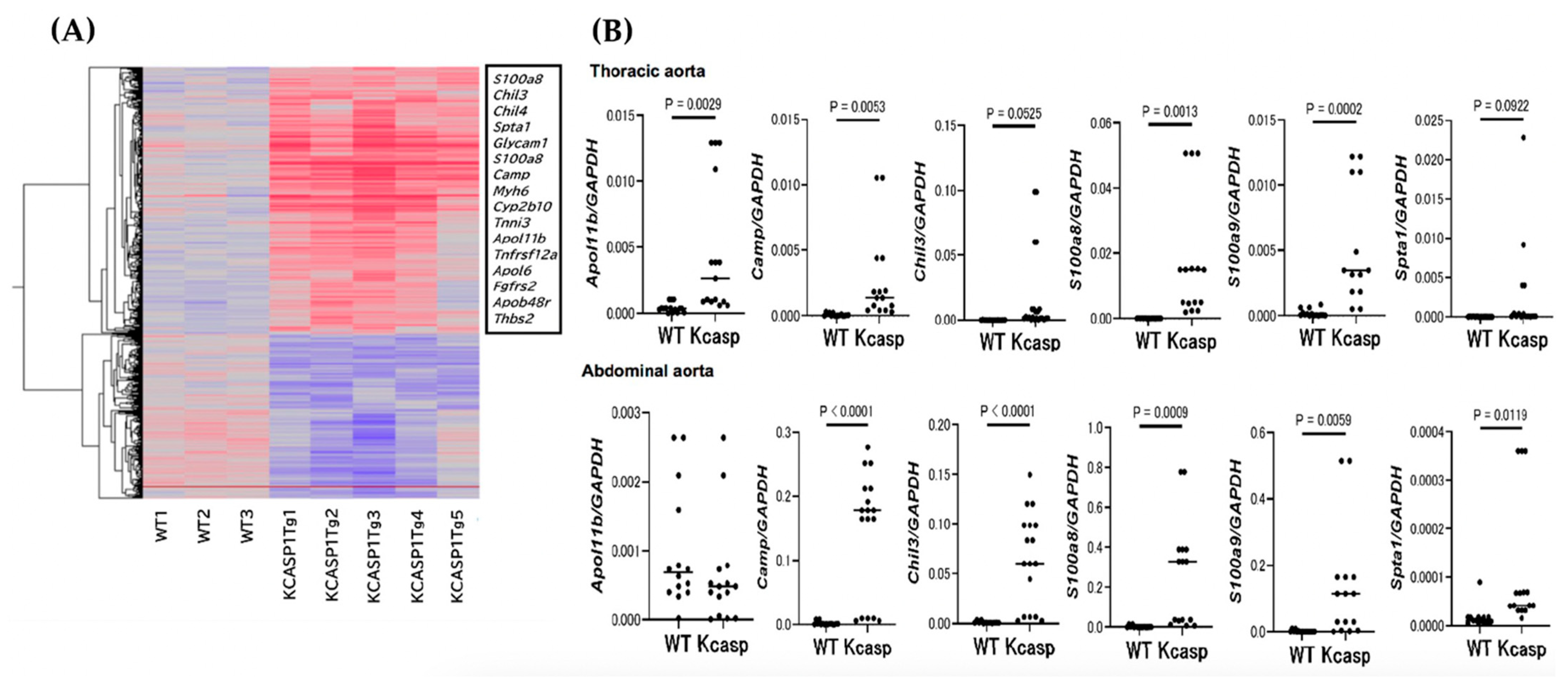
2.3. GeneChip and Real-Time Polymerase Chain Reaction Analysis for the Abdominal Aorta
2.4. Culture of Aorta-Constitute Cells
2.5. The Effect of Arteriosclerosis by IL-17 Deficiency
2.6. Snapping Tension in the Abdominal Aorta
3. Discussion
4. Materials and Methods
4.1. Mouse Study
4.2. Tissue Sampling, Histological Analysis, and Observation of the Abdominal Aorta and Adipose Tissue Using Electron Microscopy
4.3. GeneChip Analysis and Real-Time Polymerase Chain Reaction (Real-Time PCR)
4.4. Culture of Aorta-Constitute Cells
4.5. IL-17A/F Deficiency in KCASP1Tg Mice
4.6. Snapping Tension of Abdominal Aorta
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovici, B.B.; Sattar, N.; Prinz, J.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Stahle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J. Investig. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef] [Green Version]
- Fine, J.D.; Hall, M.; Weiner, M.; Li, K.P.; Suchindran, C. The risk of cardiomyopathy in inherited epidermolysis bullosa. Br. J. Dermatol. 2008, 159, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, J.D.; Johnson, L.B.; Weiner, M.; Stein, A.; Cash, S.; DeLeoz, J.; Devries, D.T.; Suchindran, C. National Epidermolysis Bullosa, R. Inherited epidermolysis bullosa and the risk of death from renal disease: Experience of the National Epidermolysis Bullosa Registry. Am. J. Kidney Dis. 2004, 44, 651–660. [Google Scholar] [CrossRef]
- Matsushima, Y.; Mizutani, K.; Goto, H.; Nakanishi, T.; Kondo, M.; Habe, K.; Isoda, K.; Mizutani, H.; Yamanaka, K. Emaciation, Congestive Heart Failure, and Systemic Amyloidosis in Severe Recessive Dystrophic Epidermolysis Bullosa: Possible Internal Complications Due to Skin-Derived Inflammatory Cytokines Derived from the Injured Skin. Dermatopathology 2020, 7, 41–47. [Google Scholar] [CrossRef]
- Herrero, L.; Shapiro, H.; Nayer, A.; Lee, J.; Shoelson, S.E. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. USA 2010, 107, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Matsushima, Y.; Mizutani, K.; Kawakita, F.; Fujimoto, M.; Okada, K.; Kondo, M.; Habe, K.; Suzuki, H.; Mizutani, H.; et al. The Stenosis of Cerebral Arteries and Impaired Brain Glucose Uptake by Long-Lasting Inflammatory Cytokine Release from Dermatitis Is Rescued by Anti-IL-1 Therapy. J. Investig. Dermatol. 2018, 138, 2280–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, K.; Nakanishi, T.; Saito, H.; Maruyama, J.; Isoda, K.; Yokochi, A.; Imanaka-Yoshida, K.; Tsuda, K.; Kakeda, M.; Okamoto, R.; et al. Persistent Release of IL-1s from Skin Is Associated with Systemic Cardio-Vascular Disease, Emaciation and Systemic Amyloidosis: The Potential of Anti-IL-1 Therapy for Systemic Inflammatory Diseases. PLoS ONE 2014, 9, e104479. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Okada, K.; Nakanishi, T.; Mizutani, K.; Matsushima, Y.; Kondo, M.; Habe, K.; Mizutani, H.; Seo, N. Skin inflammation leads immunoglobulin G aggregation and deposition in multiple organs. J. Dermatol. Sci. 2017, 88, 146–148. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Mizutani, K.; Iida, S.; Matsushima, Y.; Umaoka, A.; Kondo, M.; Habe, K.; Yamanaka, K. Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model. Int. J. Mol. Sci. 2021, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Nakanishi, T.; Momose, F.; Ichishi, M.; Mizutani, K.; Matsushima, Y.; Umaoka, A.; Kondo, M.; Habe, K.; Hirokawa, Y.; et al. IL-17A Is the Critical Cytokine for Liver and Spleen Amyloidosis in Inflammatory Skin Disease. Int. J. Mol. Sci. 2022, 23, 5726. [Google Scholar] [CrossRef]
- Umaoka, A.; Takeuchi, H.; Mizutani, K.; Seo, N.; Matsushima, Y.; Habe, K.; Hagimori, K.; Yamaguchi, Y.; Ikeda, T.; Yamanaka, K. Skin Inflammation and Testicular Function: Dermatitis Causes Male Infertility via Skin-Derived Cytokines. Biomedicines 2020, 8, 293. [Google Scholar] [CrossRef]
- Mizutani, K.; Isono, K.; Matsushima, Y.; Okada, K.; Umaoka, A.; Iida, S.; Habe, K.; Hagimori, K.; Yamazaki, H.; Yamanaka, K. Inflammatory Skin-Derived Cytokines Accelerate Osteoporosis in Mice with Persistent Skin Inflammation. Int. J. Mol. Sci. 2020, 21, 3620. [Google Scholar] [CrossRef]
- Matsushima, Y.; Mizutani, K.; Iida, S.; Ichishi, M.; Nakanishi, T.; Okada, K.; Umaoka, A.; Kondo, M.; Habe, K.; Watanabe, M.; et al. Severe skin inflammation leads to salivary gland atrophy and dysfunction. J. Dermatol. 2022, 49, 642–647. [Google Scholar] [CrossRef]
- Mizutani, K.; Shirakami, E.; Ichishi, M.; Matsushima, Y.; Umaoka, A.; Okada, K.; Yamaguchi, Y.; Watanabe, M.; Morita, E.; Yamanaka, K. Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines. Int. J. Mol. Sci. 2020, 21, 3367. [Google Scholar] [CrossRef] [PubMed]
- von Stebut, E.; Reich, K.; Thaci, D.; Koenig, W.; Pinter, A.; Korber, A.; Rassaf, T.; Waisman, A.; Mani, V.; Yates, D.; et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J. Investig. Dermatol. 2019, 139, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, K.; Tanaka, M.; Tsutsui, H.; Kupper, T.S.; Asahi, K.; Okamura, H.; Nakanishi, K.; Suzuki, M.; Kayagaki, N.; Black, R.A.; et al. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J. Immunol. 2000, 165, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Vanhollebeke, B.; Pays, E. The function of Apolipoproteins L. Cell. Mol. Life Sci. 2006, 63, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Uzureau, S.; Coquerelle, C.; Vermeiren, C.; Uzureau, P.; Van Acker, A.; Pilotte, L.; Monteyne, D.; Acolty, V.; Vanhollebeke, B.; Van den Eynde, B.; et al. Apolipoproteins L control cell death triggered by TLR3/TRIF signaling in dendritic cells. Eur. J. Immunol. 2016, 46, 1854–1866. [Google Scholar] [CrossRef] [Green Version]
- Mimmack, M.L.; Ryan, M.; Baba, H.; Navarro-Ruiz, J.; Iritani, S.; Faull, R.L.; McKenna, P.J.; Jones, P.B.; Arai, H.; Starkey, M.; et al. Gene expression analysis in schizophrenia: Reproducible up-regulation of several members of the apolipoprotein L family located in a high-susceptibility locus for schizophrenia on chromosome 22. Proc. Natl. Acad. Sci. USA 2002, 99, 4680–4685. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, T.E.; Logan, N.; Ruckerl, D.; Humbles, A.A.; Allan, S.M.; Papayannopoulos, V.; Stockinger, B.; Maizels, R.M.; Allen, J.E. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 2014, 15, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Kzhyshkowska, J.; Yin, S.; Liu, T.; Riabov, V.; Mitrofanova, I. Role of chitinase-like proteins in cancer. Biol. Chem. 2016, 397, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Song, L.; Shen, L.; Zhang, K.; Lv, Y.; Gao, M.; Ma, J.; Wan, Y.; Gai, Z.; Liu, Y. Mutational Characteristics of Causative Genes in Chinese Hereditary Spherocytosis Patients: A Report on Fourteen Cases and a Review of the Literature. Front. Pharmacol. 2021, 12, 644352. [Google Scholar] [CrossRef] [PubMed]
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadjichristos, C.; Berti, M.; Pelli, G.; James, R.W.; Mach, F.; Gabay, C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc. Res. 2005, 66, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, J.; Yokochi, A.; Maruyama, K.; Nosaka, S. Acetylcholine-induced endothelium-derived contracting factor in hypoxic pulmonary hypertensive rats. J. Appl. Physiol. 1999, 86, 1687–1695. [Google Scholar] [CrossRef] [Green Version]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, T.; Iida, S.; Maruyama, J.; Urushima, H.; Ichishi, M.; Matsushima, Y.; Mizutani, K.; Nakayama, Y.; Sugioka, K.; Nishimura, M.; et al. Arteriosclerosis Derived from Cutaneous Inflammation Is Ameliorated by the Deletion of IL-17A and IL-17F. Int. J. Mol. Sci. 2023, 24, 5434. https://doi.org/10.3390/ijms24065434
Nakanishi T, Iida S, Maruyama J, Urushima H, Ichishi M, Matsushima Y, Mizutani K, Nakayama Y, Sugioka K, Nishimura M, et al. Arteriosclerosis Derived from Cutaneous Inflammation Is Ameliorated by the Deletion of IL-17A and IL-17F. International Journal of Molecular Sciences. 2023; 24(6):5434. https://doi.org/10.3390/ijms24065434
Chicago/Turabian StyleNakanishi, Takehisa, Shohei Iida, Junko Maruyama, Hayato Urushima, Masako Ichishi, Yoshiaki Matsushima, Kento Mizutani, Yuichi Nakayama, Kyoko Sugioka, Mai Nishimura, and et al. 2023. "Arteriosclerosis Derived from Cutaneous Inflammation Is Ameliorated by the Deletion of IL-17A and IL-17F" International Journal of Molecular Sciences 24, no. 6: 5434. https://doi.org/10.3390/ijms24065434




